Page 1

User Manual
DOC022.52.80026
Refillable ORP/Redox Probe: Model MTC30101 or MTC30103
Safety information
Precautionary labels
Read all labels and tags attached to the instrument. Personal injury or damage to the
instrument could occur if not observed. A symbol on the instrument is referenced in the
manual with a precautionary statement.
Electrical equipment marked with this symbol may not be disposed of in European domestic or public disposal
systems. Return old or end-of-life equipment to the manufacturer for disposal at no charge to the user.
Specifications
Note: Specifications are subject to change without notice.
Specifications Details
Probe type Digital combination electrode with a refillable Ag/AgCl reference and a built-
in temperature sensor
Range ±1200 mV
Resolution 0.1 mV
Temperature accuracy ±0.3 °C (±0.54 °F)
Operating temperature range 0 to 80 °C (32 to 176 °F)
Storage temperature range 5 to 40 °C (41 to 104 °F)
Junction Ceramic
Reference potential versus Standard
Hydrogen Electrode
207 mV at 25 °C
Fill solution 3M KCl with saturated AgCl
Reference type Ag/AgCl (3 M KCl)
Minimum sample depth 20 mm
Dimensions Diameter: 12 mm (0.47 in.)
Length: 175 mm (6.89 in.)
Cable length: 1 or 3 m (3.28 or 9.84 ft)
Cable connection M12 digital output and connector compatible with HQd meters
Product overview
The MTC301 series probe is a refillable, combination oxidation reduction potential
(ORP/Redox) probe with a built-in temperature sensor (Figure 1). The MTC30101 or
MTC30103 probe is available with a 1 or 3 m (3.28 or 9.84 ft) cable and is intended for
laboratory use. The probe measures absolute mV values in wastewater, drinking water
