Page 1

Haemonetics® ACP™215
Automated Cell Processing System
– Operation Manual –
0123
Printed in France
Haemonetics Corporation
400 Wood Road, Braintree, MA 02184, USA P/N 85271-30, Manual revision: C
©2003, Haemonetics International. All rights reserved. January 2003
Page 2

Page 3

Preface iii
CONSUMER INFORMATION
Proprietary rights The contents of this manual are property of the Haemonetics
Haemonetics
Corporation. Any information or descriptions contained in this manual may not
be reproduced and released to any of the general public, or used in conjunction
with any professional instruction without written consent of Haemonetics Corporation, USA. Please direct any written inquiries to the appropriate address:
International Headquarters Corporate Headquarters
Haemonetics SA Haemonetics Corporation
Signy Centre, rue des Fléchères 400 Wood Road
P.O. Box 262, 1274 Signy 2, Switzerland Braintree, MA 02184, USA
Tel. [+41 22] 363 90 11 Tel. [+1 781] 848 7100
Fax [+41 22] 363 90 54 Fax [+1 781] 848 5106
®
and ACP™215 are registered trademarks of the Haemonetics
Corporation.
Legal disclaimer This manual is intended for use as a guide, uniquely for material as supplied by
the Haemonetics Corporation. It provides the operator with necessary information to safely carry out specific procedures and satisfactorily maintain Haemonetics produced equipment. The manual is to be used in conjunction with
instruction and training as supplied by qualified Haemonetics personnel.
Haemonetics guarantees its products when correctly used by a properly trained
operator. Any failure to respect the procedures as described could result in
impaired function of the equipment, as well as in injury to the operator. Haemonetics accepts no responsibility for problems resulting from failure to comply with
prescriptions as outlined by the company. Any modifications estimated as necessary by the customer should be evaluated by a Haemonetics Clinical Specialist.
Safe utilization of Haemonetics material and equipment requires the operator to
correctly handle and dispose of blood-contaminated material. The operator of
any Haemonetics equipment is responsible for understanding and implementing
the local policies and standard operating procedures concerning the handling of
blood-contaminated material, as well as blood products.
It remains solely the responsibility of the customer to fully assess and ensure the
safety of any products obtained from Haemonetics prescribed procedures, prior
to further application or use. Haemonetics declines any responsibility for choices
made by the customer concerning the utilization of products and by-products.
P/N 85271-30, Manual revision: C
Page 4

iv Preface
Haemonetics worldwide locations
Haemonetics Asia Inc.
Taiwan Branch
26F-1, No. 102 Roosevelt Road Sec. 2
Taip ei, Taiwa n
Tel. [+886 2] 2369 0722
Fax [+886 2] 2364 3698
Haemonetics GesmbH
Handelsges.m.b.H.
Berlagasse 45/B2-02
1210 Wien, Austria
Tel. [+43 1] 294 29 00
Fax [+43 1] 294 29 05
Haemonetics Belgium NV
Leuvensesteenweg 542-BP. 14
Planet II Complex
1930 Zaventem, Belgium
Tel. [+32 2] 720 7484
Fax [+32 2] 720 7155
Haemonetics BV
Naritaweg 16
Telestone 8
1043 BW Amsterdam
The Netherlands
Tel. [+31 35] 602 3425
Fax [+31 35] 602 4198
Haemonetics Medical Devices
(Shanghai) International
Trading Co. Ltd.
Room 28032, Shanghai HSBC Tower
101 Yin Cheng East Road
Shangai 200120, PRC
Tel. [+86 21] 5066 3366
Fax [+86 21] 6841 3688
Haemonetics CZ, spol. s r.o.
Ptašínského C.8
60200 Brno, Czech Republic
Tel. [+42 05] 4121 2400
Fax [+42 05] 4121 2399
Haemonetics France S.A.R.L.
46 bis, rue Pierre Curie
Z.I. Les Gatines
78370 Plaisir, France
Tel. [+33 1] 30 81 41 41
Fax [+33 1] 30 81 41 30
Haemonetics GmbH
Rohrauerstrasse 72
81477 München, Germany
Tel. [+49 89] 785 8070
Fax [+49 89] 780 9779
Haemonetics Hong Kong Ltd.
Suite 1314, Two Pacific Place
88 Queensway, Hong Kong
Tel. [+852] 2868 9218
Fax [+852] 2801 4380
Haemonetics Italia S.R.L.
Via Donizetti 30
20020 Lainate (MI), Italy
Tel. [+39 2] 9357 0113
Fax [+39 2] 9357 2132
Haemonetics Japan K.K.
Kyodo Building 3F
16, Ichiban-cho, Chiyoda-ku
Tokyo, Japan, 102-0082
Tel. [+81 3] 3237 7260
Fax [+81 3] 3237 7330
Haemonetics Scandinavia AB
Beta Huset, Ideon
Scheelegatan 17
223 70 Lund, Sweden
Tel. [+46 46] 286 2320
Fax [+46 46] 286 2321
Haemonetics (UK) Ltd.
Beechwood House
Beechwood Estate
Elmete Lane, Roundhay
Leeds LS8 2LQ, United Kingdom
Tel. [+44 113] 273 7711
Fax [+44 113] 273 4055
Haemonetics SA
Signy Centre, rue des Fléchères
P.O. Box 262
1274 Signy 2, Switzerland
Tel. [+41 22] 363 90 11
Fax [+41 22] 363 90 54
P/N 85271-30, Manual revision: C
Page 5

Table of Contents
Chapter 1 Explaining General Information
Providing an overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is the ACP 215 system ? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is the purpose of this manual ? . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is required to perform a procedure ? . . . . . . . . . . . . . . . . . . . . . . . 1-2
Understanding the use of symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found in this document. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found on the device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found on disposable packaging . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Listing ACP 215 device specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Dimensions and weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Environmental conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Power requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Chapter 2 Presenting the ACP 215 Equipment
DESCRIBING THE ACP 215 CONTROL PANEL. . . . . . . . . . . . . . . . . . . . . . 2-3
Display screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Keypad. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
DESCRIBING THE ACP 215 CABINET COMPONENTS . . . . . . . . . . . . . . . . 2-4
Centrifuge system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Pumps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Pressure monitors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Valves. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Air detectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Optical line sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Solution bag pole . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Power entry module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Power cord. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Biohazard waste bag . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
DESCRIBING THE SHAKER AND THE PRINTER . . . . . . . . . . . . . . . . . . . . . 2-8
Shaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9
P/N 85271-30, Manual revision: C
Page 6

vi Table of Contents
Chapter 3 Maintaining the ACP 215 Equipment
CLEANING PROCEDURES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Listing tools and cleaning supplies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Exterior surfaces, valves, shaker and printer . . . . . . . . . . . . . . . . . . . . . . 3-3
Pressure monitors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Air detectors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Optical line sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Pumps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Fluid detector. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Centrifuge components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Filter screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
CUSTOMER SERVICE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Clinical training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Field service. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Returned Goods Authorization system . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Haemonetics
®
Cleaning and maintenance record . . . . . . . . . . . . . . . . . 3-8
Chapter 4 Ensuring Safety and Quality for an ACP 215
Procedure
HANDLING THE ACP 215 EQUIPMENT . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Storing the device and material . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Inspecting the material . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
PREVENTING PROBLEMS DURING AN ACP 215 PROCEDURE . . . . . . . . 4-3
Understanding the risk of hemolysis. . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Avoiding the consequences of flow restriction . . . . . . . . . . . . . . . . . . . . 4-3
Avoiding bowl misalignment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Avoiding overheating due to mechanical situations . . . . . . . . . . . . . . . . 4-4
WARNINGS FOR THE OPERATOR. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Electrical shock hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Leakage current control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Mechanical hazards/rotating parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Power outlet connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Communicable disease precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Chapter 5 Troubleshooting for the ACP 215 Device
LISTING SYSTEM ERROR MESSAGES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
LISTING INTERRUPT SAFETY SYSTEM MESSAGES . . . . . . . . . . . . . . . . . . . 5-5
P/N 85271-30, Manual revision: C
Page 7

Chapter 1
Explaining General Information
Providing an overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is the ACP 215 system ?. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is the purpose of this manual ?. . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
What is required to perform a procedure ? . . . . . . . . . . . . . . . . . . . . . . . 1-2
Understanding the use of symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found in this document . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found on the device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Symbols found on disposable packaging. . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Listing ACP 215 device specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Dimensions and weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Environmental conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
Power requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6
P/N 85271-30, Manual revision: C
Page 8

