Page 1
HI 4103
CADMIUM
COMBINATION
HI 4003
CADMIUM
Instruction Manual
HI 4003HI 4003
HI 4003
HI 4003HI 4003
HI 4103HI 4103
HI 4103
HI 4103HI 4103
Cadmium Ion
Selective Electrode
Half-cell
Combination
1
Page 2

HI 4003 Cadmium Half-cellHI 4003 Cadmium Half-cell
HI 4003 Cadmium Half-cell
HI 4003 Cadmium Half-cellHI 4003 Cadmium Half-cell
HI 4103 Cadmium Combination ElectrodeHI 4103 Cadmium Combination Electrode
HI 4103 Cadmium Combination Electrode
HI 4103 Cadmium Combination ElectrodeHI 4103 Cadmium Combination Electrode
I. I.
Introduction:Introduction:
I.
Introduction:
I. I.
Introduction:Introduction:
The Hanna HI 4003 and HI 4103 are ion selective electrodes designed for the measurement of cadmium ions in
aqueous solutions. The HI 4003 is a solid state half-cell
sensor that requires a separate reference. The HI 4103 is a
combination ion selective electrode.
IIII
SpecificationsSpecifications
II.
Specifications
IIII
SpecificationsSpecifications
Type: Solid State electrode with
a Cadmium sulfide/ silver
sulfide membrane.
++
2
+
Ion(s) measured: Cadmium (Cd
++
)
Measurement range: 0.1 M to 1X 10
-7
M
11,200 to 0.01 ppm
Interfering ions: Silver and mercury must
be absent. Any ion that forms a more insoluble sulfide than
cadmium sulfide will interfere with the electrode response.
These include lead (Pb2+)and cupric ions (Cu2+). Oxidation
of the cadmium surface by ferric ion( Fe3+) will severely
poison the electrode. Ions that can form complexes with
cadmium ions will reduce the measured concentration.
Examples include bromide, chloride and EDTA.
Operating Temperature: 0-80°C
Operating pH: 2 to12 pH
concentration dependant
(4.5-7.5 recommended)
Dimensions: 12 mm (OD) X 120 mm
nominal insertion
(0.47” X 4.72”)
Connection: BNC
2
Page 3