and general applications. The probe measures ORP/Redox in wastewater, drinking water
and general applications. A 59 mL bottle of reference electrolyte filling solution (3M KCl
solution saturated with AgCl) is included with the probe.
1
Page 2
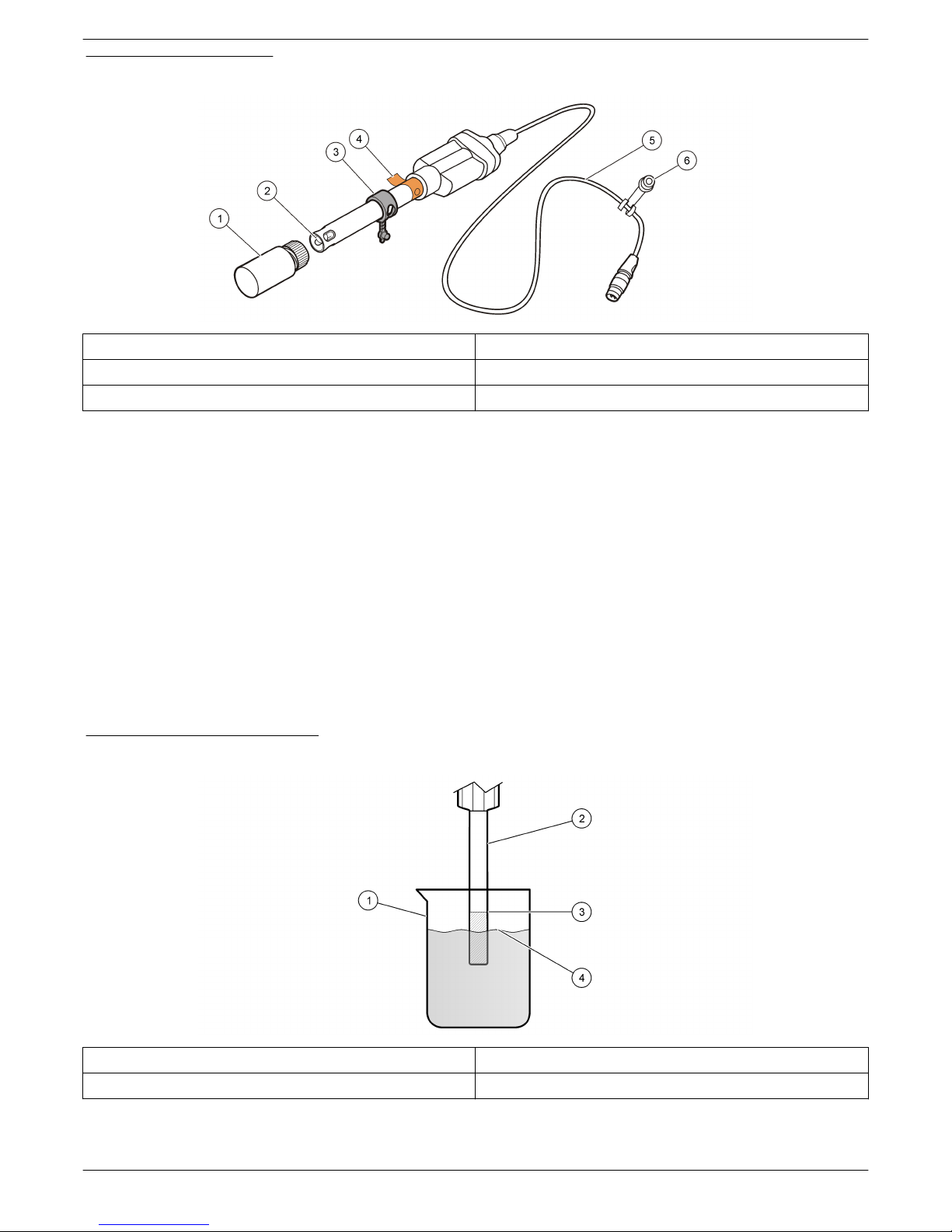
Figure 1 Probe overview
1 Probe soaker bottle 4 Protective tape and filing-hole
2 Reference junction, platinum and temperature sensor 5 1 or 3 meter (3.28 or 9.84 ft) cable
3 Filling-hole cap 6 Probe soaker bottle holder
Preparation for use
Prepare the probe for use before calibration or sample measurement.
1. Turn the probe soaker bottle cap counter-clockwise to loosen the cap.
2. Remove the soaker bottle from the probe.
3. Rinse the reference junctions and electrode with deionized water thoroughly to
remove the viscous storage/filling solution completely. Blot dry with a lint-free cloth.
4. Remove the protective tape from the filling hole before initial use (refer to Figure 1
on page 2). Dispose of the protective tape.
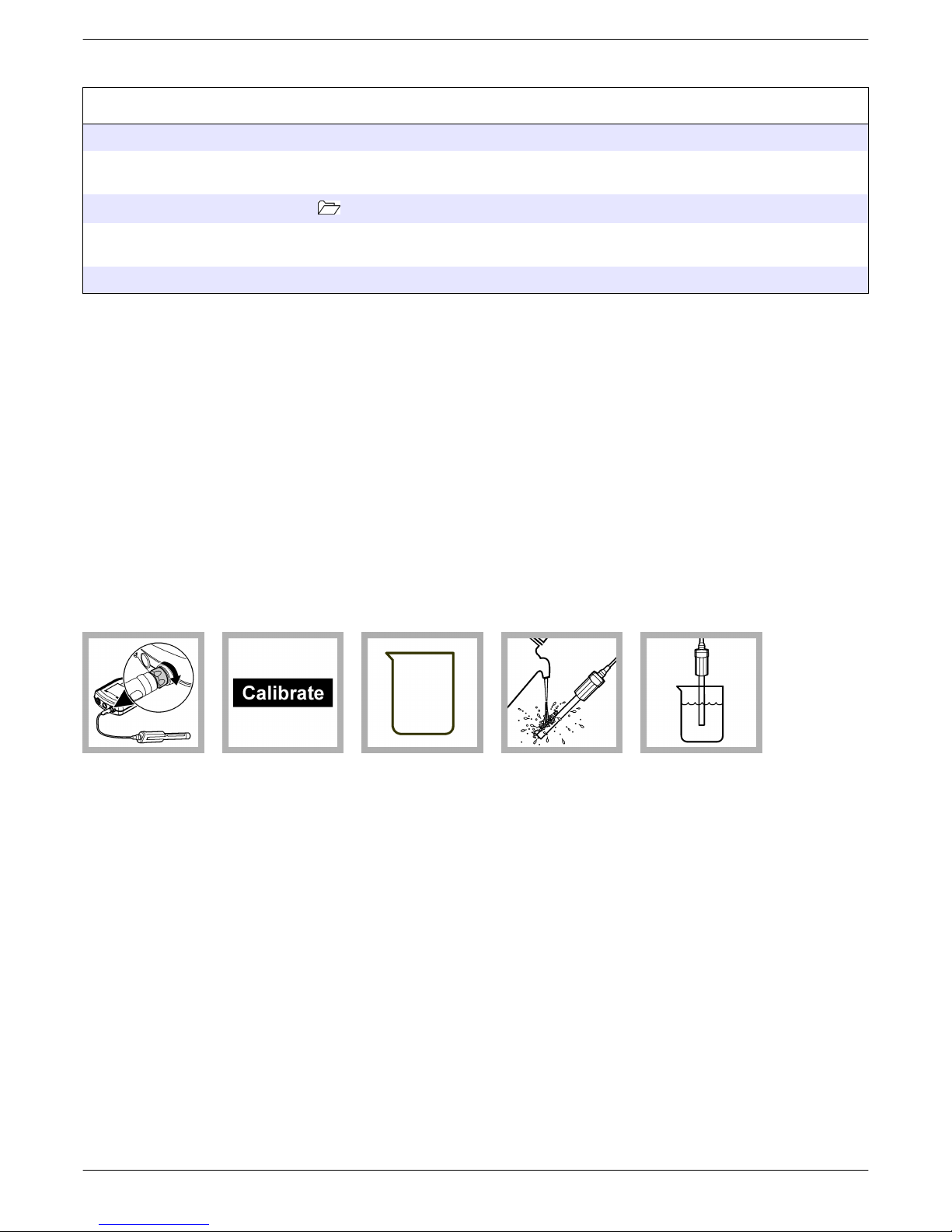
5. Add filling solution to the probe as necessary (refer to Fill the probe on page 11).
The filling solution must be above the standard solution or sample level during
measurement (Figure 2).
6. Make sure that the filling hole is open during measurement for the proper flow of the
filling solution.
Figure 2 Measurement method
1 Container 3 Filling solution level
2 Probe body 4 Standard solution or sample level
2
Page 3
Calibration
Before calibration:
The probe must have the correct service-life time stamp. Set the date and time in the meter before the probe is attached.
