Page 1
Instruction Manual
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
HI 3833
Phosphate
Test Kit
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read this instruction manual carefully before using
the chemical test kit. It will provide you with the necessary
information for correct use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
• 1 beaker (20 mL);
• 1 color comparator cube;
• HI 3833-0 powder reagent (100 pcs).
Note: Any damaged or defective item must be returned in
SPECIFICATIONSSPECIFICATIONS
its original packing materials.
Range 0 to 5 mg/L (ppm) PO
Smallest Increment 1 mg/L (ppm) PO
Analysis Method Colorimetric
Sample Size 10 mL
Number of Tests 100
Case Dimensions 220x145x55 mm (8.7x5.7x2.1")
Shipping Weight 160 g (6 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Phosphates are widely introduced into the environment from
such sources as agricultural fertilizers, cleaning and laundering products, boiler water conditioners, and drinking
water treatment aids.
At high levels, phosphates stimulate the growth of photosynthetic organisms which may contribute to eutrophication
of lakes, rivers, and ponds. This makes it important to
monitor and control phosphate discharges into the environment.
Phosphates can be classified as ortho, condensed or organically bound. As with existing test kits on the market, the
Hanna Phosphate Test Kit will only determine ortho phosphate levels.
NOTE: mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTION CHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTION CHEMICAL REACTION
The orthosphosphate level in mg/L (or ppm) is determined
by a colorimetric method. Ammonium molybdate and potassium antimonyl tartrate react in acid medium with
orthophosphate to form a phosphomolybdate complex, that
is reduced to intensely colored molybdenum blue by ascorbic acid. The color intensity of the solution determines the
phosphate concentration.
ISTR3833C 12/99 PRINTED IN ITALY
3–
4
3–
4
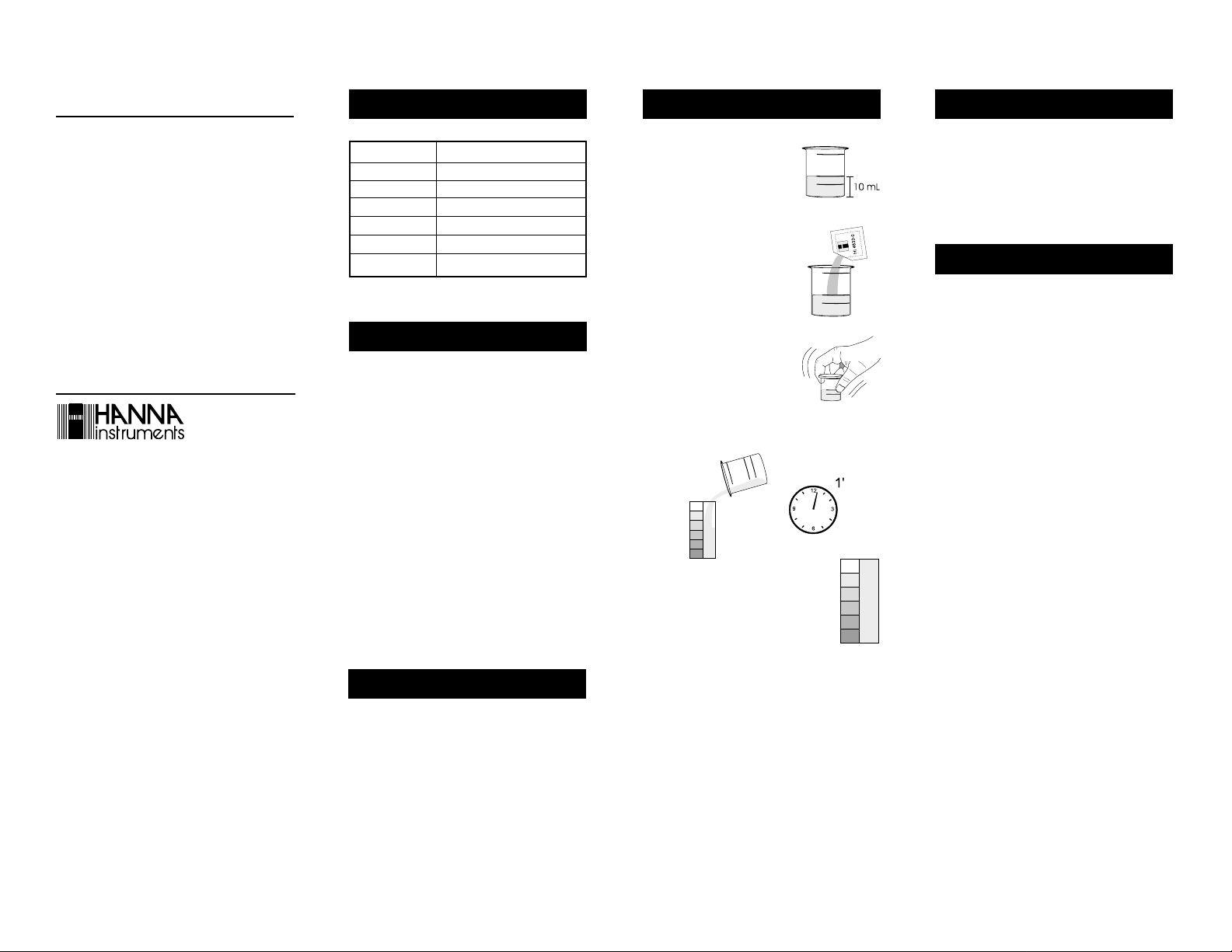
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
• Remove the cap from the plastic
vessel. Rinse the plastic vessel with
water sample, fill it to 10 mL
mark.
• Add 1 packet of reagent HI 3833-0.
• Replace the cap and mix solution
until solids dissolve.
• Remove the cap and transfer the solution into the color
comparator cube. Let set for 1 minute.
• Determine which color matches the solution
in the vessel, and record the results as
mg/L (or ppm) PO
3-
.
4
• 1987 Annual Book of ASTM Standard, Volume 11.01
Water (1). Pages 651-652.
• Standard Methods for the Examination of Water and
Wastewater, 16th Edition, 1985, Pages 445-446.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
 Loading...
Loading...