Page 1
Instruction Manual
HI 3843
Bleach
Test Kit
Specifications
Range 50 to 150 g/L as Chlorine (Cl2)
Smallest Increment 5 g/L (0.5%) as Chlorine (Cl2)
Analysis Method Iodometric method- Titration
Sample Size 1 mL
Number of Tests 100 (average)
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 485 g (17.1 oz.)
Instructions
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
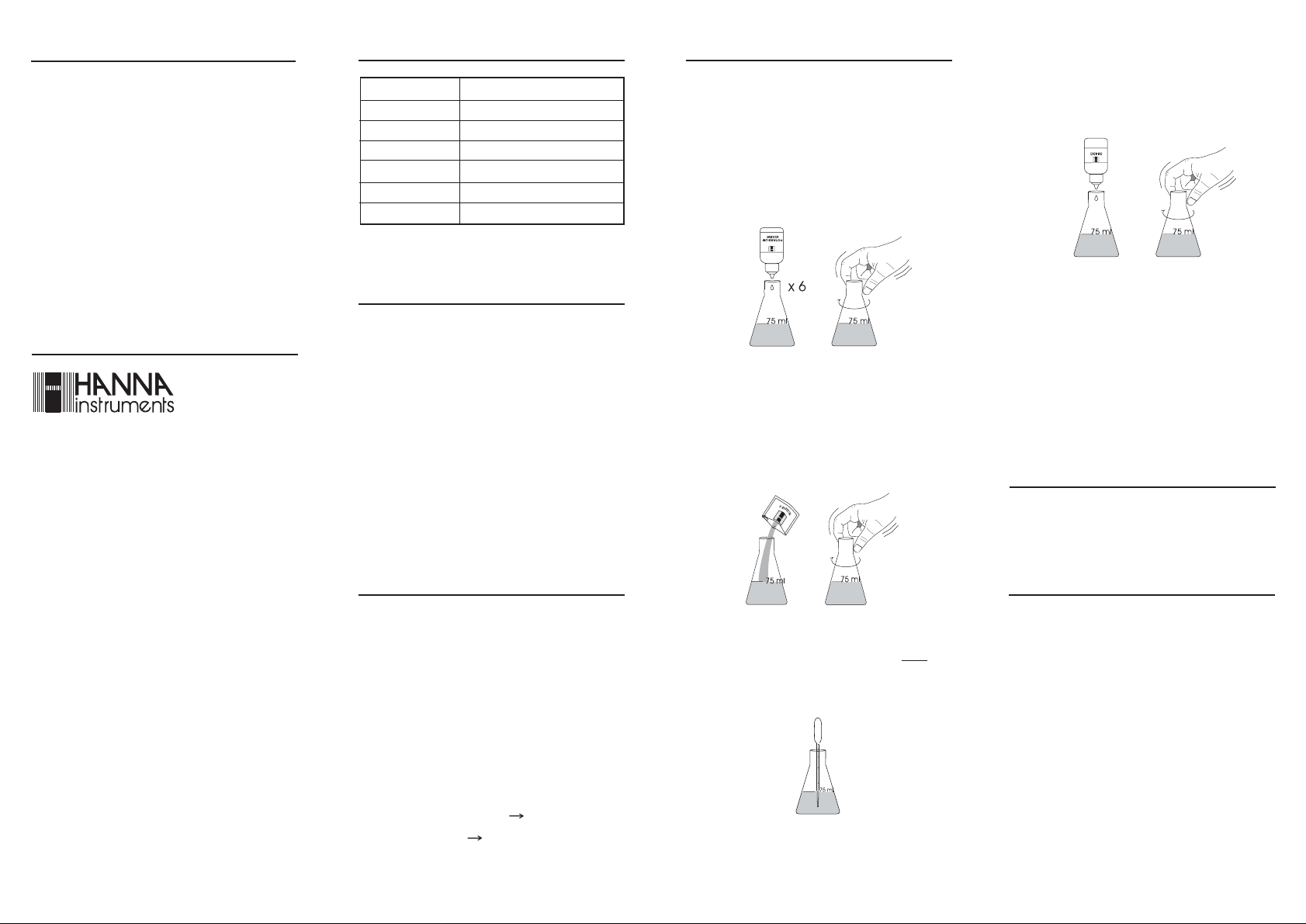
• Fill the Erlenmeyer flask with about 70-75 mL of tap
water (the residual chlorine in the tap water will not
affect the test).