It is not necessary to recalibrate when moving a calibrated probe from one HQd meter to another if the additional meter is
configured to use the same calibration options.
To view the current calibration, push , select View Probe Data, then select View Current Calibration.
If any two probes are connected, push the UP or DOWN arrow to change to the single display mode in order to show the
Calibrate option.
Prepare the probe for use (refer to Preparation for use on page 2).
Calibration notes:
• Additional standards can be selected in the Calibration Options.
• Do not dilute ORP/Redox standards. Use fresh ORP/Redox standard for calibration.
• ZoBell’s redox potential is temperature dependent. The HQd calibration routine
factors in this temperature dependency allowing accurate calibrations within the
temperature range of 0 to 30 °C (32 to 86 °F). Light’s solution should be read at 25 °C
(77 °F). Custom ORP/Redox calibration solution values and temperature are userdefined.
• The calibration is recorded in the probe and the data log. The calibration is also sent
to a PC, printer or flash memory stick if connected.
• Air bubbles under the sensor tip when submerged can cause slow response or error
in measurement. If bubbles are present, gently shake the probe until bubbles are
removed.
• If a calibration error occurs, refer to Troubleshooting on page 12.
Calibration procedure:
1. Connect the
probe to the
meter. Make sure
that the cable
locking nut is
securely
connected to the
meter. Turn on the
meter.
2. Push
Calibrate. The
display shows the
ORP/Redox
standard solution
that is necessary
for calibration.