1-2 Explaining General Information
PROVIDING AN OVERVIEW
What is the ACP 215 system ?
What is the purpose of this manual ?
In the tradition of its innovative technology, Haemonetics has produced the
ACP 215 - a compact, lightweight, automated cell processing system which is
simple to operate.
The ACP 215 system can perform the following types of procedures:
! Prepare collected red blood cell products with glycerol for freezing.
! Deglycerolize thawed red blood cell products, removing extra cellular
components, and resuspend the red blood cells in an additive solution.
! Wash red blood cell units stored in commonly used AC and additive solu-
tions to remove plasma and extra cellular components.
The ability to process-control the addition and removal of cryroprotectant solution as well as wash banked blood in a closed system significantly improves the
safety and availability of blood products.
This manual is intended to supply anyone involved in using the ACP 215 equipment with information to attain:
! An awareness of the purpose of the device and the implications of its
procedures.
! An understanding of how to safely operate the system, correctly install the
appropriate disposable material, and troubleshoot any difficulties.
! An ability to consistently apply the principles behind safe operation,
What is required to perform a procedure ?
P/N 85271-30, Manual revision: C
The following material is required to perform an ACP 215 procedure:
! The ACP 215 device, to process the collected red blood cell products.
! A dedicated shaker, to properly mix the blood with procedure-specific
! A disposable set designed for the selected procedure.
! Appropriate solutions.
! A printer, to provide the operator with printed procedure data.
! A sterile connection device, to ensure proper tubing welds.
proper maintenance and correct handling, to ensure optimal results.
solutions.
Page 9
Explaining General Information 1-3
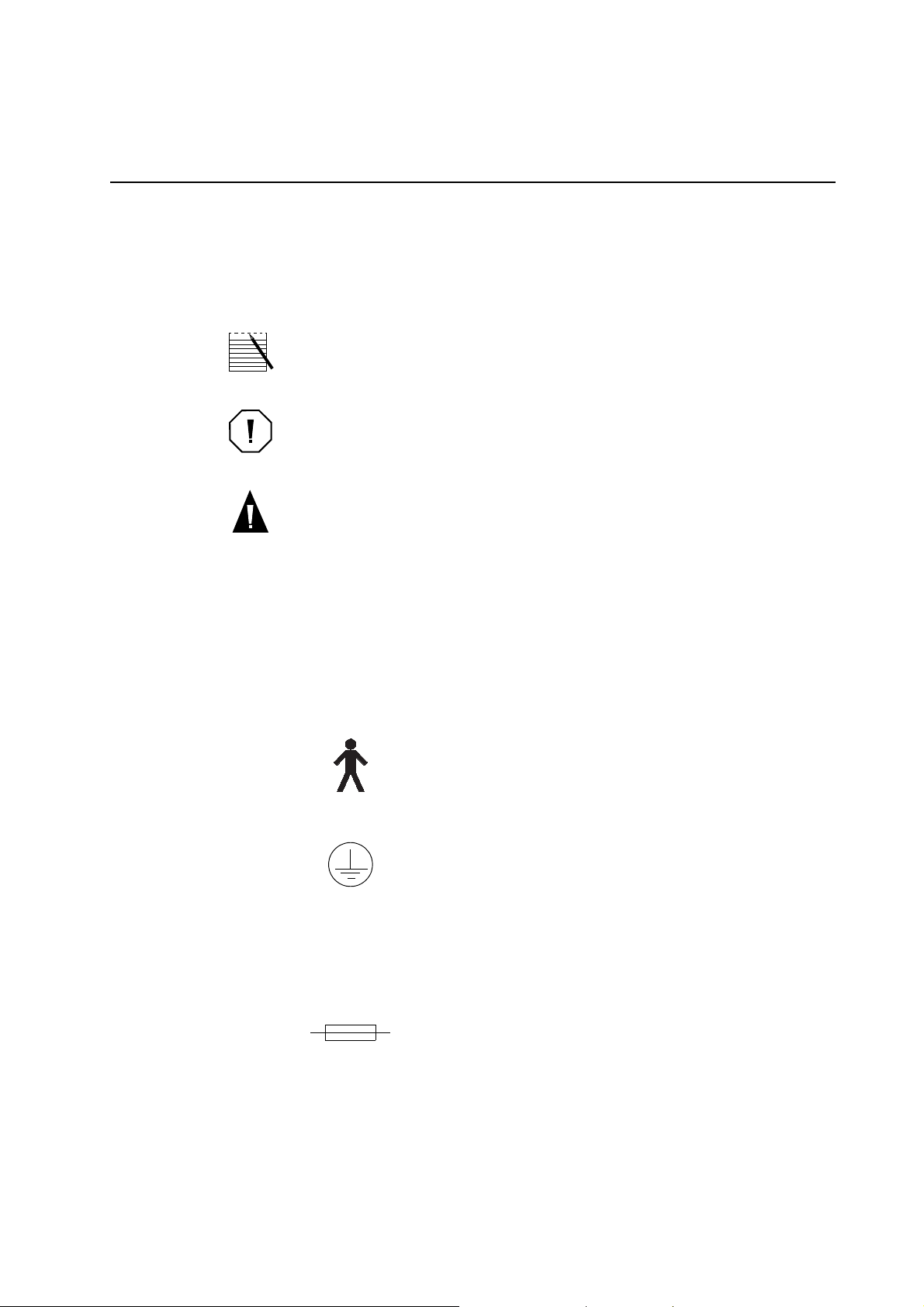
UNDERSTANDING THE USE OF SYMBOLS
Symbols found in this document
Symbols found on the device
The terms Note, Caution and Warning are used in this manual with the following
symbols to emphasize certain details for the operator.
Note: provides useful information regarding a procedure or operating technique
when using Haemonetics material.
Caution: advises the operator against initiating an action or creating a situation which could result in damage to equipment, or impair the quality of the
by-products; personal injury is unlikely.
Warning: advises the operator against initiating an action or creating a situation
which could result in serious personal injury to either the operator or the
recipient of the product.
! Text preceded by this bullet indicates an action for the operator.
" Text preceded by this bullet indicates an item on a list of information for
the operator.
The following symbols are found on the ACP 215 device.
Typ e B