III. III.
Theory of OperationTheory of Operation
III.
Theory of Operation
III. III.
Theory of OperationTheory of Operation
::
:
::
The HI 4103 or HI 4103 cadmium electrodes are potentiometric devices used for the rapid determination of free cadmium ions in samples and as a detector for the titration of
cadmium with EDTA.
Cadmium sensors are “electrodes of
the third kind” because they detect cations which also form
low-solubility salts with sulfide anions that also form lowsolubility salts with silver.
The electrode functions as a sensor or ionic conductor. The
HI 4003 requires a separate reference electrode to complete
its electrolytic circuit. The HI 4103 incorporates a reference
electrode. The mixed cadmium sulfide/ silver sulfide membrane produces a potential change due to changes in the
sample’s cadmium ion activity. When the ionic strength of
the sample is fixed by the addition of ISA, the voltage is
proportional to the concentration of cadmium ions in solution and the electrode follows the Nernst equation.
E= E
+ 2.3 RT/nF log A
a
ion
E= observed potential
Ea= Reference and fixed internal voltages
R= gas constant (8.314 volt coulomb/K Mole)
n= Charge on cadmium ion (2+)
A i=ion activity in sample
T= absolute temperature in K
F= Faraday constant (9.648 x 104 coulomb/mole)
3
Page 4
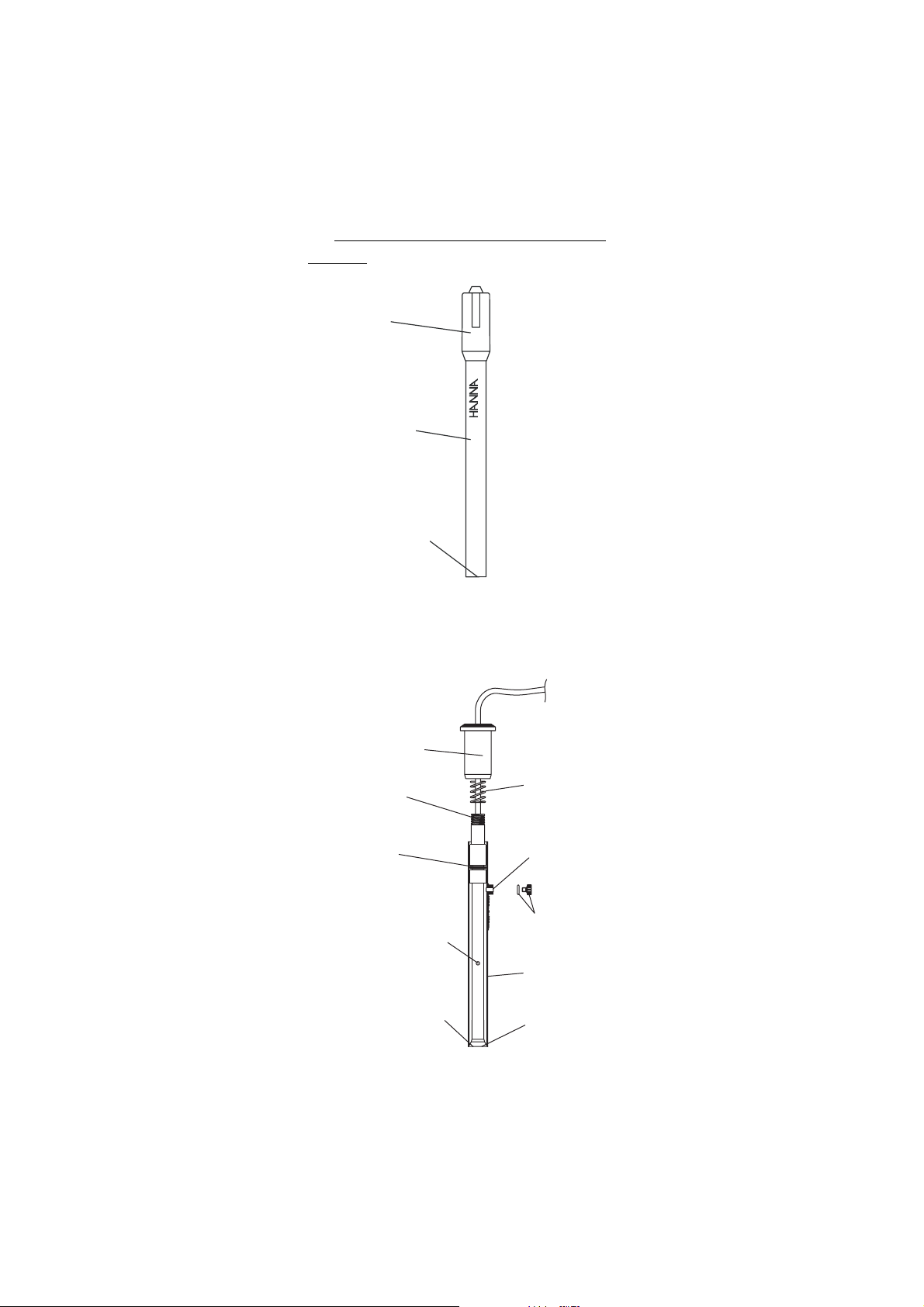
IV. IV.
Design elements of the HI 4003 and HI 4103Design elements of the HI 4003 and HI 4103
IV.
Design elements of the HI 4003 and HI 4103
IV. IV.
Design elements of the HI 4003 and HI 4103Design elements of the HI 4003 and HI 4103
electrodeselectrodes
electrodes
electrodeselectrodes