3. Add the fresh
ORP/Redox
standard solution
to a beaker or an
appropriate
container.
4. Rinse the
probe with
deionized water.
Blot dry with a lintfree cloth.
5. Put the probe
in the standard
solution so that
the temperature
sensor is
completely
submerged. Stir
gently. Shake the
probe from side to
side in the sample
to refresh the
reference junction.
3
Page 4
6. Push Read.
Stir gently. The
display will show
"Stabilizing" and a
progress bar as
the probe
stabilizes in the
sample. The
display shows the
standard solution
value and the mV
offset when the
reading is stable.
7. Push Done to
view the
calibration
summary.
8. Push Store to
accept the
calibration and go
back to the
measurement
mode.
Sample measurement
Before measurement:
The probe must have the correct service-life time stamp. Set the date and time in the meter before the probe is attached.
If complete traceability is necessary, enter a sample ID and operator ID before measurement. Refer to the HQd meter
manual for more information.
Regular calibration is required for the best measurement accuracy (refer to Calibration on page 3).
Prepare the probe for use (refer to Preparation for use on page 2).
Make sure that the platinum disc is clean and smooth (refer to Clean the probe on page 10).
Measurement notes:
• Data is automatically stored in the data log when Press to Read or Interval is
selected in the Measurement Mode. When Continuous is selected, data will only be
stored when Store is selected.
• Air bubbles under the sensor tip when submerged can cause slow response or error
in measurement. If bubbles are present, gently shake the probe until bubbles are
removed.
• If a measurement error occurs, refer to Troubleshooting on page 12.
Measurement—direct method procedure:
4
Page 5
1. Connect the
probe to the
meter. Make sure
that the cable
locking nut is
securely
connected to the
meter. Turn the
meter on.
2. To significantly
reduce the
stabilization time
for reducing-type
samples, put the
platinum disc in
Reducing Solution
for ORP
Electrodes for
3-10 minutes
before the initial
sample
measurement.
3. Rinse the probe
with the sample.
4. Put the probe in
the sample and
stir gently. Make
sure that the
reference
junctions are
completely
submerged. Do
not put the probe
on the bottom or
sides of the
container. Shake
the probe from
side to side in the
sample to refresh
the reference
junction.
5. Push Read.
The display will
show "Stabilizing"
and a progress
bar as the probe
stabilizes in the
sample. The
display will show
the lock icon when
the reading
stabilizes. If
necessary for the
application, record
the sample pH
and temperature.
6. Repeat steps
3-6 for additional
measurements.
7. When
measurements
are done, store
the probe (refer to
Storage
on page 11).
Measurement—conversion to SHE reference procedure:
For some applications, it is customary to report redox potential readings relative to the
standard hydrogen electrode (SHE), also called normal hydrogen electrode (NHE). To do
this, select the value in Table 1 that corresponds to the temperature of the solution
measured. Substitute that value Eref into the equation and solve for Eh:
Eh = E + E
ref
where:
Eh = oxidation reduction potential of the sample relative to the SHE
E = potential developed by the ORP/Redox electrode
E
ref
= potential developed by the reference electrode portion relative to the SHE
(Table 1).
Table 1 shows the potentials, E
ref
, developed by the reference electrode portion relative
to the SHE at various temperatures.
Table 1 Standard potential of reference electrode
Temperature (°C) Electrode potential in mV (E
ref
)
80 163.1
75 167.7
5
Page 6

Table 1 Standard potential of reference electrode (continued)
Temperature (°C) Electrode potential in mV (E
ref
)
70 172.1
65 176.4
60 180.3
55 184.4
50 188.4
45 192.3
40 196.1
35 199.8
30 203.4
25 207.0
20 210.5
15 214.0
10 217.4
5 220.9
0 224.2
Measurement—oxidation titrations procedure:
Oxidation-reduction, or redox titrations, give a simple, reliable method to identify many
substances in a solution. A redox titration consists of adding to an unknown sample, small
increment of a titrant that converts the unknown to a different oxidation state. After each
addition of titrant, the ORP/Redox electrode develops a potential proportional to the
logarithm of the ratio of the activities of the two oxidation states.