Protection against electric shock, particularly regarding: allowable leakage current and reliability of the protective earth
connection.
~
Protective earth (ground)
Used to identify any terminal intended for connection to an
external conductor, for protection against electrical shock in
case of a fault.
Alternating current
Used to indicate on the rating plate that the device is suitable for
alternating current only.
Fuse symbol
Used to identify fuse boxes or the location of a fuse box.
P/N 85271-30, Manual revision: C
Page 10
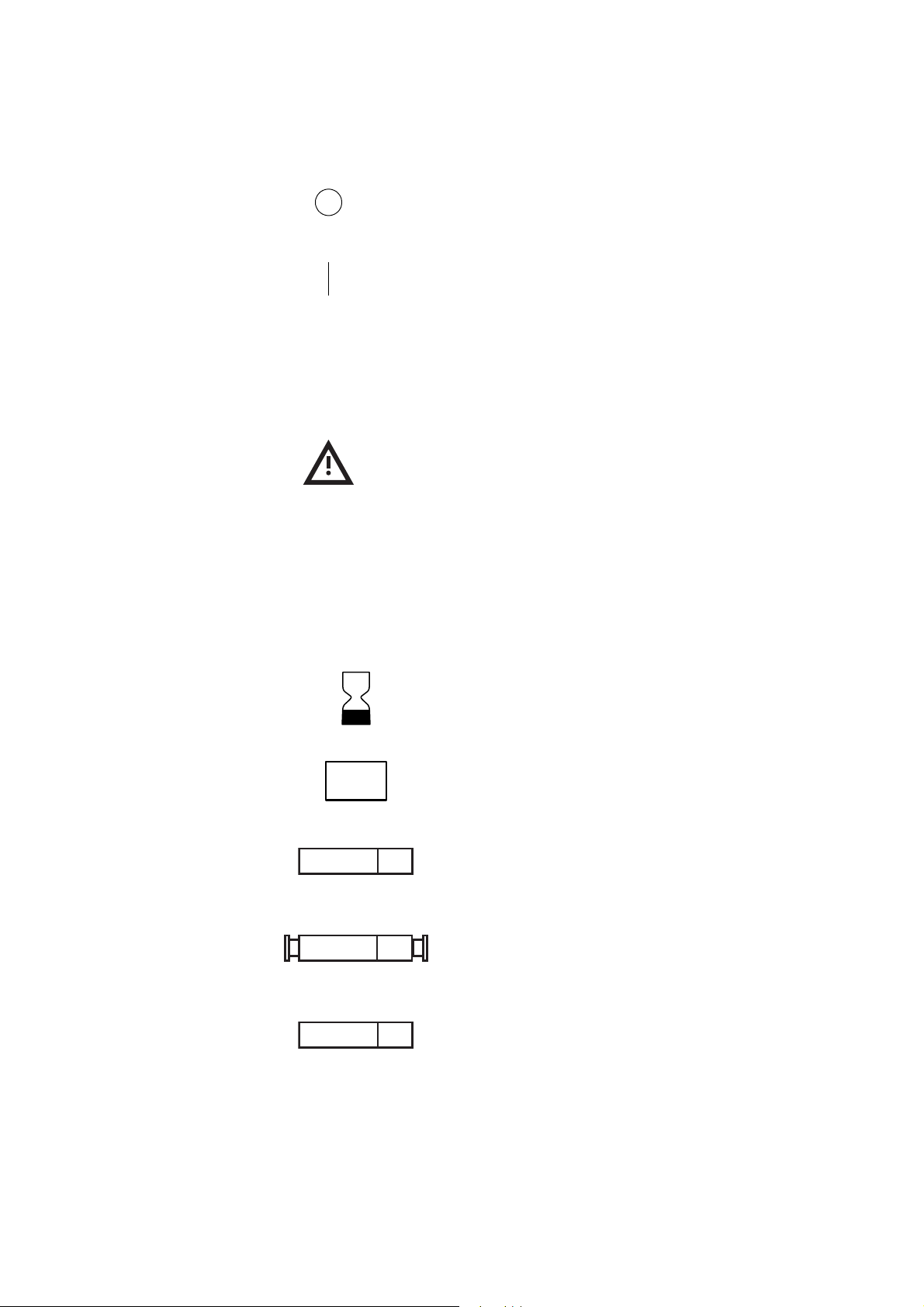
1-4 Explaining General Information
Power OFF
Position of the main power switch indicating disconnection from
the mains.
Power ON
Position of the main power switch indicating connection to the
mains.
Protection against ingress of liquid
IPX1
Indicates that the enclosure of the device is designed to provide
a specified degree of protection against harmful ingress of water
or liquid into the equipment (under applicable conditions).
Attention (Consult accompanying documents)
Symbols found on
disposable packaging
The following symbols are used by Haemonetics on disposable set packaging.
REF
LOT
STERILE EO
STERILE
EO
Catalog number
Expiration date
Lot Number
Contents sterile by exposure to ethylene oxide
Fluid path sterile by exposure to ethylene oxide
STERILE R
P/N 85271-30, Manual revision: C
Contents sterile by exposure to gamma irradiation
Page 11
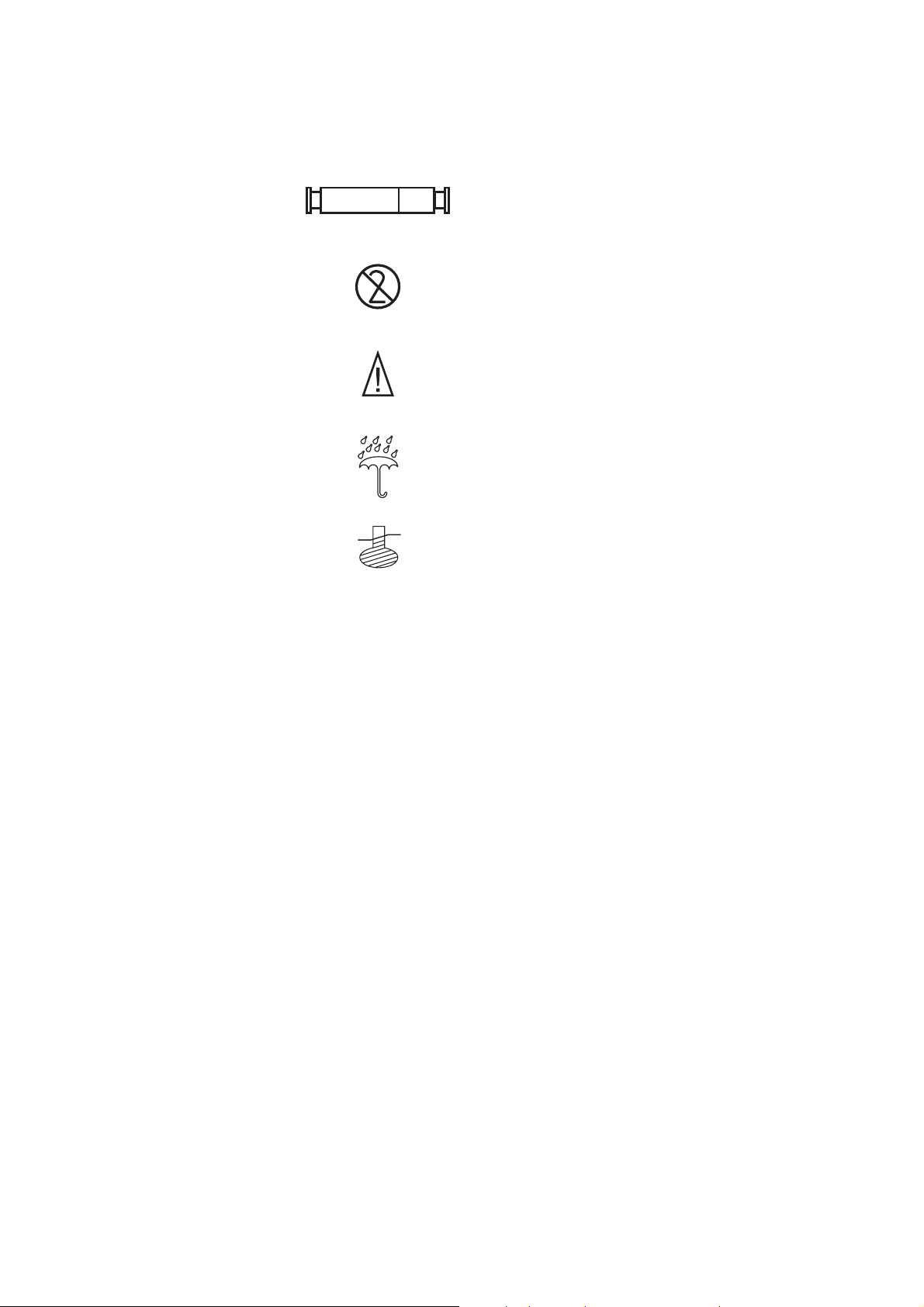
Explaining General Information 1-5
STERILE R
-yy˚C
yy˚C
Fluid path sterile by exposure to gamma irradiation
Do not reuse
Caution: consult operator manual for instructions
KEEP DRY [storage conditions]
Storage conditions, temperature level
P/N 85271-30, Manual revision: C
Page 12
1-6 Explaining General Information
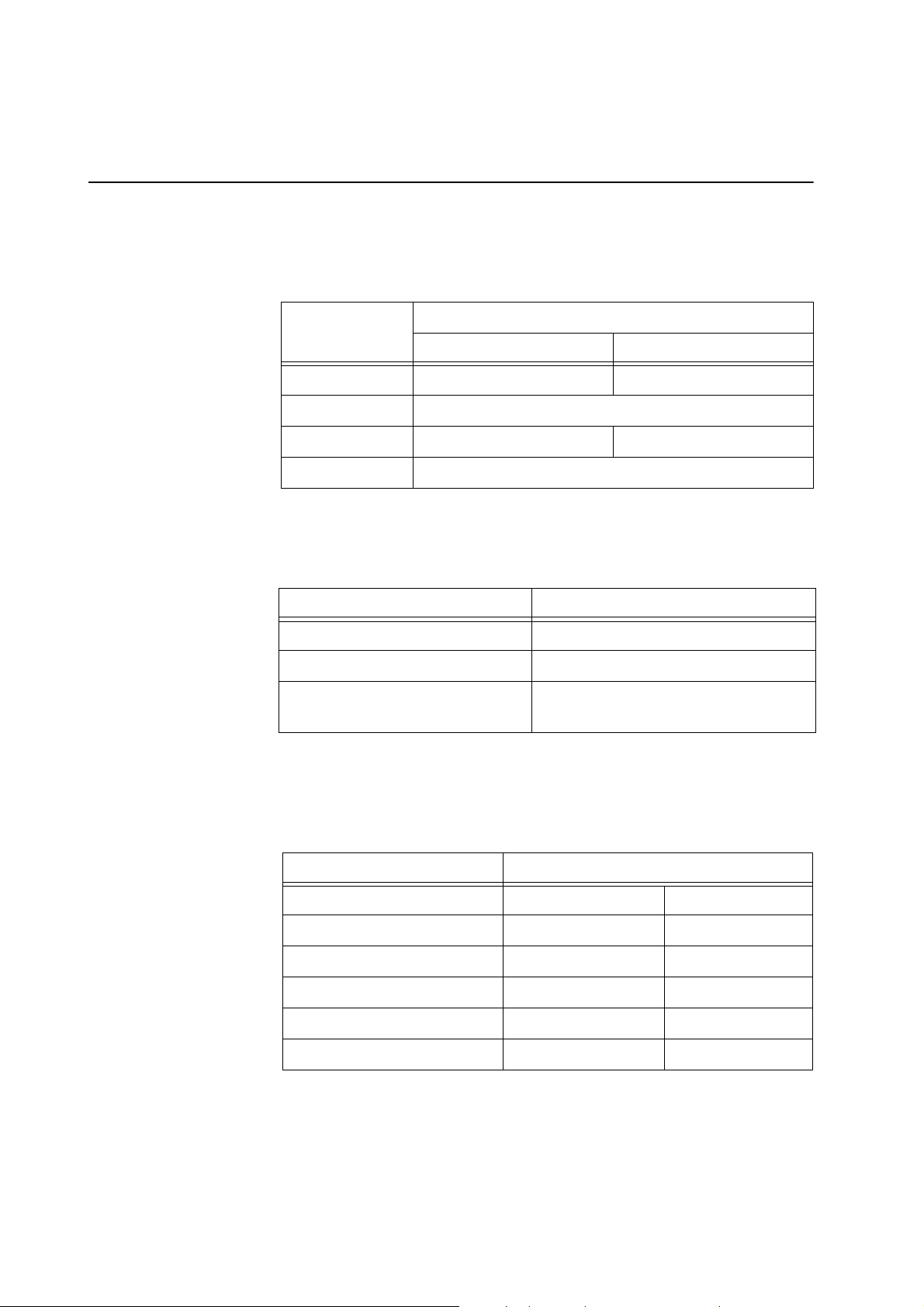
LISTING ACP 215 DEVICE SPECIFICATIONS
Dimensions and weight
Environmental conditions
The approximate weight and dimensions of the ACP 215 device are as follows:
Value (approximate)
Characteristic
Cabinet cover open Cabinet cover closed
Height 67.5 cm [26.5 inches] 43 cm [17 inches]
Width 55 cm [21.5 inches]
Depth 55 cm [21.5 inches] 30.5 cm [12 inches]
Weight 25 kg [56 lb]
The following environmental conditions should be respected pertaining to operation and storage of the ACP 215 device.
Condition Value
Operating Temperature 18° C to 35° C
Humidity 95% maximum, non-condensing
Tested storage temperature range 5° C to 45° C, non-condensing environ-
ment
Power requirements
P/N 85271-30, Manual revision: C
The power is supplied to the device from an external AC power source. The
power source must be properly grounded. Haemonetics will regulate the proper
voltage setting upon installation.
Characteristics Values (relative to input voltage)
Input voltage 230 VAC ± 10% 110 VAC ± 10%
Operating current ~1.9 A ~ 2.6 A
Maximum input current 2.5 A 5.0 A
Fuse rating F2.5 A @ 250 V F5.0 A @ 250 V
Operating frequency range 50 - 60 Hz 50 - 60 Hz
Maximum leakage current 500 µA 100 µA
Page 13