Cap
HI 4003
Sensor
Handle
Sensing
Membrane
Upper Cap
Upper
Threads
O-Ring
CADMIUM
Spring
Fill Hole
Ceramic Junction
on Inner Stem
Liquid junction
O-Ring and
Plug
Outer Sleeve
Sensing
Membrane
4
Page 5
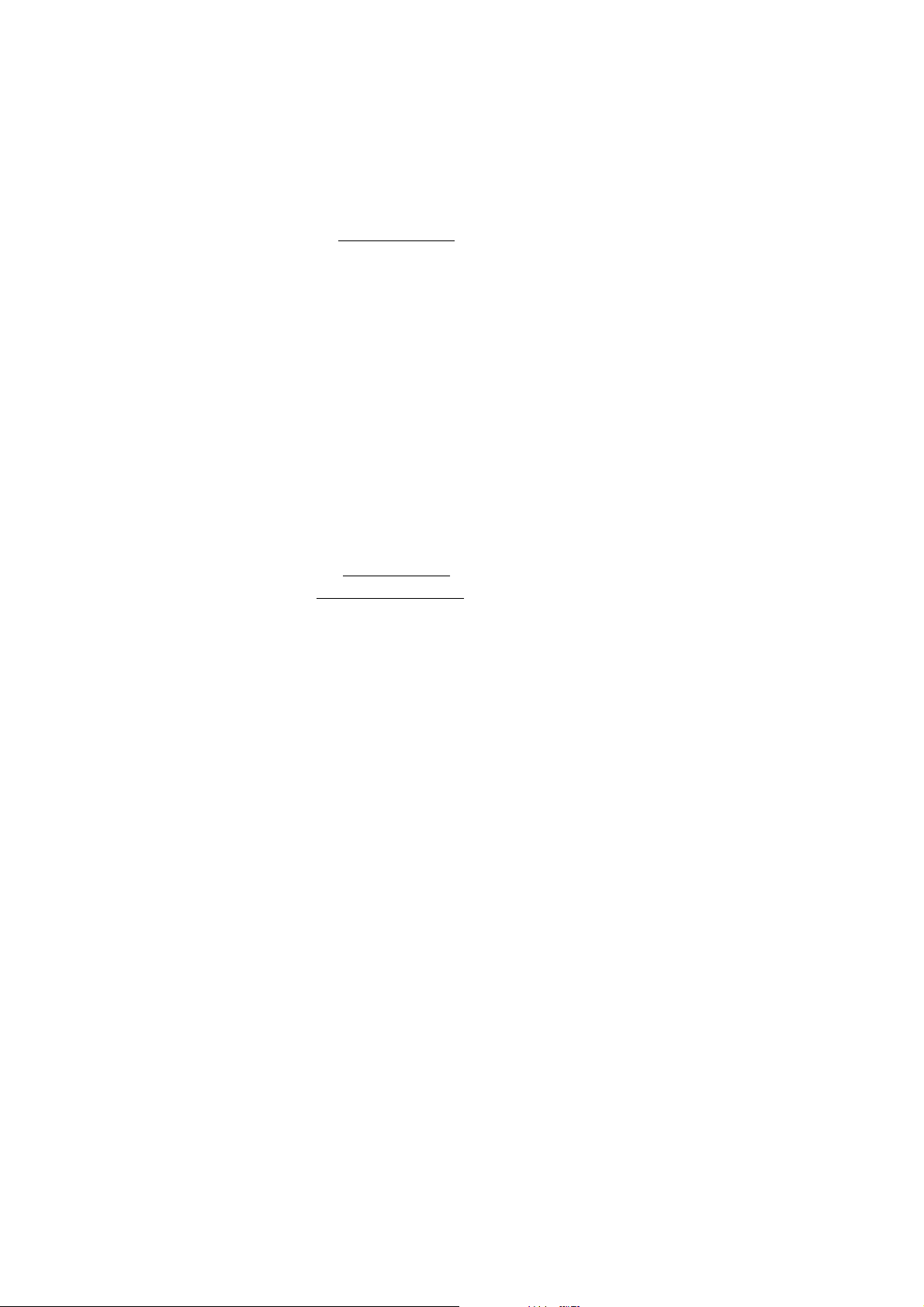
V. V.
Equipment required:Equipment required:
V.
Equipment required:
V. V.
Equipment required:Equipment required:
• Hanna HI 5315 Double Junction Reference Electrode
with HI 7072 Fill Solution for use with HI 4003 halfcell.
• Hanna HI 4222 pH/ISE/mV meter or other suitable
ion or pH/mV meter. (Note: log/linear graph paper is
useful if an ISE (ion) meter is not available).
• Hanna HI 180 Magnetic Stirrer or equivalent with TFE
coated stirring bars (HI 731320). Note: isolate beakers from stirrer motor heat by placing insulating
material such as foam or cork between them.
• Hanna HI 76404 Electrode Holder or equivalent.
• Plastic beakers (HI 740036P) or other suitable mea-
surement vessel.
VI. VI.
Solutions RequiredSolutions Required
VI.
Solutions Required
VI. VI.
Solutions RequiredSolutions Required
For Cadmium Measurements
0.1 M cadmium standard, (500 mL) HI 4003-01
ISA, (500 mL) HI 4000-00
For direct cadmium measurements- Using volumetric pipettes and glassware, make dilutions of HI 4003-01 to
bracket the concentration of the samples. Note: 0.1M cadmium standard is equivalent to 11,200 ppm. Standards
with concentrations < 10
-3
M (112 ppm) should be prepared daily. Store in tightly closed plastic bottle. To 100
parts standard or sample add 2 parts of ISA.
Please note: cadmium compounds are toxic by ingestion or
skin contact. Take appropriate precautions.
5
Page 6