At the inflection, or end point, the titrant has completely oxidized or reduced the unknown,
causing a sharp change in the logarithm of the ratio of the activities of the two oxidation
states. A corresponding sharp change in the potential is developed by the platinum
electrode. Often several oxidizing or reducing species can be precisely identified in the
same solution by a single titration with several inflection points.
The following directions represent a general procedure for doing a redox titration once the
sample is prepared for measurement.
6
Page 7

1. Fill a 10 mL
burette with a
standard titrant
solution with a
normality that is
5-10 times that of
the sample.
2. Connect the
probe to the
meter.
3. Pipet a 50 mL
sample into a
150‑mL beaker.
Stir with a
magnetic stirrer
throughout the
titration.
4. Add titrant in
0.5 to 1 mL
increments.
Record the
potential after
each addition.
Near the end
point, when large
potential changes
are seen, add
increments of 0.1
to 0.2 mL.
Continue the
titration 3 to 4 mL
past the end point.
5. Plot the
electrode potential
versus volume of
added titrant and
fit a smooth curve
through the points.
The end point is
the point of
inflection (the
point of greatest
slope).
6. Calculate the
normality of the
sample, Nx, in
equivalents per
liter:
Nx = (Vt x Nt) / V
x
where:
Nt = normality of
titrant (Eq/L)
Vt = volume of
titrant at end point
(mL)
Vx = Volume of
sample (mL)
Run Check Standard
The Run Check Standard feature validates the instrument performance between sample
measurements. Use the Run Check Standard feature for a periodic or a user-defined
interval measurements of a traceable standard solution. Set the criteria for check
standards from the MTC301 probe Settings menu.
Note: Access control must be set to off or a valid password must be used to change the Run Check
Standard options.
1. Push
. The Full Access Options menu shows.
2. Select Run Check Standard.
Note: Select the correct probe if two probes are connected to the meter.
3. Use the standard solution shown on the display.
4. Rinse the probe with deionized water. Dry the probe with a no-lint cloth.
7
Page 8

5. Put the probe in the standard solution. Make sure that the reference junction is fully in
the standard. Move the probe up or down or lightly shake the probe to remove air
bubbles.
6. Push Read. The display shows "Stabilizing" and a progress bar as the reading
stabilizes. The display shows the value of the check standard and Check Standard
Passed or Check Standard Failed.
7. If the display shows Check Standard Passed, the check standard measurement is in
the accepted limits. Select Done to continue the sample measurement.
8. If the display shows Check Standard Failed, the measurement is out of the accepted
limits. A calibration is recommended. Make sure that the limits are set correctly at the
MTC301 probe Settings menu. If the acceptance criteria is set to "Cal Expires on
Failure: Yes", the display shows the calibration icon and a question mark until the
probe is calibrated again. To correct the probe calibration and status indicator,
calibrate the probe (refer to Calibration on page 3).
Advanced operation
Parameter-specific settings can be changed through the Full Access Options menu.
Details about menu navigation, available options and how to change them are given in
the screens, tables and procedures throughout this section.
The settings that can be changed are shown in Table 2.
Table 2 Parameter-specific settings
Setting Options
Measurement Options
• Response time
• Upper and lower range limits (defines mV limits per method)
Calibration Options
• Standard
• Calibration reminder
• Offset limit
• Standard value (if Custom option is selected)
Check Standards Options
• Standard (temperature compensated for ZoBell’s and Light’s solutions)
• Check standard reminder
• Acceptance criteria
• Standard value (at 25 °C if Custom option is selected)
Change measurement options
Methods are groups of default or user-defined settings relevant to specific applications. If
the meter is set to the default method and the Modify Current Settings option is chosen, a
prompt for a new name is shown after the changes are entered. The settings are saved
with this name to distinguish them from the default method settings, which cannot be
changed. A saved method can be used instead of multiple adjustments to the individual
settings. Changes made to a user defined method are automatically saved with the
existing name. Multiple methods can be saved for the same probe on each meter.
