Gyrus Acmi PlasmaKinetic SuperPulse User Manual

PlasmaKinetic SuperPulse Generator
USER MANUAL
Manufactured for:
Gyrus ACMI,
136 Turnpike Road
Southborough
MA 01772-2104
USA
Gyrus Medical Ltd
St Mellons
Cardiff
CF3 0LT
United Kingdom
Rx only
Customer Service USA:
Customer Service: 1-888-524-7266
Technical Service: 1-800-621-3739
www.gyrusacmi.com
744000
Part Number: 144012-MB
2011-02
0344


………OVERVIEW OF THE GYRUS ACMI SUPERPULSE GENERATOR
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL OVERVIEW
Part Number: 144012-MB
This user manual will familiarize you with the controls and output functions available from your Gyrus
ACMI SuprePulse Generator and instruct you on its proper use.
Copyright
Gyrus Medical, Ltd. 2010. All rights reserved. No part of this
publication may be reproduced, transmitted, transcribed, stored
in retrieval systems, or translated into any language or computer
language, in any form, or by any means: electronic, mechanical,
magnetic, optical, or otherwise, without the prior written
permission of Gyrus ACMI.
Patents
This product may be covered by one or more of the following US
patents:
5,944,715; 6,004,319; 6,013,076; 6,015,406; 6,045,549;
6,056,746; 6,074,386, 6,090,106, 6,093,186; 6,152,143;
6,131,579; 6,179,803; 6,210,355; 6,210,405; 6,228,081;
6,234,178; 6,261,286; 6,293,942; 6,303,134; 6,364,877;
6,416,491; 6,416,509; 6,482,202; 6,517,535; 6,371,926;
6,682,501, 6,893,435,6,984,231, 7,214,224, 7,211,081,
7,195,627.
Associated Patents are in place in other countries
OVERVIEW OF THE GYRUS ACMI SUPERPULSE GENERATOR
The Gyrus ACMI SuperPulse Generator forms a versatile platform for Urology and General surgical use.
Ensure that the contents of this User Manual are read and understood before proceeding to use the
Gyrus ACMI SuperPulse Generator.
OVERVIEW OF THE GYRUS ACMI SUPERPULSE GENERATOR
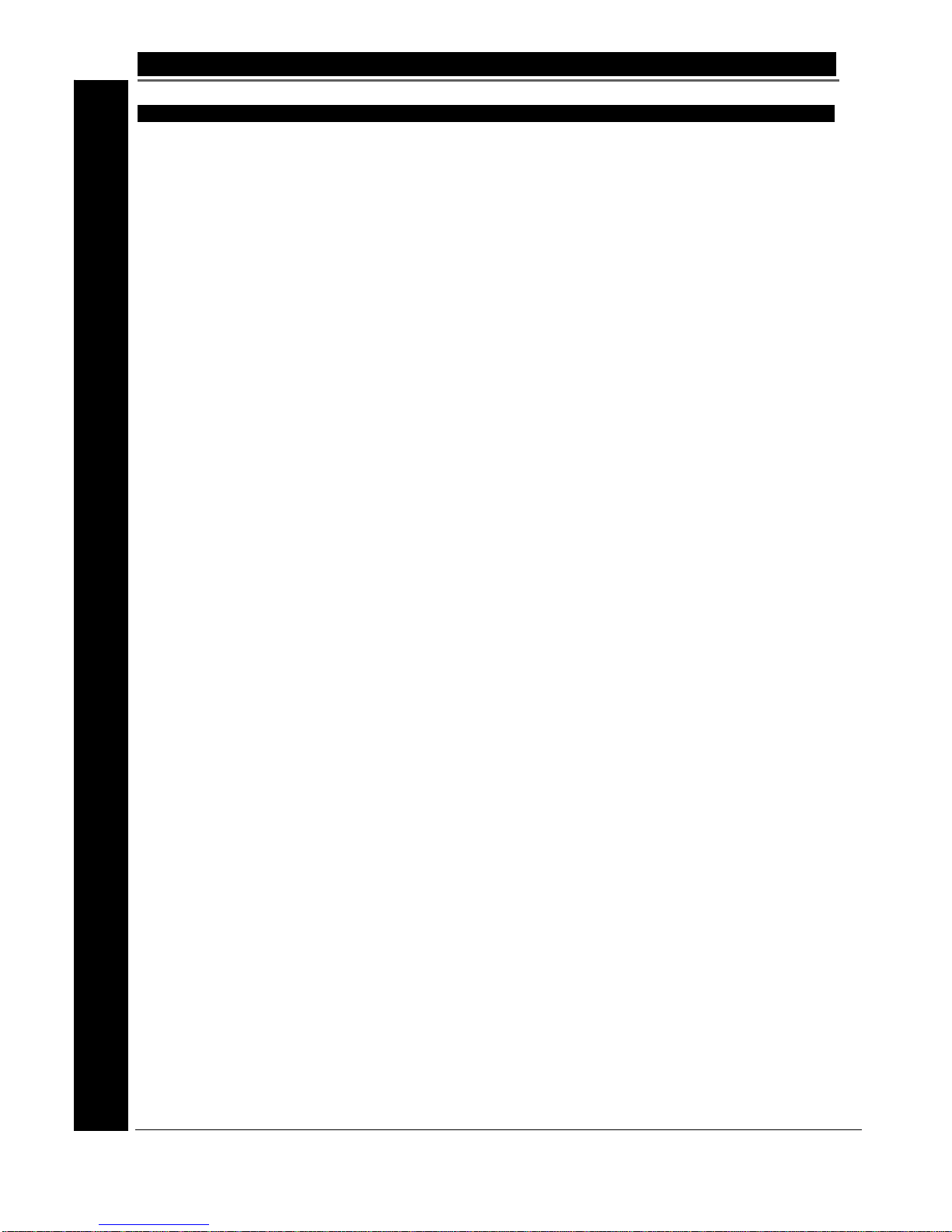
……………………………….TABLE OF CONTENTS .
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL TABLE OF CONTENTS
Part Number: 144012-MB
SECTION PART PAGE .
1 INTRODUCTION