VIIVII
General GuidelinesGeneral Guidelines
VII.
General Guidelines
VIIVII
General GuidelinesGeneral Guidelines
• Concentrated samples (>0.10 M) should be diluted
before measurement. Multiply the final result by the
corresponding dilution factor.
• Calibration standards and sample solutions should
have the same ionic strength.
• For high ionic strength samples, use standard addi-
tion or titration methods.
• Calibration standards and sample solutions should
be at same temperature.
• The magnetic stirrer may generate heat. Thermally
insulate beaker containing standard or sample from
magnetic stirrer by placing cork or other insulative
sheet between beaker and stirrer plate.
• Calibration standards and sample solutions should
be stirred at the same rate using identical sized TFE
coated stir bars.
• Rinse electrode pair with distilled or deionized water
between samples and gently dab dry with soft disposable absorbent toweling. Do not rub electrodes.
• Avoid Cd(OH)
precipitation or hydrogen ion interfer-
2
ence by maintaining pH of samples between 4.5 and
7.5 pH. Use Nitric acid or sodium hydroxide to adjust
pH.
• Presoaking the cadmium sensor tip in a dilute stan-
dard will optimize response. Use concentrations approximately10-3M.
• Presoaking the cadmium tip in a mild reducing solu-
tion such as ascorbic acid for a few minutes may be
helpful when oxidation of the membrane surface is
an issue between sample measurments.
• A scratched, pitted, or oxidized pellet surface can cause
drift, a loss of low level response, or poor repeatability.
Optimum response can be restored by removing the
damaged surface with the microabrasive strip HI 4000-
70. Use gloves to protect skin. Cadmium dust is toxic.
• Avoid large changes in temperature (thermal shock)
as it may damage the sensor.
6
Page 7