1. Make sure a probe is connected to the meter.
2. Push and select MTC301 Settings.
8
Page 9

3. Select Modify Current Settings.
4. Select Measurement Options and update the settings:
Option Description
Response Time Sets the response time—
• Fast (2 mV/minute)
• Medium (1 mV/minute) (default)
• Slow (0.5 mV/minute)
The response time affects the speed of the measurement by adjusting the
stabilization criteria.
Measurement
Limits
Sets the measurement limits—Lower limit (default: -1200.0 mV) or Upper
limit (default: 1200.0 mV).
The measurement limits can be set to match the acceptable values for
the sample. When the measurement is above the upper limit setting or
below the lower limit setting, the meter shows an "Out of limits" message.
This message is an alert to a potential problem with the process
conditions.
5. If prompted, enter a name for the new method settings. Additional changes made to
the settings of an existing method are automatically saved with the same method
name.
6. Push EXIT until the meter returns to the measurement mode.
Change calibration options
1. Make sure a probe is connected to the meter.
2. Push and select MTC301 Settings.
3. Select Modify Current Settings.
4. Select Calibration Options and update the settings:
Option Description
Standard Sets the calibration standard—
• ZoBell’s (221mV – 25 °C)
• Light’s (468mV – 25 °C)
• Custom
Temperature compensated for ZoBell’s solution.
Standard values are shown on the Calibration Options screen.
Light’s solution is characterized at 25 °C.
Custom standard values and temperature are user-defined.
Offset Limits Sets the offset limits—±1 mV to 250 mV (default: ±25 mV).
The offset must fall within set limits for successful calibration.
Standard
Value
When Standard is set to Custom, sets the values for the custom calibration
standard— -1200.00 to 1200.0 mV (default: +221.0 mV). Custom standards
are characterized at 25 °C.
5. Select Calibration Reminder and update the settings:
Option Description
Reminder
Repeat
Meter will make an audible sound when a calibration is due and repeat the
sound at the selected interval—Off (default), 1 d, 7 d or 30 d.
Expires Calibration expires after the selected time—Immediately, Reminder +
30 min, Reminder + 1 h, Reminder + 2 h or Continue Reading.
Note: The meter cannot be used to read samples after calibration has
expired unless Continue Reading is selected.
9
Page 10

6. If prompted, enter a name for the new method settings. Additional changes made to
the settings of an existing method are automatically saved with the same method
name.
7. Push EXIT until the meter returns to the measurement mode.
Change check standard options
1. Make sure a probe is connected to the meter.
2. Push and select MTC301 Settings.
3. Select Modify Current Settings.
4. Select Check Standards Options and update the settings:
Option Description
Standard Sets the check standard—
• ZoBell’s (221 mV – 25 °C) (default)
• Light’s (135 mV – 25 °C)
• Custom
Temperature compensated for ZoBell’s solution.
Standard value for check standard.
Standard value is shown on Check Standard Options screen.
Lights solution is characterized at 25 °C.
Custom standard values and temperature are user-defined.
Standard
Value
When Standard is set to Custom, enter the standard value using the up/down
arrow keys— -1200.0 to 1200.0 mV (default: 221.0 mV).
The value and temperature for custom check standard are user-defined.
5. Select Check Standard Reminder and update the settings:
Option Description
Reminder Repeat Sets the time interval for the check standard reminder—Off (default), 1 d,
7 d or 30 d.
Allow Defer Allows the postponement of check standard reminders—Yes (default) or
No.
6. Select Acceptance Criteria and update the settings:
Option Description
Acceptance Limits Sets the tolerance limits for check standard—-±1 mV to 25 mV
(default: ±10 mV).
Cal Expires on Failure Recalibration required if check standard fails—Yes or No (default).
7. If prompted, enter a name for the new method settings. Additional changes made to
the settings of an existing method are automatically saved with the same method
name.
8. Push EXIT until the meter returns to the measurement mode.
Maintenance
Clean the probe
Clean the probe when:
• Drifting/inaccurate readings or slow stabilization time occurs as a result of
contamination on the platinum disc or the probe being left dry for extended periods of
time.