Explaining General Information 1-7
Caution: The ACP 215 device must be operated in an environment compatible to
the requirements of the IEC 60601-1-2 Standard, Electromagnetic compatibility.
Mobile RF communication equipment not approved by Haemonetics and
portable communication equipment can affect the ACP 215 device. Any accessories and cables not approved by Haemonetics used in conjunction with the
device may increase hazards and influence compatibility with EMC requirements. Therefore, non-approved accessories and cables must not be used.
In addition, the ACP 215 device and accessories must not be placed directly adjacent to, or top of other equipment, unless specifically approved by Haemonetics.
P/N 85271-30, Manual revision: C
Page 14

Page 15

Chapter 2
Presenting the ACP 215 Equipment
DESCRIBING THE ACP 215 CONTROL PANEL . . . . . . . . . . . . . . . . . . . . . . 2-3
Display screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
DESCRIBING THE ACP 215 CABINET COMPONENTS . . . . . . . . . . . . . . . . 2-4
Centrifuge system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Pumps. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Pressure monitors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Valves. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Air detectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Optical line sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Solution bag pole . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Power entry module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Power cord . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Biohazard waste bag. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
DESCRIBING THE SHAKER AND THE PRINTER . . . . . . . . . . . . . . . . . . . . . 2-8
Shaker. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Printer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9
P/N 85271-30, Manual revision: C
Page 16

2-2 Presenting the ACP 215 Equipment
P/N 85271-30, Manual revision: C
Figure 2-1, The ACP 215 device with the shaker
Page 17

Presenting the ACP 215 Equipment 2-3
DESCRIBING THE ACP 215 CONTROL PANEL
The control panel is located on the inside of the ACP 215 cabinet cover. It consists
of a display screen and a keypad, containing groups of keys used to operate the
device. There is a protective plastic coating on the keypad, which allows for efficient cleaning and disinfecting.
Display screen The ACP 215 display screen provides procedural information and posts the
volume of the processed solutions, pump speed, and procedure time. It is used
to guide the operator through the procedure and display warning messages.
1. Display screen
2. Start/Stop keys
3. Pump control keys
4. Programming keys
5. Shaker key
1.
2.
3.
Figure 2-2, ACP 215 control panel
Keypad START and STOP keys
These two keys are active throughout the entire procedure and will provide the
operator with the basic control of starting or interrupting the procedure.
PUMP control keys
These keys allow the operator to START, to STOP, or to increase (arrow up) or
decrease (arrow down) the speed of the pumps during a procedure. The pump
control keys are active when the pump is actually rotating.
PROGAMMING keys
The MODIFY PROGRAM, YES, NO, SAVE PROGRAM keys will allow the operator to adjust a limited set of parameters, which are presented in each relevant
procedure operation manual.
SHAKER key
This key is used only to start and stop the shaker; speed or amplitude cannot be
modified.
5.
4.
P/N 85271-30, Manual revision: C
Page 18

2-4 Presenting the ACP 215 Equipment
DESCRIBING THE ACP 215 CABINET COMPONENTS
1. Centrifuge system
2. Solution pump
3. Blood pump
4. DPM
5. SPM
6. Red valve
7. White valve
8. Green valve
9. Blue valve
10. Yellow valve
11. Orange valve
12. SLAD
13. BLAD
14. Line Sensor
15. Solution bag pole
2.
3.
4.
12.
13.
6. 7.
11.
10.
9.
1.
14.
5.
8.
Figure 2-3, ACP 215 top deck view
15.
Centrifuge system
The centrifuge chuck, seated in the centrifuge well, is designed to hold the
disposable blow molded bowl (BMB) in place during operation and spin the bowl
up to 8000 revolutions per minute (rpm).
The centrifuge lid, referred to as the cover, contains a locking knob; it will secure
the disposable bowl in the chuck while isolating the spinning bowl from the operator. The centrifuge drain tube, located at the rear of the device, must be
connected at all times to the biohazard waste bag supplied by Haemonetics.
When installing a bowl, the operator should exert a downward pressure on the
head of the bowl and ensure that the bowl is completely seated. The bowl will
be completely secured once the operator has locked the centrifuge lid.
To remove a bowl at the end of a procedure, the operator should open the centrifuge lid and pull upward on the bowl.
Warning: It is unlikely that a properly installed centrifuge bowl will become
unaligned as it spins. If the operator does notice anything unusual, under no
circumstances, should the operator attempt to open the centrifuge lid if the
bowl is still spinning. The bowl must come to a complete stop before attempting
to open the centrifuge for any reason.
Fluid detector
The centrifuge well is equipped with an electronic fluid detection system
designed to detect the presence of liquid. The detector is mounted on the wall of
the centrifuge well. The ACP 215 safety system will automatically stop the centrifuge and the pumps if there is contact between liquid of any sort and the fluid
detector.
P/N 85271-30, Manual revision: C
Page 19