• Gas bubbles may form from solution out-gassing due
to temperature change. Gently tap body of sensor to
dislodge them from sensing membrane.
HI 4003
• Remove protective cover from sensor tip.
HI 4103
• Remove the protective plastic wrap that covers the
ceramic junction before assembling sensor for the first
time.
• HI 7072 reference fill solution should be added daily
to electrolyte reservoir before electrode use.
• During measurement always operate electrode with
the fill hole open.
• During normal use, fill solution will slowly drain out
of the tapered cone junction at the lower portion of the
electrode. Excessive loss (>4 cm drop within 24 hours)
is not normal. If this occurs verify cap is tightened and
the interface between the internal cone and outer
body is free of debris.
• Add fill solution daily to maintain a good head pres-
sure. For optimum response, this level should be maintained and not be allowed to drop more than 2-3 cm
(1-inch) below fill hole. Fill solution must cover the
ceramic found on the inner stem.
• If an erratic measurement occurs, check to see if for-
eign matter is seen trapped near the internal cone.
Drain, wipe off outer cone while depressing cap, release cap and refill with fresh fill solution.
7
Page 8

VIII. VIII.
Electrode PreparationElectrode Preparation
VIII.
Electrode Preparation
VIII. VIII.
Electrode PreparationElectrode Preparation
HI 4003
1. Remove protective cover from sensor tip.
2. Prepare reference electrode by filling outer electrolyte
reservoir with HI 7072.
3. Place sensor and reference electrodes into electrode
holder and connect cable connectors to meter.
HI 4103
1. Unwrap plastic film seal found over ceramic junction
on inner stem and discard. This is only used for shipping and long term storage.
2. Rinse inner stem with deionized water making certain
to wet the o-ring found on the inner stem.
Remove
Water
Deionized
3. Reassemble electrode by gently pushing the inner
assembly into the outer body, sliding spring down
cable, and screwing cap into place.
4. Remove fill hole cover and o-ring on fill hole spout.
5. Using the dropper pipette provided, add a few drops
HI 7072 fill solution to the electrode, wetting the oring and rinsing out the fill solution chamber.
8
Parafilm
Page 9

6. Holding the body of the electrode gently press upper
cap with your thumb. This permits the fill solution to
drain out of the body. Release cap and verify electrode returns to its original position. (You may need to
gently assist for this to occur).
COMBINATION
CADMIUM
HI 4103
7. Tighten the electrode cap onto the body and fill electrode body until fill solution volume is just below fill
hole.
8. Position electrode in a Hanna HI 76404 electrode
holder (or equivalent) and connect plug to meter.
9
Page 10

IX. IX.
Quick Check of Electrode SlopeQuick Check of Electrode Slope
IX.
Quick Check of Electrode Slope
IX. IX.
Quick Check of Electrode SlopeQuick Check of Electrode Slope
• Connect sensors to pH/mV/ISE meter
• Place meter in mV mode.
• Place 100 mL of deionized water into a beaker with
stir bar and 2 mL of HI 4000-00 ISA. Add stir bar and
place on magnetic stirrer.
• Place electrodes into prepared sample.
• Add 1 mL of the stock standard HI 4003-01 (or .01 M
or .001 M standard by dilution) to the beaker.
Record the mV value when reading has stabilized.
• Add an additional 10 mL of standard HI 4003-01 (or
same dilute standard) to the solution. Record the mV
when reading has stabilized. This value should be
more positive than the previous noted.
• Determine the difference between the two mV values.
An acceptable value for this slope is +27 ± 4 mV
near 25°C.
X. X.
Corrective actionCorrective action
X.
Corrective action
X. X.
Corrective actionCorrective action
• Verify protective cap has been removed (HI 4003).
• Verify plastic film has been removed from inner stem
(HI 4103).
• Verify electrodes are connected properly to meter and
meter is powered.
• Verify standard has been properly stored. Remake
standards if appropriate.
• If the sensor slope just misses the suggested slope
window, soaking the sensor in a dilute standard may
solve the problem. (<10
-3
M standard).
• A scratched, pitted or oxidized sensing surface can be
polished with HI 4000-70 polishing strip. Cut off
approximately 1 inch of the micro-abrasive strip. Wear
protective gloves. Wet the frosted side with deionized
water and place against damaged membrane of the
electrode. Place your gloved thumb against the shiny
backing and slowly rotate back and forth while
applying gentle pressure. Continue polishing until
you are satisfied with the surface. If dark deposits
10
Page 11