1A Overview of the SuperPulse
Generator 1-1
1B Comparison with Conventional Electrosurgery 1-1
1C Indications for Use 1-1
1D Contraindications for Use 1-1
1E System Description 1-2
1F Instrument Description 1-2
1G Connector Cable Description 1-3
1H Dual Footswitch Connector Cable 1-3
2 PATIENT AND OPERATING ROOM SAFETY
2A General 2-1
2B Servicing/Equipment Disposal 2-2
2C Fire/Explosion 2-2
2D Before Surgery 2-3
2E During Surgery 2-4
2F After Surgery 2-5
2G EMC Classification 2-5
3 INSTALLATION
3A Responsibility of the Manufacturer 3-1
3B Generator Power Requirements 3-1
3C Grounding of the Generator 3-1
3D Routine Maintenance of the Superpulse Generator 3-1
3E Medical Electrical Systems 3-1
4 GENERAL INFORMATION
4A Gyrus ACMI SuperPulse Generator Indicators and Displays 4-1
4B Output Mode Selection and Power Controls 4-3
4C SuperPulse Generator Indicators, Set-Up and Malfunction Displays 4-5
4D Changing the Display Language 4-9
4E Enabling Additional Instruments via a PIN code 4-10
5 BEFORE SURGERY
5A Power up the Generator 5-1
5B Select and Connect the Connector Cable 5-1
5C Attach Gyrus ACMI PK Instrument to the PK Connector Cable 5-2
6 DURING SURGERY
6A Recommendations During Surgery 6-1
6B PK Default Settings 6-1
6C Activation: Output Selection and Audible Tones 6-1
6D Changing Output Mode and Power Setting during Surgery 6-2
6E Changing Instruments During Surgery 6-2
6F Changing Accessories Between Procedures 6-3
7 TECHNIQUES TO OPTIMIZE PERFORMANCE 7-1
8 AFTER SURGERY
8A Following Surgery 8-1
8B Cleaning the Footswitch 8-1
8C Cleaning the Generator 8-1
8D Cleaning and Sterilizing the PK Connector Cable 8-2
9 OPERATING ROOM TROUBLESHOOTING 9-1
10 PERFORMANCE SPECIFICATIONS 10-1
11 ERROR AND FAULT CODES 11-1
12 EXPLANATION OF SYMBOLS 12-1
13 PERIODIC EQUIPMENT SAFETY CHECKS 13-1
14 EMC TABLES 14-1
15 LIMITED WARRANTY 15-1
TABLE OF CONTENTS

SECTION 1 INTRODUCTION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 1-1
Gyrus Medical Ltd, Gyrus Medical Inc and are referred to as Gyrus ACMI in this user manual.