Presenting the ACP 215 Equipment 2-5
Pumps Located on the left side of the ACP 215 top deck are two pumps which use peri-
staltic movements to displace fluids through the disposable set tubing.
Figure 2-4, ACP 215 pump rotor
Blood Pump
The blood pump will rotate during certain procedures simultaneously with the
solution pump to direct solution to the RBC bag. As the bowl is filling, the blood
pump directs the RBC product to the bowl.
Pressure monitors
At the end of certain procedures, the blood pump directs the final product from
the bowl to the final product bag.
Solution Pump
The solution pump will rotate at certain points during the procedures to transfer
solution to the RBC bag.
As the bowl is filling, or when transferring the final product from the bowl back
to the RBC bag or the final product bag, the solution pump is stopped.
There are two electronically controlled pressure monitors on the ACP 215 device,
which function with the correlating filter on the disposable set to measure pressure in the disposable tubing. The pressure monitors provide feedback to the
system about the flow of blood components.
The draw pressure monitor (DPM), located on the left side of the top deck,
measures pressure in the blood line tubing.
The system pressure monitor (SPM), located on the right side of the top deck,
measures pressure in the effluent tubing. This measurement verifies that the sterile
seal, between the head and the body of the centrifuge bowl, remains intact.
If a pressure monitor detects that pressure is outside of acceptable limits, the ACP
215 safety system will interrupt the procedure and provide an explanatory
message.
P/N 85271-30, Manual revision: C
Page 20

2-6 Presenting the ACP 215 Equipment
Valves There are six pneumatic pinch valves located on the ACP 215 top deck which
automatically control the flow of fluids through the disposable set tubing. These
valves are color-coded to facilitate accurate tubing installation.
The ACP 215 safety system will control the valves during the self-diagnostic tests.
Once the operator has selected a procedure, the appropriate valves will automatically open, in preparation for installing the disposable set tubing. In case of
power failure, the valves revert to a closed status.
Air detectors The ACP 215 device is equipped with two ultrasonic sensors designed to detect
the presence of air, bubbles or foam in the fluids flowing through the disposable
set tubing. The solution line air detector (SLAD) is located to the right of the solution pump. The blood line air detector (BLAD) is located to the right of the blood
pump.
In the case of an occlusion in the tubing, or an interruption in the fluid flow, the
pump(s) will stop and a message will be displayed with information about the
condition.
Figure 2-5, Example of an air detector
Optical line sensor
Located on the right side of the ACP 215 top deck is the optical line sensor which
monitors the blood components passing through the effluent tubing.
Caution: The line sensor will not provide accurate readings if the optical lens it
is obstructed in any way; thus the lens must be cleared of any extraneous substances to ensure proper functioning of the system.
Further information about specific functions of the line sensor is provided in each
procedure operation manual.
Solution bag pole Located on right side of the ACP 215 cabinet is a height-adjustable pole, used to
hang solution bags during the procedure. There is a knob located on the base of
the pole that permits the operator to adjust the height of the pole by pulling the
knob outward to extend or retract the pole.
P/N 85271-30, Manual revision: C
Page 21

Presenting the ACP 215 Equipment 2-7
Power entry module
1. ON/OFF power switch
2. Power input receptacle
The power entry module (PEM) is located on the left side panel of the device.
Externally, the module consists of an ON/OFF power switch and a power-input
receptacle for the power cord. In the case of an emergency, the power switch can
be used to stop all device function.
Internally, the PEM contains the fuse panel. It will interrupt power supply to the
system in the event of an electrical current surcharge. The design of the PEM also
provides a filter-effect for the device against the effects of a power surge.
1.
2.
Figure 2-6, Power entry module (PEM)
Power cord The power cord provided by Haemonetics is designed to connect the ACP 215
device with an external power source via the power-input receptacle, located on
the power entry module on the side panel.
Biohazard waste bag
The biohazard waste bag is designed to collect any biologically contaminated
material from the centrifuge well in the rare case of a spill or leak. Two biohazard
waste bags are supplied with the delivery of each ACP 215 device.
A bag must be attached at all times to the centrifuge drain tube, located at the
rear of the device. The bag must hang freely, with the clamp open, visible to the
operator.
Warning: The biohazard waste bags are not to be used to collect or store blood
products. When a bag contains evacuated waste products, it must be clamped,
removed and properly disposed of, according to the local policies concerning
biologically contaminated material. A new bag must be placed before resuming
operation.
P/N 85271-30, Manual revision: C
Page 22

2-8 Presenting the ACP 215 Equipment
DESCRIBING THE SHAKER AND THE PRINTER
1. Shaker connector port
2. Printer connector port
2
1
Figure 2-7, ACP 215 rear panel
Shaker During certain procedures, a shaker device manufactured by Haemonetics prop-
erly mixes the blood in the RBC bag with the added solutions. The ACP 215
device provides power to the shaker and controls the shaker operation. The RBC
bag is secured on the shaker platform with magnets.
The operator should connect the shaker to the rear panel of the ACP 215 device
as follows.
" Place the shaker device to the left of the ACP 215 device.
" Attach the shaker power cord to the connector port labeled SHAKER on
the rear panel of the ACP 215 device.
" Secure the connection using the two thumb screws.
Note: When ordering any additional shaker magnets from Haemonetics, the
following part number should be specified.
! ACP 215 shaker magnets, P/N 09437-00.
P/N 85271-30, Manual revision: C
Page 23

Presenting the ACP 215 Equipment 2-9
Printer The printer is a satellite unit which provides a procedure summary printout auto-
matically at the end of the procedure. Examples of a printout are provided in each
procedure operation manual. The ACP 215 device controls the printer, however
the printer has an independent power source via a wall unit adapter that converts
AC into DC.
The printer is connected to the rear panel of the device through an RS232 serial
port, using a 9 pin connector. The function of each pin is defined as follows.
Pin Number Definition
1 RS-232C Data Carrier Detect
2 RS-232C Receive Data
3 RS-232C Transmit Data
4 RS-232C Data Terminal Ready
5 RS-232C RS-232C Ground
6 RS-232C Data Set Ready
7 RS-232C Request to Send
8 RS-232C Clear to Send
9 RS-232C Ring Indicator
Connecting the printer
The operator should connect the printer to the rear panel of the device as follows.
" Attach one end of the printer serial cable to the serial port labeled PRINT-
ER on the rear panel of the ACP 215 device.
" Secure the printer cable to the rear panel connector using the two thumb
screws.
" Attach the other end of the printer serial cable to the serial port located on
the rear panel of the printer.
" Secure the cable in to the printer using the two thumb-screws.
" Insert the DC plug of the printer AC adaptor into the power supply jack lo-
cated at the rear of the printer.
" Plug the printer AC adaptor power cord into an electric outlet.
" Place the printer to the right of the ACP 215 device.
" Power-on the printer.
" Ensure that there is enough paper remaining in the printer.
P/N 85271-30, Manual revision: C
Page 24

2-10 Presenting the ACP 215 Equipment
To remove the printer AC adaptor, the operator should:
" Power off the printer first.
" Unplug the AC adaptor and the DC plug.
The operator should not touch the pins of the DC plugs.
Note: Further information about replacing the paper or cleaning the printer is
provided in the Operation Manual for the DPU-414 Thermal Printer, Seiko
Instruments Inc.
P/N 85271-30, Manual revision: C
Page 25

Chapter 3
Maintaining the ACP 215 Equipment
CLEANING PROCEDURES. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Listing tools and cleaning supplies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Exterior surfaces, valves, shaker and printer . . . . . . . . . . . . . . . . . . . . . . 3-3
Pressure monitors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Air detectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Optical line sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Pumps. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Fluid detector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Centrifuge components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Filter screens. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
CUSTOMER SERVICE. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Clinical training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Field service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Returned Goods Authorization system . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Haemonetics
®
Cleaning and maintenance record . . . . . . . . . . . . . . . . . 3-8
P/N 85271-30, Manual revision: C
Page 26