appear on polishing strip move the paper slightly
and continue polishing.
• If the membrane is damaged, the response becomes
extremely sluggish, or the slope of the electrode has
decreased significantly, and procedures above have
not helped, the sensor should be replaced.
XI. XI.
Direct Calibration and MeasurementDirect Calibration and Measurement
XI.
Direct Calibration and Measurement
XI. XI.
Direct Calibration and MeasurementDirect Calibration and Measurement
This method is a simple procedure for measuring many
samples. A direct reading ISE meter (HI 4222 or equivalent) determines concentration of the unknown by a direct
reading after calibrating the meter with the standards. The
meter is calibrated with two or more freshly made standards that are in the linear measurement range of the
unknowns. Two mL of ISA (HI 4000-00) is added to each
100 mL volume of standard or sample. More calibration
standards are required in non-linear regions. Unknowns
are read directly.
Samples with concentration greater than 0.1 M should be
diluted to be within the working range of the electrodes.
The final result must be multiplied by the corresponding
dilution factor to determine the actual concentration.
A pH/mV meter in mV mode with semi-log graph paper
may also be used. Two or more freshly prepared standards
that are in the measurement range of the unknowns are
measured in mV mode on the meter.
These values are plotted on the semi-log paper and the
points are connected to form a straight-line curve. When
samples are measured, their mV values are converted to
concentration by following the mV to the concentration axis
on the semi-log plot.
11
Page 12

Procedure
1) Follow sections VIII and IX to prepare sensors for
measurement.
2) Follow section VI to prepare standards / solution.
Standards should bracket and fall within the range of
interest.
Two mL HI 4000-00 ISA is added to 100 mL of both
samples and standards. Add stir bar and mix before
taking measurements.
3) Follow section VII; General Guidelines to optimize test
set-up.
4) During calibration it is best to start with lower concentration samples first. Wait for a stable measurement
before recording values. Slightly longer equilibrations are required at lower concentrations .
5) To prevent carry over and contamination of samples,
rinse sensors with deionized and dab dry with
absorbant laboratory tissue between samples.
Typical Linearity of HI 4003 and HI 4103 Cadmium Sensors
-100
-120
-140
-160
-180
mV
-200
-220
-240
-260
-280
1.0 2.0 3.0 4.0 5.0 6.0 7.0
XII. XII.
Other Measurement TOther Measurement T
XII.
Other Measurement T
XII. XII.
Other Measurement TOther Measurement T
-LOG [M]
echniquesechniques
echniques
echniquesechniques
Known Addition (for Cd2+)
An unknown concentration can be determined by adding a
known amount (volume and concentration) of measured
ion to a known volume of the sample. A mV value is taken
before and after the addition of standard, and using the
equation provided, the unknown concentration is found.
12
Page 13

This technique is called Known Addition. The method can
use an ideal sensor slope, but actual determined slopes at
the temperature of measurement should be used if known.
The volume and concentration of the added standard must
cause a mV change of at least 8 mV. This method is
preprogrammed in the Hanna HI 4222 pH/ISE/mV meter,
which simplifies the method greatly. The method works
well for samples with high ionic strengths.
Example: Cadmium ion determination in samples with
concentrations less than 1 X 10
-3
M using known addition.
1. A 50 mL sample of unknown concentration (Vsample)
is placed in a clean plastic beaker with a cadmium
sensor with 1 mL of HI 4000-00 ISA (V
ISA=1 mL
) a. The
stable mV value (mV 1) is recorded after the sample
is mixed.
2. 5mL (Vstd) of 10-1M standard (Cstd) is added to the
beaker and the mV value increases as does the con-
centration. ∆E is calculated as mV2-mV1. The un-
known cadmium concentration in the original sample
(Csample) can then be determined by the following
equation.
C
sample
=
C
standardVstandard
(V
)10
T
∆E/S
- (VS’)
V
V
S’
sample
(V
sample+Vstandar d+VISA
(V
sample+VISA
)= V
S’
)= V
T
3. The procedure can be repeated with a second standard addition to verify slope and operation of the
method.
Titration of cadmium
A cadmium electrode may be used as an indicator to follow
the progress and detect the endpoint of a complexation
titration of cadmium ions with EDTA standard. During the
titration the sensor follows the decreasing cadmium concentration while small additions of EDTA titrant are added.
The cadmium ion reacts with the EDTA and removes
13
Page 14