• Measurement values are outside the calibration/measurement range of the probe
even after a calibration is done using freshly prepared standards.
10
Page 11

Note: After cleaning is done, condition the platinum electrode in representative sample before use.
For general cleaning (including oils, greases and organics):
1. Rinse the probe with deionized water and blot dry with a lint-free cloth.
2. Put the probe sensor and platinum disc in Electrode Cleaning Solution or warm
detergent solution for up to 15 minutes.
Note: The platinum disc can be polished using a soft cloth or cotton swab with detergent
solution.
3. Rinse the probe sensor and platinum disc with deionized water. Blot dry with a lintfree cloth.
For inorganic deposits:
1. Put the platinum disc in a solution of 0.1 M hydrochloric or nitric acid solution for up to
15 minutes.
2. Rinse the probe sensor and platinum disc with deionized water. Blot dry with a lintfree cloth.
Fill the probe
Add filling solution to the probe when the filling solution level is low (refer to Preparation
for use on page 2). Refer to Specifications on page 1 for the applicable filling solution.
1. If the filling hole is closed, remove the filling-hole cap from the filling hole (refer to
Product overview on page 1).
2. Remove the cap from the tip of the filling solution bottle.
3. Hold the bottle so that the tip is down. Put the tip of the bottle in the filling hole.
4. Slowly squeeze the bottle and fully fill the probe.
Note: Fully fill the probe for the best performance.
5. Put the probe into storage if not used immediately (refer to Storage on page 11).
6. Keep the filling solution bottle and cap for later use.
Note: If the dispensing tip becomes clogged, remove the dispensing tip and soak the tip in
warm water. Fully dry and assemble the tip.
Storage
For the best probe performance, do not let the reference junction dry out.
Short-term and long-term storage
Note: The probe can be stored in a sample for up to 2 hours if the sample pH is not high.
1. Put the filling-hole cap in the filling hole (refer to Figure 1 on page 2).
2. Rinse the probe with deionized water. Dry the probe with a lint-free cloth.
3. Fill the probe soaker bottle half full with Hach Electrode Storage Solution or 3 M
potassium chloride (KCl) solution.
4. Loosen the soaker bottle cap and put the soaker bottle on the probe.
5. Turn the soaker bottle cap clockwise to tighten the soaker bottle cap.
6. Make sure that the solution in the soaker bottle completely covers the reference
junction holes.
11
Page 12

Troubleshooting
Message or symptom Possible cause Action
Probe not supported Software not updated To download the most current version of the
software, refer to the applicable product page on the
manufacturer's website.
Refer to the HQd Series meter manual for specific
instructions for the meter model.
HQd meter does not support IntelliCAL
®
probe
Contact a Technical Support Representative.
Connect a probe or probe
requires service
Probe not connected correctly Disconnect, then connect the probe. Tighten the
locking nut.
Software not updated To download the most current version of the
software, refer to the applicable product page on the
manufacturer's website.
Refer to the HQd Series meter manual.
Large number of methods stored on the
probe
Continue to let the probe connect. Do not disconnect
the probe.
Damaged probe Make sure there is connectivity with another probe or
meter to confirm isolated issue with probe. Contact a
Technical Support Representative.
Standard not recognized
error
Storage cap not removed Remove the storage cap from the probe.
Incorrect or contaminated standard
solution
Use fresh standard solution as specified in the
method.
mV reading is same for all
solutions
Storage cap not removed Remove the storage cap from the probe.
Electrical issue Contact a Technical Support Representative.
Slow stabilization time Tape not removed from the filling hole Remove the tape from the filling hole.
Contaminated filling solution Drain and replace the filling solution in the probe with
fresh solution.
Filling hole is closed Open the filling hole cap while in use.
12
Page 13

Message or symptom Possible cause Action
Slow stabilization time Contaminated platinum sensor Clean the probe (refer to Clean the probe
on page 10).
Probe not conditioned/pre-treated for
reducing type samples
To significantly reduce the response time for
reducing type samples the platinum disc must
undergo the following:
1. Make sure that the platinum disc is clean and
smooth.