• Add 6 drops of Potassium Iodide Solution and swirl
gently to mix.
• Slowly add drops of HI 3843C-0 Bleach Reagent while
swirling after each drop and counting the drops until
the solution changes from yellow to colorless.
Always hold the dropper vertically, swirling the titrated
solution after each addition.
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instructions carefully before using the
chemical test kit. It will provide you with the necessary
information for correct use of the kit.
Remove the chemical test kit from the packing material
and examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage,
notify your Dealer or the nearest Hanna office immediately.
Each kit is supplied with:
Potassium Iodide Solution, 1 bottle with dropper (30 mL);
•
HI 3843B-0 Bleach Reagent, packets (100 pcs);
•
HI 3843C-0 Bleach Reagent, 2 bottles with dropper
•
(60 mL);
1 glass Erlenmeyer flask (125 mL);
•
25 plastic pipettes (1 mL).
•
Note: Any damaged or defective item must be returned in
its original packing materials.
Significance and Use
Hypochlorites are common bleaching agents to whiten textile
or paper and to disinfect solutions. Sodium hypochlorite
solution has been traditionally used for the treatment of
pool water, since it is an inexpensive and readily available
form of chlorine. The solution usually contains 10 to 15 per
cent available chlorine (equivalent to 100 to 150 g/L), but
it rapidly loses its strength during storage. In addition,
since it is greatly affected by heat, light, pH and heavy
metals, it needs to be monitored regularly.
Chemical Reaction
The available chlorine refers to the chlorine liberated by
the action of dilute acid on the hypochlorite:
OCl- + Cl- + 2H+ = Cl2 + H2O
An iodometric titration method is used in this test kit. The
hypochlorite solution is treated with potassium iodide and
strongly acidified with acid (Step 1). The amount of iodine
generated is equivalent to the chlorine in the sample. The
concentration of iodine is then calculated by titration of
thiosulfate ions that reduce the iodine back to iodide ions
(Step 2).
Step 1: OCl- + 2H+ + 2I
Step 2: I2 + 2S2O
ISTR3843R2 09/03
–
2–
3
2I– + S4O
Cl- + I2 + H2O
2–
6
• Add 1 packet of HI 3843B-0 Bleach Reagent and
swirl gently to dissolve. After adding this reagent to
your sample and mixing, check the pH of the solution
in the Erlenmeyer flask: the pH should always
be below 3.
Otherwise, add packets of HI 3843B-0 reagent, one at
a time, until the pH value drops below 3.
• Add 1 mL of your sample to the Erlenmeyer flask using
the plastic pipette. Dispense the sample below the
solution level in the flask. If hypochlorite is present, the
solution will turn a dark orange color.
Note:
Use the plastic pipettes for about 5 times each,
rinsing them with tap water after every test. Discard
when they become brittle and use a new one.
• To obtain the concentration in % of Chlorine in your
sample, multiply by 0.5 the number of drops of the
titration reagent HI3843C-0 used to turn the solution
colorless.
# of DROPS * 0.5 = % Chlorine
• The result obtained can also be expressed in g/L by
multiplying the % number by 10.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Bibliography
Vogel’s, Textbook of quantitative Chemical Analysis, 5th ed.
Longman scientific & technical.
Health and Safety data Sheets
The chemicals contained in this test kit may be hazardous if
improperly handled. Read Health and Safety Data Sheets
before performing the test.
Page 2

 Loading...
Loading...