the free ions from the measurement pool.
Cd2+ + EDTA <—>Cd-EDTA
The EDTA reacts with the cadmium ion forming a complex.
The EDTA also can react with a host of other metals. Working
at a pH between 4 and 6 optimizes the pH of the Cd-EDTA
complex, and also discourages complexing with other ions
that form stable complexes at high pH. Consult a
comprehensive Analytical Chemistry Treatise for techniques
concerning your particular interferent. The end point is
assumed to be at the inflection of the mV versus titrant
volume curve. Measurements may be automated by use of
the Hanna Titrator HI 901 or titrated manually.
Notes: .
• Addition of ISA is suggested (1part/50parts sample).
• Use of the an acetic acid buffer may increase the end
point break. Prepare buffer by diluting glacial acetic
acid (17.4 M) to 1M and adjust to pH 5 with KOH.
Add 1part buffer to 10 parts sample.
• The EDTA titrant should be 10X more concentrated
than the expected cadmium concentration.
• Competing metals will need to be identified and
removed or masked.
14
Page 15

XIII.XIII.
pHpH
XIII.
pH
XIII.XIII.
pHpH
HI 4103 and HI 4003 electrodes may be used in solutions
with values between 4.5 and 7.5 pH. Samples above pH
7.5 may form Cd(OH)
. Adjust these samples with nitric
2
acid.
XIV. SXIV. S
torage and Care of the HI 4003 andtorage and Care of the HI 4003 and
XIV. S
torage and Care of the HI 4003 and
XIV. SXIV. S
torage and Care of the HI 4003 andtorage and Care of the HI 4003 and
HI 4103 sensors HI 4103 sensors
HI 4103 sensors
HI 4103 sensors HI 4103 sensors
The HI 4003 and HI 4103 sensor can be stored in very
dilute standards (<10-3M) for short periods of time.
The HI 4003 should be stored dry with it’s protective
cap on for longer periods. For longer term storage of the
model HI 4103 combination electrode ;Drain and wash
of salts with distilled or deionized water. Unscrew the
upper cap and move outer sleeve up cable. Wrap the
ceramic junction on the inner stem with Parafilm® or
other sealing wrap. Place the protective cap provided
over the dried membrane and store in original storage
box provided with electrode.
2+2+
2+
XVXV
. .
Conversion TConversion T
XV
.
Conversion T
XVXV
. .
Conversion TConversion T
ables For Cdables For Cd
ables For Cd
ables For Cdables For Cd
2+2+
MultiplyMultiply
Multiply
MultiplyMultiply
Moles/L (M) to ppm (mg/L) 1.124 x 10
ppm (mg/L) to M (moles/L) 8.896 x 10
15
5
-6
Page 16

MAN4103 02/07
WARRANTY WARRANTY
WARRANTY
WARRANTY WARRANTY
Hanna Instruments Ion Selective Electrodes are warranted
to be free of defects in material and workmanship for 6
months from date of purchase when used for their intended
purpose and maintained according to instructions. If they
fail to work when first used contact your dealer immediately.
Damage due to accidents, misuse, misapplication, tampering
or lack of prescribed maintenance is not covered.
Hanna Instruments reserves the right to modify the design,
construction or appearance of its products without advance
notice.
16
 Loading...
Loading...