Page 1
Instruction Manual
HI 3854
Zinc Test Kit
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 0 to 3.0 mg/L (ppm) as Zinc
Smallest Increment 0.6 ppm
Analysis Method Colorimetric
Sample Size 20 mL
Number of Tests 100
Case Dimensions 230x59x70 mm (9.0x2.3x2.8")
Shipping Weight 250 g (8.8 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
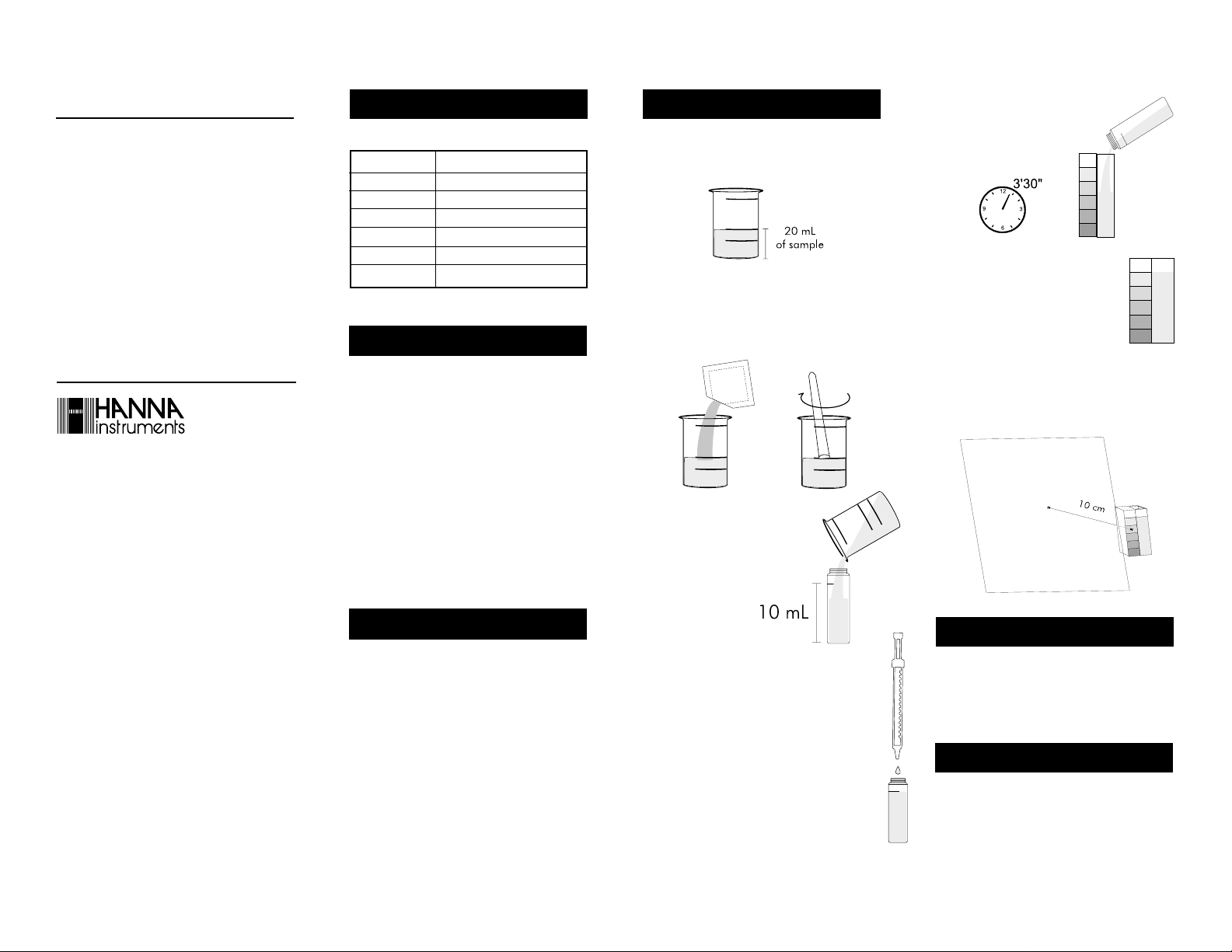
• Fill the plastic vessel with 20 mL of the sample, up to
the mark.
• Add 1 packet of reagent HI 3854A-0 and mix, using
the plastic spoon, until the powder is completely dissolved.
• Wait 3 minutes and 30 seconds to allow color to
develop. Fill the color comparator cube with 5 mL of
the reacted sample.
• Determine which color best
matches the solution in the
cube and record the result as
mg/L (ppm) of zinc.
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instructions carefully before using the
chemical test kit. It will provide you with the necessary
information for correct use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
HI 3854A-0 Reagent, packets (100 pcs);
•
HI 93731B-0 Zinc Reagent B (Cyclohexanone), 2
•
bottles (60 mL);
1 color comparator cube;
•
1 glass cuvet (10 mL) with HDPE plastic stopper ;
•
1 syringe (1 mL);
•
1 calibrated plastic vessel (20 mL);
•
1 plastic spoon.
•
Note: Any damaged or defective item must be returned in
its original packing materials.
Zinc is widely used in alloys (brass, bronze, and dye-casting
alloys), in galvanizing iron and other metals, also as a
fungicide. It is also an essential growth element in human
diet. But with concentrations higher than 5 mg/L, it gives
a bitter taste to water and opalescence to alkaline water.
Zinc can enter the domestic water supply from the deterioration of galvanized iron and dezincification of brass.
Note:
mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
Zinc reacts with the zincon reagent to form a brownishgreen to blue complex in a solution buffered at alkaline pH.
Since other metals can form colored complexes with zincon,
cyanide is added to complex zinc and any other heavy
metal present. Then, cyclohexanone is added to selectively
free zinc from its cyanide complex so that it can react with
zincon to form the final blue colored product. The amount of
color developed is proportional to the concentration of zinc
present in the aqueous sample.
ISTR3854 12/99 PRINTED IN ITALY
• Pour 10 mL of the solution from the vessel
into the glass cuvet,
up to the mark.
• Add 0.5 mL of HI 93731B-0 reagent by means of
the syringe. Close the cuvet with the HDPE plastic
stopper and mix for 15 seconds.
• It is better to match the color with a white sheet at
about 10 cm behind the comparator.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Standard Methods for the Examination of Water and Waste-
th
water, 18
Edition, 1992
APHA/AWWA/WEF.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
 Loading...
Loading...