3-2 Maintaining the ACP 215 Equipment
CLEANING PROCEDURES
To ensure consistent performance of the device, the operator needs to primarily
perform routine cleaning of the components. A record of routine cleaning should
be kept along with any routine or preventive service maintenance performed by
a Haemonetics representative. An example of a cleaning and routine maintenance form is provided at the end of this chapter. Annual preventive maintenance should be performed by a Haemonetics service representative.
The frequency and type of cleaning each ACP 215 device will depend on the
number of procedures performed. Special cleaning needs may arise and should
be dealt with promptly. Haemonetics recommends the following routine
cleaning schedule for each device, based on an average of three procedures per
day, or approximately sixty per month. Adjustments should be made to the
cleaning schedule according to the actual number of procedures performed.
! Daily: Clean the exterior surfaces.
! Weekly: Clean the pressure monitors, air detectors, optical sensors (line
sensor and optical bowl sensor), the fluid detector and the inside of the
centrifuge well.
Listing tools and cleaning supplies
! Monthly: Clean the pump rotors and the pump wells.
! Quarterly: Clean the filter screens.
Warning: To eliminate the potential danger of electrical shock, the operator
must clean the ACP 215 device only when it is disconnected from an external
power source.
A tool kit has been included with each device containing the following articles.
! Phillips-head screwdriver, to remove the retainer plate from the air vent.
! Allen (hexagonal head) wrench, to remove the pump heads from the pump
assembly.
! A brush, to remove loose debris within the roller assembly.
! Silicon lubricant, used for the O-ring gasket of the centrifuge chuck.
The following list describes the basic material required for routine cleaning.
! Disinfectant cleaning solution, specific for blood borne pathogens.
! Clear warm water.
! 70% Isopropyl alcohol.
! Lint-free gauze or cloth for cleaning and drying.
! Cotton swabs.
! Protective gloves.
! Spray bottle, recommended for cleaning the centrifuge well.
P/N 85271-30, Manual revision: C
Page 27

Maintaining the ACP 215 Equipment 3-3
Exterior surfaces, valves, shaker and printer
Pressure monitors
The exterior cabinet, keypad, display screen, valves, shaker and printer should
be wiped daily, as well as following any spill, using an appropriate cleaning solution.
The pressure monitors (DPM/SPM) should be cleaned in the following manner.
" Depress and hold the white ring as if installing the disposable filter.
" Wipe the silver tube thoroughly, using a circular motion and warm water
only.
" Dry the tube and release the pressure on the ring.
Caution: It is very important to use only water on the pressure monitor rod.
Alcohol or cleaning solution residue could cause a reaction with the plastic
material of the corresponding disposable set filter and affect the function of the
filter.
Air detectors The air detectors (SLAD/DLAD) are designed with a groove to hold the disposable
tubing. The contents of the tubing are monitored by the sensors which are located
internally on either side of this groove. If a procedure is interrupted due to an air
detector alarm, the operator should remove the tubing and clean the groove
before continuing the procedure.
Optical line sensor
The operator should use only warm water and lint-free gauze to clean and dry
inside of the tubing grove. The groove should be kept free of any particles, such
as powder residue from disposable gloves, since this could lead to an erroneous
detection of air.
Caution: Alcohol may cause the plastic housing to degrade and must not be used
to clean the air detectors.
The line sensor contains two very small lenses which are centered on either side
of the disposable tubing groove. The lenses must be kept completely free of particles or debris which could produce inaccurate readings and influence the device
performance.
The operator should carefully pass lint-free gauze moistened with warm water
only through the groove to clean, then dry the lenses.
Caution: If cleaning solution should come into contact with the optical sensor
lenses, the lenses should be cleaned immediately with lint-free gauze and warm
water, then thoroughly dried. Dried cleaning solution can leave an opaque film
on the lens.
P/N 85271-30, Manual revision: C
Page 28

3-4 Maintaining the ACP 215 Equipment
Pumps The pump rotors should be removed from the well with the Allen/hexagonal-
head wrench. Debris should be removed from the rotors and the pump wells on
a routine basis, as well as after any spills to contribute to efficient operation.
For routine cleaning, the operator should:
" Remove the pump rotor from the housing, using the hexagonal head
wrench to remove the pump screw.
" Wipe the rotor and remove all debris from the rollers, using warm water
and lint-free cloth or gauze.
" Dry with lint free cloth (or compressed air, if available).
" Clean and dry the pump well using the same method.
" Ensure that all of the rollers spin freely and replace the pump rotor in the
well, aligning the cross pin in the rotor with the pump shaft.
" Replace and tighten the hexagonal head screw.
In the case of a fluid spill, the same cleaning method should be followed;
however, disinfectant cleaning solution should be used, followed by a clear
water rinse. The pump rotor should not be immersed in water.
Fluid detector The fluid detector is located inside of the centrifuge well. The surface of the
detector should be cleaned using a cotton swab moistened with 70% alcohol.
Centrifuge components
The centrifuge well, chuck, hinged lid and locking knob can be wiped routinely
using the cleaning solution and a lint-free cloth.
After a major cleaning, the operator should apply a small amount of the silicon
lubricant to the O-ring gasket to prevent it from cracking. It is not necessary to
remove the gasket when applying the lubricant.
Cleaning after a blood spill
In the case of a blood spill, the operator should:
" Power off the device and disconnect it from the external power source
before cleaning.
" Ensure that the equipment waste bag is attached to the drain tube and
hangs freely.
" Wipe the centrifuge lid with cleaning solution.
" Clean the centrifuge chuck and well using the disinfectant solution and a
lint-free cloth until all traces of blood components are removed.
" Lubricate the O-ring gasket with a small amount of the silicon lubricant.
Haemonetics recommends that the operator wear protective gloves to avoid
direct contact with the cleaning solution and/or any spilled blood which may be
present.
P/N 85271-30, Manual revision: C
Page 29

Maintaining the ACP 215 Equipment 3-5
In the case of a larger spill, fluid and/or blood may be evacuated into the
biohazard waste bag. The operator should complete the following additional
steps and contact the local Haemonetics representative for further instructions
before using the device.
" Wipe away as much blood as possible with gauze pads.
" Irrigate the centrifuge drain holes with cleaning solution, until the drain
tube is rinsed clear of the spilled material.
" Remove the bag and replace it with a new bag.
" Dispose of the used waste bag according to local established policies
concerning the disposal of biohazard waste.
Note: A 50 ml syringe attached to a 20 cm section of disposable tubing placed in
the drain holes can be used for irrigation. The biohazard waste bag should be
monitored to avoid overfilling.
Warning: An authorized Haemonetics technician should perform a leakage
current control after any major fluid spill involving the ACP 215 device. Leakage
current represents a primary indication of electrical shock hazard and should
be checked according to guidelines as described in local standard operating
procedures.
Filter screens The ACP 215 device is equipped with filter screens on the bottom of the cabinet,
which eliminate dust from incoming cool air. The filters should be cleaned
routinely, especially if dust becomes visible on the screens.
To clean the filters, the operator should:
" Remove the retainer plates using a Phillips-head screwdriver.
" Remove the filter screens from the panel.
" Rinse the screens under running water - DO NOT use any cleaning agents.
" Gently squeeze the screens to remove excess water.
" Place the screens on a clean, dry cloth and allow to dry completely.
" Reinsert the screens into the panel, ensuring that all openings are
completely covered by the filter.
" Replace the retainer plates and tighten the screws.
Warning: To avoid electrical shock, the filter screen should be completely dry
before it is reinstalled on the ACP 215 cabinet.
P/N 85271-30, Manual revision: C
Page 30

3-6 Maintaining the ACP 215 Equipment
CUSTOMER SERVICE
Clinical training Haemonetics employs a staff of Clinical Specialists to provide training in the use
of the ACP 215 equipment. The local Haemonetics representative will schedule
staff training upon delivery of the ACP 215 equipment and should be contacted
to organize further instruction when needed.
Field service Haemonetics maintains a worldwide network of company-trained service repre-
sentatives responsible for responding to technical needs concerning equipment.
These technical specialists are available to diagnose and repair any malfunctions,
as well as provide routine annual or semi-annual maintenance of the equipment,
including leakage current tests. If service beyond the routine maintenance and
cleaning described in this manual is required, the local Haemonetics representative should be contacted to provide specific instruction.
Returned Goods Authorization system
Haemonetics seeks to provide its customers with equipment and material which
respects the highest established standards of quality in design and manufacturing.
If for any reason merchandise must be returned to the company, the customer
should refer to the Haemonetics Returned Goods Authorization (RGA) system
procedure to ensure proper handling and subsequent analysis of the material.
The customer should contact the local Haemonetics Representative (or the
Haemonetics Customer Service Department) and provide the following information:
! Product list number, lot number and manufacture date.
! Number of articles to be returned.
! Description of defect.
! Number of parcels being shipped.
The Haemonetics Representative may ask for additional details, depending on
the nature of the problem. The customer should be prepared to provide a thorough description of the problem encountered, as well as the product information
listed above.
If a contaminated disposable set must be returned by courier services, the
Haemonetics Representative may provide specific instructions concerning preparation for shipping blood-contaminated products. In addition to the Haemonetics guidelines, the consumer should strictly follow the local standard operating
procedure related to the shipment of blood-contaminated materials and thus
minimize any potential health hazards involved.
P/N 85271-30, Manual revision: C
Page 31