2. Put the platinum disc in Reducing Solution for
ORP Electrodes for 3-10 minutes before sample
analysis.
3. Rinse the probe with sample, then measure.
Low sample temperature or temperature
difference between samples
Check the sample temperature. The lower the
temperature or the greater the difference of
temperatures between samples, the longer the
response time.
Platinum electrode not conditioned for
reducing-type samples
1. Make sure that the platinum disc is clean and
smooth.
2. Put the platinum disc in Reducing Solution for
ORP Electrodes for 3-10 minutes before sample
analysis.
3. Rinse the probe with sample, then measure.
Air bubbles around inner reference
electrode
Gently tap the probe with hand or shake the probe
while holding the probe downward in the
solution/sample to remove any air bubbles in the
reference junction holes.
Out of range Measure value is outside the
calibration/measurement range of the
probe
Calibrate again using freshly prepared standards.
Clean the probe and calibrate again.
Make sure that the sample is within the range of the
probe.
Air bubbles around inner reference
electrode
Gently tap the probe with hand or shake the probe
while holding the probe downward in the
solution/sample to remove any air bubbles in the
reference junction holes.
13
Page 14

Message or symptom Possible cause Action
Drifting/Inaccurate
readings
Contaminated platinum disc Clean the probe (refer to Clean the probe
on page 10).
Clogged reference Thoroughly rinse the reference junction holes with
deionized water. Gently tap the probe with hand
while holding the probe downward to remove any air
bubbles.
Improper storage conditions The probe may not function correctly if the probe has
been left dry for extended periods of time.
1. Clean or condition the probe and attempt to
recalibrate the probe.
2. If recalibration fails, attempt to recondition the
reference junctions by putting the probe tip in a
3.0 M KCl storage solution for 1-2 hours.
3. Rinse the probe with deionized water before use.
Electromagnetic Forces (EMF) such as
voltaic cells, thermoelectric devices,
electrical generators, resistors and
transformers.
Do not test in areas where EMF is present. For
testing in process units (i.e. spot checking), make
sure that the equipment is grounded.
Air bubbles around inner reference
electrode
Gently tap the probe with hand or shake the probe
while holding the probe downward in the
solution/sample to remove any air bubbles in the
reference junction holes.
Out of limits Check standard value is outside of limits
set in the current method
Make sure that the standard is within the limits of the
current method.
Create a new method that expands the acceptable
limits.
Measurement value is outside of
measurement limits set in the current
method.
Make sure that the sample is within the limits of the
current method.
Create a new method with an expanded range.
Calibration adjustment offset value
outside the limits set in the current
method
Make sure that the standard is within the limits of the
current method.
Create a new method that expands the acceptable
limits.
Storage cap not removed Remove the storage cap.
Temperature out of range Calibration temperature value is outside
of range
Make sure that the sample temperature is within the
range of the probe.
Make sure that the temperature sensor is working
correctly.
Measured temperature is outside range
of the probe
Make sure that the standard temperature is within the
range of the probe.
Make sure that the temperature sensor is working
correctly.
Check standard temperature value is
outside of range
Make sure that the Check Standard temperature is
within the range of the probe.
14
Page 15

15
Page 16

HACH COMPANY World Headquarters
P.O. Box 389, Loveland, CO 80539-0389 U.S.A.
Tel. (970) 669-3050
(800) 227-4224 (U.S.A. only)
Fax (970) 669-2932
orders@hach.com
www.hach.com
HACH LANGE GMBH
Willstätterstraße 11
D-40549 Düsseldorf, Germany
Tel. +49 (0) 2 11 52 88-320
Fax +49 (0) 2 11 52 88-210
info@hach-lange.de
www.hach-lange.de
HACH LANGE Sàrl
6, route de Compois
1222 Vésenaz
SWITZERLAND
Tel. +41 22 594 6400
Fax +41 22 594 6499
©
Hach Company/Hach Lange GmbH, 2010, 2013, 2014. All rights reserved. Printed in U.S.A. 07/2014, Edition 3
 Loading...
Loading...