Page 1
Instruction Manual
HI 3855
Cyanide
Test Kit
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instructions carefully before using the
chemical test kit. It will provide you with the necessary
information for correct use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
HI 3855A-0 Cyanide Reagent, 1 bottle (17 g);
•
HI 3855B-0 reagent, packets (100 pcs)
•
HI 3855C-0 reagent, packets (100 pcs)
•
• 1 checker disc;
• 2 glass vials with caps;
1 plastic pipette (3 mL);
•
• 1 spoon
Note: Any damaged or defective item must be returned in
.
its original packing materials.
;
;
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 0 to 0.30 mg/L (ppm) as Cyanide
Smallest Increment 0.01 mg/L in the 0.00-0.15 range
0.05 mg/L in the 0.15-0.30 range
Analysis Method Colorimetric
Sample Size 10
Number of Tests 100
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 580 g (20.4 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Cyanide refers to all of the CN groups in cyanide-compounds
that can be determined as the cyanide ion CN
natural waters the molecular HCN form predominates. In
solutions of metal cyanides, the CN group may also be
present as a complex of varying stability.
Cyanides are extensively used for extraction of silver/gold
ores, metal-cleaning and electroplating baths, coke ovens
and other chemical processes. There are mainly two chemical
treatments to remove cyanides from waste-waters: one is
chlorination and the other is the alkaline method.
CAUTION: cyanides and their solutions liberate very
toxic gases when in contact with acids!
Note:
mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
Cyanides react with the pyridine-pyrazolone reagent to form
a blue complex in neutral buffered solution. The absorbance
of this colored product is proportional to the concentration of
cyanide present in the aqueous sample.
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
Note: Temperature is a very important
parameter for this test method. For
best results the sample should have
a temperature not exceeding 20°C.
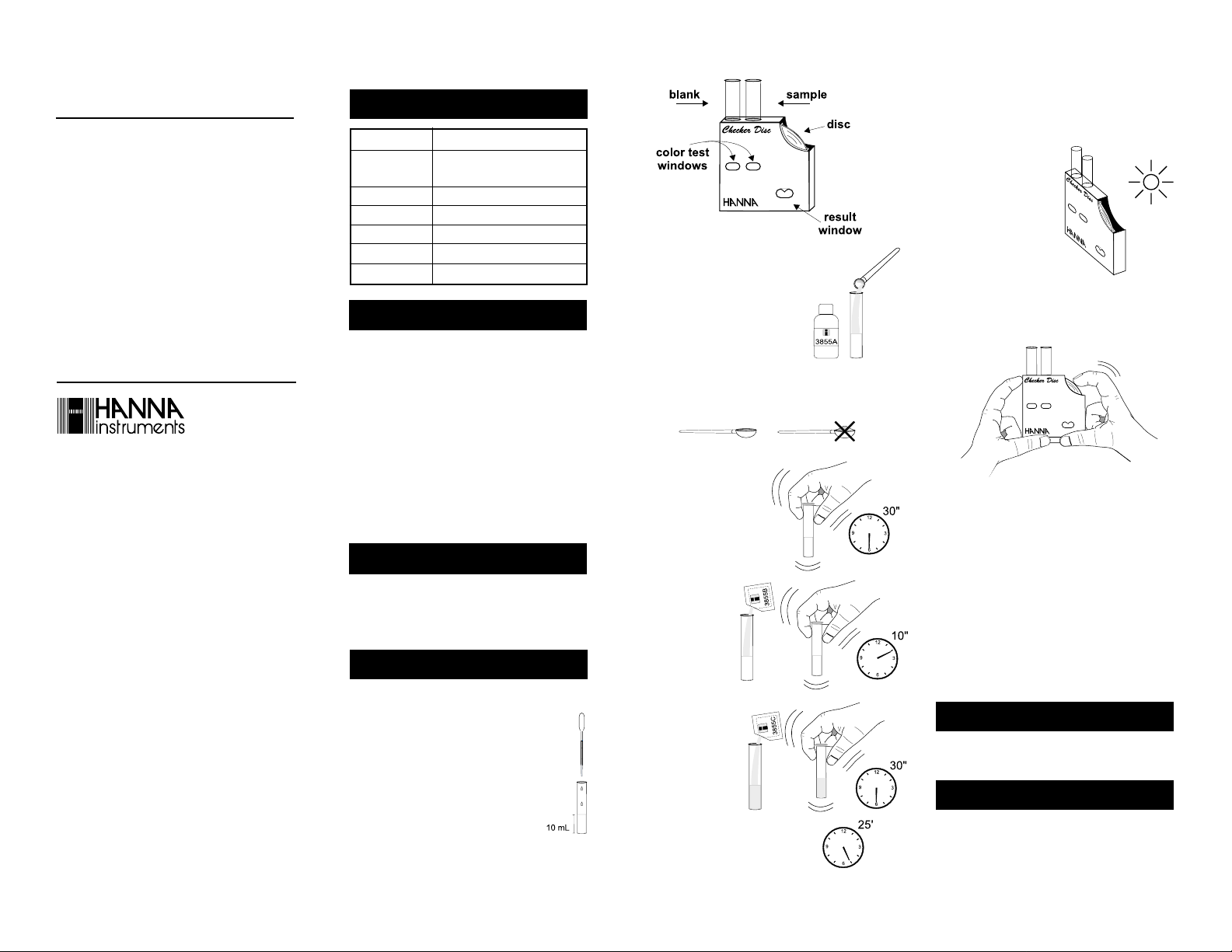
• Using the plastic pipette, fill each glass
vial with 10 mL of sample, up to the
mark.
• Insert one of them into the left hand
ISTR3855 10/99 PRINTED IN ITALY
opening of the checker disc. This is the blank.
mL
-
. In most
• Add to the other glass vial 1
level spoon of HI 3855A-0 Cyanide Reagent. Remember to close
the reagent bottle immediately
after use.
Note: Pay attention to the way the
spoon is filled:
- do not overfill it;
- do not press the powder.
• Replace the cap immediately, to prevent the escape
of chlorine gas which is developed during the reaction,
and shake gently for 30
seconds.
• Wait for 30 seconds
and add the content of one packet
of HI 3855B-0 reagent. Replace the
cap and shake gently for 10 seconds.
• Immediately add
the content of one packet of HI
3855C-0 reagent,
replace the cap and
shake vigorously until the powder has
completely dissolved.
Keep the vial
capped.
• Wait for 25 minutes to allow color to
develop. This is the reacted sample.
Note: Shake the vial 4 or 5 times vigorously during the first
20 minutes of the reaction time. The result is not
affected by undissolved reagent powder.
• Remove the cap and insert
the reacted sample into the
right hand opening of the
checker disc.
• Hold the checker disc so
that a light source illuminates the samples from the
back windows.
• Rotate the disc while looking at the color test windows
and stop when you find the color match. Read the
value in the result window and record it in mg/L (or
ppm) of Cyanide as CN
For best results: Intensely colored samples will make the color
matching determination difficult and they should be
adequately treated before performing the test. Suspended matter in large amounts should be removed
by prior filtration.
Oxidizing (like chlorine) or reducing agents (such as
sulfide or sulfur dioxide) are known to interfere with the
test. Distillation will remove these.
Samples with high pH values should be adjusted to
approximately pH 7 before testing.
Caution: Ultraviolet radiation may cause fading of colors.
When not in use, keep the disc protected from light, in
a cool and dry place.
Standard Methods for the Examination of Water and Waste-
th
water, 18
edition, 1992
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
-
.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
 Loading...
Loading...