Maintaining the ACP 215 Equipment 3-7
In some cases, it may be necessary to dispose of the contaminated goods after
reporting the problem to the Haemonetics representative. This should be done
according to the locally established guidelines pertaining to the disposal of
biologically contaminated material.
Warning: Haemonetics products must be properly cleaned and packed prior to
their return. It remains an important responsibility of the customer to reduce
this serious potential health hazard, by being aware of the risks involved in the
shipping, handling and testing of this material.
P/N 85271-30, Manual revision: C
Page 32

Cleaning and maintenance record
®
Action January Febr. March April May June July August Sept. Oct. Nov. Dec.
Clean cabinet, control panel, valves, shaker and
printer
Clean air detectors
Clean pump rotors
Clean centrifuge cover and well
Haemonetics
Device serial number: ................................................................................. Year: .....................................................................................
Clean optical bowl sensor
Clean optical line sensor
Clean air filters
Inspect O-ring: apply silicon lubricant
Clean DPM and SPM
Verify biohazard waste bag
Maintenance performed by:
(date and initials)
Reviewed by:
(date and initials)
Annual preventive maintenance should be scheduled by a supervisor when appropriate and performed by a Haemonetics service representative or a qualified engineer.
Annual maintenance performed by: ................................................................................................ Date performed: ................................................................................................
Reviewed by: .................................................................................................................................. Date/initials: ......................................................................................................
Page 33

Chapter 4
Ensuring Safety and Quality for an ACP 215 Procedure
HANDLING THE ACP 215 EQUIPMENT . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Storing the device and material. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Inspecting the material . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
PREVENTING PROBLEMS DURING AN ACP 215 PROCEDURE . . . . . . . . . 4-3
Understanding the risk of hemolysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Avoiding the consequences of flow restriction. . . . . . . . . . . . . . . . . . . . . 4-3
Avoiding bowl misalignment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Avoiding overheating due to mechanical situations. . . . . . . . . . . . . . . . . 4-4
WARNINGS FOR THE OPERATOR . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Electrical shock hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Leakage current control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Mechanical hazards/rotating parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Power outlet connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Communicable disease precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
P/N 85271-30, Manual revision: C
Page 34

4-2 Ensuring Safety and Quality for an ACP 215 Procedure
HANDLING THE ACP 215 EQUIPMENT
Safe and successful ACP 215 operation will depend in part on the proper routine
handling of the ACP 215 equipment. The operator should be aware of the problems which could result if the device or disposable material is stored, installed or
used incorrectly.
Storing the device and material
Inspecting the material
The ACP 215 device must not be operated or stored in an area where flammable
gases or vapors are present. The ACP 215 disposable set material should be kept
in a dry, well-ventilated area and isolated from any chemical vapors. The operator
should handle the disposable set elements with clean, dry hands or gloves.
Prior to installation, the operator should complete a visual inspection of the
disposable set elements and control for twisted or flattened sections.
After installing the disposable set, the operator should verify the correct placement of the individual elements, prior to initiating a procedure. It is important that
the tubing remain free of any twists or occlusions which could cause a flow
obstruction.
Warning: Upon completion of any procedure, the used disposable should be
considered as biologically contaminated. It must be disposed of according to
local standard operating procedure for the removal of such material and should
not be mixed with non-biologically contaminated waste.
Installation Installation of the disposable set is explained in detail in each procedure opera-
tion manual.
P/N 85271-30, Manual revision: C
Page 35

Ensuring Safety and Quality for an ACP 215 Procedure 4-3
PREVENTING PROBLEMS DURING AN ACP 215 PROCEDURE
Understanding the risk of hemolysis
Avoiding the consequences of flow restriction
Hemolysis involves the destruction of red blood cell membranes, with the release
of free hemoglobin into the plasma portion of the blood. Free hemoglobin does
not have the capacity to transport oxygen and can produce serious problems. The
remnants of the red cell can stimulate clot formation and damage the vascular
nature of the lungs and the kidneys. This could lead to respiratory complications
and/or renal failure.
Hemolysis of red cells can occur during a procedure in the rare event of a
mechanically induced situation, such as overheating or excessive pressure.
Warning: Forcing a pump to work against a severe flow restriction can lead to
hemolysis, and thus, consequently high levels of free hemoglobin. It is important
that the operator remain attentive to this fact in the case of any “high pressure”
alarms during operation.
If there is any suspicion that hemolysis has occurred, the operator should follow
the local SOP applicable in this type of situation. The operator should also
contact the local Haemonetics representative if there is any suspicion of a
mechanically induced problem.
As the bowl is filling, a flow restriction in the effluent tubing can create pressure
on the outlet port of the disposable bowl. This unrelieved pressure can deform
the rotary seal of the disposable bowl. If the functional characteristics of the
rotary seal are altered, the increased friction and excessive heat can make the
contents of the bowl unsuitable for further use.
As the bowl is emptying, a flow restriction in the effluent tubing can cause the
pressure in the centrifuge bowl to drop severely. This sudden drop in pressure
could potentially produce hemolysis.
To eliminate these potential problems, the operator should not clamp the effluent
tubing.
P/N 85271-30, Manual revision: C
Page 36

4-4 Ensuring Safety and Quality for an ACP 215 Procedure
Avoiding bowl misalignment
Avoiding overheating due to mechanical situations
An improperly installed disposable bowl can become misaligned as it spins. This
can create excessive friction, and consequently overheat the bowl contents. The
operator should follow the bowl seating procedure to ensure proper placement
of the bowl in the centrifuge.
Warning: The operator must not use any bowl which cannot be properly seated
in the centrifuge chuck. Overheating can occur, subsequently lead to hemolysis
and make any blood being processed unsafe for reinfusion. During operation
the operator should interrupt the ACP 215 procedure if an abnormality or noise
appears, related to the spinning bowl.
Overheating could also result from a mechanical or maintenance-related
problem, such as a defective bearing or seal within the centrifuge well. In this
case, the operator should contact the local Haemonetics representative and
discontinue use of the device until it is serviced.
Warning: If any component of the ACP 215 device has become overheated
during a procedure, and thus overheats the blood being processed, the blood
components cannot be considered safe for re-infusion.
P/N 85271-30, Manual revision: C
Page 37

Ensuring Safety and Quality for an ACP 215 Procedure 4-5
WARNINGS FOR THE OPERATOR
Electrical shock hazards
Leakage current control
The operator should always use the ACP 215 device with clean, dry hands, or
gloves. The internal parts of the device contain various electrical components.
Contact with any of these components, when the device is connected to an
external powered source, could result in an electrical shock to the operator.
The operator should never remove any of the ACP 215 cabinet panels. Maintenance requiring access to the inner cabinet remains the responsibility of a
Haemonetics-trained technician.
Each ACP 215 device receives a careful inspection for leakage current prior to
leaving the factory. Haemonetics recommends that a control be performed for
leakage current by an authorized representative as part of the annual preventative
maintenance. It remains the responsibility of the facility to ensure that this control
is performed.
In the event of any major spill in which fluid may enter the cabinet, or an important voltage surge, the operator is responsible to ensure that a leakage current test
is performed before re-using the device. The control is necessary to avoid the risk
of electrical shock and should be conducted by an authorized Haemonetics
representative.
Mechanical hazards/rotating parts
Power outlet connection
As with any equipment containing rapidly rotating parts, the potential for severe
injury exists if contact is made, or if clothing becomes entangled with the moving
parts. The ACP 215 device contains a safety feature, designed to prevent the
centrifuge from spinning if the system has not been properly secured. However,
the operator should respect the usual precautions taken when working with
equipment containing rotating mechanical parts.
It is not permitted to power the device using a power cord not supplied by
Haemonetics, a multiple portable socket outlet or an extension cord.
P/N 85271-30, Manual revision: C
Page 38

