Page 1
Instruction Manual
SPECIFICATIONS
INSTRUCTIONS
HI 3835
Salinity Test Kit
www.hannainst.com
SPECIFICATIONS
Dear Customer,
Thank you for choosing a Hanna Product. Please read the
instructions carefully before using the chemical test kit. It
will provide you with the necessary information for correct
use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
• Diphenylcarbazone Indicator, 1 bottle with dropper
(15 mL);
• Nitric Acid Solution, 1 bottle with dropper (30 mL);
• HI 3835-0 Reagent Titrant Solution, 1 bottle (120
mL);
• 1 plastic vial;
• 1 calibrated syringe (1 mL) with tip.
Note: Any damaged or defective item must be returned in
its original packing materials.
Range 0 to 40 g/kg (ppt)
Analysis Method Titrametric
Sample Size 1 mL
Number of Tests 110 (average)
Case Dimensions 200x120x60 mm (7.9x4.7x2.4")
Shipping Weight 460 g (17.2 oz.)
SIGNIFICANCE AND USE
Salinity is defined as the total solids in water after all
carbonates have been converted to oxides, all bromide and
iodide have been replaced by chloride and all organic
matter has been oxidized. The value is in g/kg or ppt (parts
per thousand). The monitoring of salinity is essential for
industrial waste and seawater. The Hanna Test Kit measures
salinity using a fast and efficient titrametric method. The
test requires only a few simple and safe steps to obtain a
result. The components are contained in a compact case,
which makes it perfect for on-site tests.
CHEMICAL REACTION
The salinity level in g/kg is determined by a mercuric
nitrate titration method. The pH is lowered to approximately
3 by addition of nitric acid. Mercuric ions react with chloride
ions to form mercuric chloride. When excessive mercuric ions
are present, it complexes with diphenylcarbazone to form a
purple solution. The color change from yellow to violet
indicates the endpoint.
Hg(NO3)2+2Cl
ISTR3835R4 04/02 PRINTED IN ITALY
–
→ HgCl
+2NO
2
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
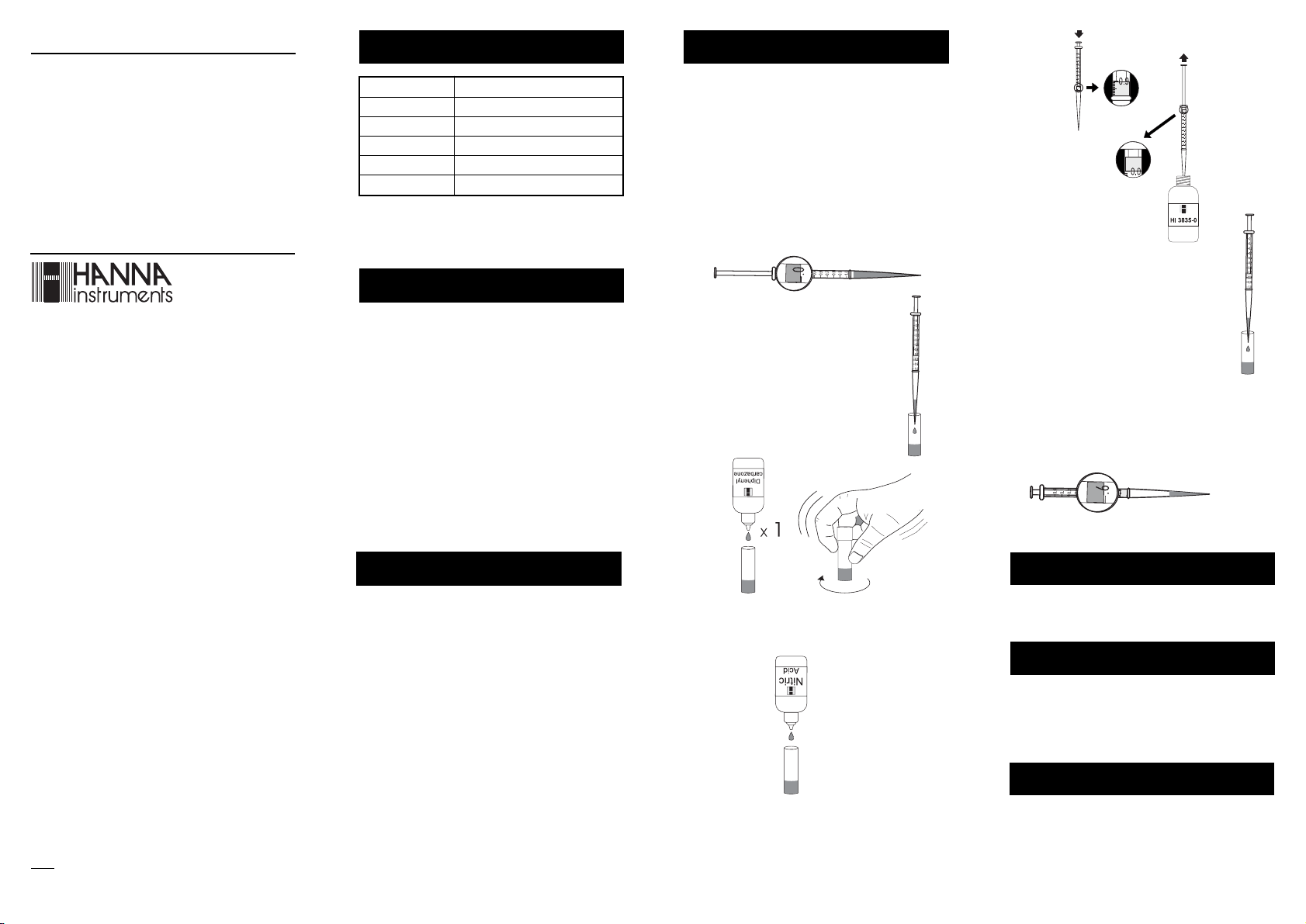
Note: Push and twist pipet tip onto tapered end of syringe
ensuring an air-tight fit.
Note: Use separate pipet tips for sampling and titration.
• Take the titration syringe and push plunger completely
into the syringe. Insert tip into water sample and pull
plunger out until the lower edge of the plunger seal is
on the 0 mL mark of the syringe.
• Place syringe tip into the plastic vial and
slowly add the titration solution drop by
• Add the sample in the syringe to the
plastic vial.
• Add 1 drop of Diphenylcarbazone
Indicator and cap the vial. Swirl the
solution. The solution will become a
violet color.
drop, swirling to mix after each drop.
Continue adding titration solution until the
solution in the vial changes from yellow to
violet.
• Read off the milliliters of titration solution from the
syringe scale, and multiply by 40 to obtain salinity in
g/kg (ppt).
x 40
ACCESSORIES
• Remove the cap. While swirling the vial, add Nitric Acid
Solution drop by drop until the solution turns yellow.
HI 3835-100 Spare reagents (100 tests)
REFERENCES
Standard Methods for the Examination of Water and Wastewater, 16th Edition, 1985.
–
3
HEALTH AND SAFETY
• Take the titration syringe and insert a new pipet tip.
Push plunger completely into the syringe. Insert tip into
HI 3835-0 Reagent Titrant Solution and pull plunger
out until the lower edge of the plunger seal is on the 0
mL mark of the syringe.
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
Page 2

 Loading...
Loading...