4-6 Ensuring Safety and Quality for an ACP 215 Procedure
Communicable disease precautions
Despite testing and screening to detect communicable diseases such as hepatitis,
syphilis or HIV, the risk remains that the blood being processed may be infected.
The operator must take the appropriate precautions when handling blood products and disposing of blood-contaminated material, to ensure personal safety as
well as the safety of others who may come in contact with the material.
Proper handling of blood contaminated material
If a leak or blood spill should occur, it should be cleaned immediately. The operator should follow the local standard operating procedure outlining the steps to
follow and product(s) to be used for the disinfection of blood-contaminated material.
If any blood-contaminated material must be returned to Haemonetics for further
inspection, the operator should consult the RGA Procedure as described in the
chapter Maintaining the ACP 215 Equipment.
Proper disposal of biologically contaminated materials
Any disposable material used during an ACP 215 procedure is considered as
biologically contaminated. It must be disposed of according to local standard
operating procedure for the removal of such material and should not be mixed
with non-biologically contaminated waste.
P/N 85271-30, Manual revision: C
Page 39

Chapter 5
Troubleshooting for the ACP 215 Device
LISTING SYSTEM ERROR MESSAGES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
LISTING INTERRUPT SAFETY SYSTEM MESSAGES . . . . . . . . . . . . . . . . . . . 5-5
P/N 85271-30, Manual revision: C
Page 40

5-2 Troubleshooting for the ACP 215 Device
LISTING SYSTEM ERROR MESSAGES
When the ACP 215 device is powered-on, several internal tests are performed.
Any test failure will interrupt the test sequence and a system error message will
be displayed. If a message of this type is displayed, the operator should record the
message, power off the device and contact Haemonetics Field Service.
If for any reason the system has been powered off, or an unexpected power
failure has occurred during a procedure, there is no automatic recovery procedure. The operator should follow the local SOP applicable in this type of situation.
The following table lists the system error messages with a corresponding code
number and relevant operator actions.
System Error Message Corrective Action
CPU OPERATION ERROR
CODE = 4
Please POWER OFF
ROM TEST FAILURE
CODE = 5
Please POWER OFF
CALIBRATION CRC FAILURE
CODE = 7
Please POWER OFF
DPM FAILED 20% ANALOG TEST
CODE = 10
Please POWER OFF
DPM FAILED 50% ANALOG TEST
CODE = 10
Please POWER OFF
DPM FAILED 80% ANALOG TEST
CODE = 10
Please POWER OFF.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
SPM FAILED 20% ANALOG TEST
CODE = 11
Please POWER OFF
SPM FAILED 50% ANALOG TEST
CODE = 11
Please POWER OFF
P/N 85271-30, Manual revision: C
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
Page 41

Troubleshooting for the ACP 215 Device 5-3
System Error Message Corrective Action
SPM FAILED 80% ANALOG TEST
CODE = 11
Please POWER OFF
XXX* VALVE POSITION FAULT
CODE = 12
PLEASE POWER OFF
* XXX DENOTES THE COLOR OF THE VALVE
WATCHDOG ERROR
CODE = 14
Please POWER OFF
WATCHDOG ERROR
CODE = 15
Please POWER OFF
LINE SENSOR VOLTAGE TOO LOW
CODE = 50
Please POWER OFF
LINE SENSOR VOLTAGE TOO HIGH
CODE = 51
Please POWER OFF
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
CONFIGURATION CRC FAILURE
CODE = 52
Please POWER OFF
SPM FILTER INTERLOCK FAILURE
CODE = 502
Please POWER OFF
DPM FILTER INTERLOCK FAILURE
SPM FILTER INTERLOCK FAILURE
CODE = 504
Please POWER OFF
DPM FILTER INTERLOCK FAILURE
CODE = 507
Please POWER OFF
AIR DETECTOR FAULT: [SLAD]
CODE = 509
Please POWER OFF
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
P/N 85271-30, Manual revision: C
Page 42

5-4 Troubleshooting for the ACP 215 Device
System Error Message Corrective Action
AIR DETECTOR FAULT: [BLAD]
CODE = 510
Please POWER OFF
DPM OUT OF TOLERANCE
CODE = 514
Please POWER OFF
SPM OUT OF TOLERANCE
CODE = 515
Please POWER OFF
CPU RELAY CLOSING FAILURE
CODE = 701
Please POWER OFF
CPU RELAY OPENING FAILURE
CODE = 702
Please POWER OFF
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
P/N 85271-30, Manual revision: C
Page 43

Troubleshooting for the ACP 215 Device 5-5
LISTING INTERRUPT SAFETY SYSTEM MESSAGES
Specific inputs are checked at 50 ms intervals during an ACP 215 procedure. If
an error is detected, all peripherals are shut down, and an error message is
displayed.
For most these of situations, the procedure can be automatically recovered.
However, in some of these situations, the operator will be required to power off
the device and the procedure cannot be recovered.
The following table list these messages which may be displayed during a procedure and the relevant operator actions.
Interrupt Safety System Message Corrective Action
CENTRIFUGE OVERSPEED
PRESS YES TO CONTINUE
CENTRIFUGE UNDERSPEED
PRESS YES TO CONTINUE
CENTRIFUGE COVER UNLOCKED
PRESS YES TO CONTINUE
DPM FILTER NOT INSTALLED
PRESS YES TO CONTINUE
SPM FILTER NOT INSTALLED
PRESS YES TO CONTINUE
1. Press the YES key to continue.
2. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Press the YES key to continue.
2. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Verify the position of the cover lock and ensure that it is in
the locked position.
2. Press the YES key to resume the procedure.
3. If the message persists, stop the procedure and contact
Haemonetics Field Service
1. Ensure that the DPM filter is correctly installed on the draw
pressure monitor and reinstall if necessary.
2. Press the YES key to resume the procedure.
3. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Ensure that the SPM filter is correctly installed on the
system pressure monitor and reinstall if necessary.
2. Press the YES key to resume the procedure.
3. If the message persists, stop the procedure and contact
Haemonetics Field Service
XXX* VALVE POSITION FAULT
PRESS YES TO CONTINUE
* XXX DENOTES THE COLOR OF THE VALVE
BLOOD PUMP POSITION FAULT
PRESS YES TO CONTINUE
1. Press the YES key to continue.
2. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Ensure that the tubing is properly installed in the blood
pump and reinstall if necessary.
2. Press the YES key to resume the procedure.
3. If the message persists, stop the procedure and contact
Haemonetics Field Service
P/N 85271-30, Manual revision: C
Page 44

5-6 Troubleshooting for the ACP 215 Device
Interrupt Safety System Message Corrective Action
SOLUTION PUMP POSITION FAULT
PRESS YES TO CONTINUE
BLOOD PUMP WRONG DIRECTION
PRESS YES TO CONTINUE
SOLUTION PUMP WRONG DIRECTION
PRESS YES TO CONTINUE
CHECK SHAKER CONNECTION
PRESS YES TO CONTINUE
FLUID IN CENTRIFUGE DETECTED
Please POWER OFF
1. Ensure that the tubing is properly installed in the solution
pump and reinstall if necessary.
2. Press the YES key to resume the procedure.
3. If the message persists, stop the procedure and contact
Haemonetics Field Service
1. Press the YES key to continue.
2. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Press the YES key to continue.
2. If the message persists, stop the procedure and contact
Haemonetics Field Service.
1. Ensure that the shaker connector is attached to the rear panel of the ACP 215 device.
2. Press the YES key to resume the procedure
3. If the message persists, stop the procedure and contact
Haemonetics Field Service.
If there is a spill in the centrifuge, the centrifuge and pump(s)
will stop.
1. Power off the ACP 215 device.
2. Refer to the appropriate section of the manual for guidance
on handling a blood spill.
3. Contact Haemonetics Field Service.
WATCHDOG ERROR
Please POWER OFF
SOFTWARE WATCHDOG FAULT
INVALID STATE
CODE = 802
Please POWER OFF
SOFTWARE WATCHDOG FAULT
CURRENT MODULE ID = XXX*
EXPECTED MODULE ID = XXX
CURRENT STATE = XXX
CODE = 800
Please POWER OFF
* XXX DENOTES THE VALUE DISPLAYED
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
P/N 85271-30, Manual revision: C
Page 45

Troubleshooting for the ACP 215 Device 5-7
Interrupt Safety System Message Corrective Action
=============================
INTERNAL SOFTWARE ERROR
MODULE: XXX*
LINE: XXX
STATE: XXX
PARAM: XXX
=============================
* XXX DENOTES THE VALUE DISPLAYED
INTERNAL SOFTWARE ERROR
CALL HAEMONETICS FIELD SERVICE
1. Power off the ACP 215 device.
2. Contact Haemonetics Field Service.
1. Power off the ACP 212 device.
2. Contact Haemonetics Field Service.
P/N 85271-30, Manual revision: C
 Loading...
Loading...