This user’s manual will familiarize you with the controls and output functions available from your
SuperPulse Generator and instruct you on its proper use.
1A. Overview of the SuperPulse Generator
Bipolar electrosurgery is a familiar tool widely employed in surgery. Based on similar
principles, the Gyrus ACMI PlasmaKinetic (PK) technology combined with Gyrus ACMI PK
instruments provides more effective coagulation and cutting of tissues than when similar
instruments are used with other electrosurgical generators.
A key feature of the Gyrus ACMI SuperPulse Generator is the capability of the generator to
identify the type of Gyrus ACMI PK instrument connected to it. The identification of the instrument
causes the generator to operate in the Plas maKinetic (PK) mode that selects a default output
designed to produce the desired electrosurgica l effect for that partic ular instrument. Th is feature
provides additional convenience for the user of the device. The user can change this default to
obtain a wider range of PlasmaKinetic outputs from the device.
1B. Comparison with Conventional Electrosurgery
Conventional bipolar electrosurgery outputs are rarely optimized to the performance
characteristics of specific instruments. This can reduce the speed of clinical effect, increase
the thermal margin around the application site and result in tissue sticking to the instrument.
The following describes the features of the PlasmaKinetic Generator when used with Gyrus
ACMI PK instruments that distinguishes it from conventional bipolar generators.
• Vapor Pulse Coagulation (VPC)
VPC produces controlled coagulation of vascular pedicles using vapor-focused pulses
of energy. VPC has been specifically tailored for delivery through Gyrus ACMI PK
Instruments. Once tissue to be coagulated is grasped in a PK Instrument, the tissue is
uniquely coagulated using the pulses of PlasmaKinetic energy compared to the
continuous output employed in conventional bipolar generators. This feature provides
controlled and repeatable outcomes under a variety of surgical situations.
• PlasmaKinetic Tissue Cutting (PK)
In the PK output mode, the RF energy is used to create an electrical arc into the tissue
located between the electrodes of the Gyrus ACMI PK instrument. This provides the
tissue the tissue cutting through the vaporization and a level of hemostasis dependent
on the selected PK output level. This mode utilizes the conductive properties of the
tissue itself and any fluid released from the tissue during application of the RF energy.
1C. Indications for Use
The SuperPulse Generator is a general surgical electrosurgical device intended for use with
bipolar instruments used in open, endoscopic and laparoscopic surgical procedures involving
the coagulation and cutting of soft tissue. The device is intended for use by qualified medical
personnel trained in the use of electrosurgical equipment.
1D. Contraindications for Use
The use of this device is contraindicated in patients with the following conditions:
Circumcision procedures
Electrosurgery should not be used for circumcision.
Patients with Pacemakers
Use with caution in the presence of internal or external pacemakers. Interference from an
electrosurgical current can cause a pacemaker to enter an asynchronous mode or can block
the pacemaker effect entirely. For further information, consult the pacemaker manufacturer or
hospital Cardiology Department.
SECTION 1 INTRODUCTION
SECTION 1 INTRODUCTION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 1-2
1E. System Description
The SuperPulse Generator (figure 1.1) is designed for use in surgery for the coagulation and
cutting of soft tissue.
A typical system setup would comprise of the following items:
• Generator
• Footswitch (744010) or two 744010 Footswitches connected via connector cable
710003
• Re-usable Connector Cable
• Gyrus ACMI PK surgical instruments and other bip olar surgical instruments.
Fig 1.1
1F. Instrument Description
The instruments below are designed and rated to be used with the Generator. For
convenience and to improve safety during use, all the dedicated instruments have an internal
classification code, which is interrogated by the Generator when the instrument is attached.
Default settings and power set adjustment limits are then set appropriately for that particular
instrument.
Instruments are supplied sterile and are single use only.
SECTION 1 INTRODUCTION
SECTION 1 INTRODUCTION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 1-3
Instrument types:
Model No Description Connector Cable
PlasmaKinetic Molly Forceps 3300PK 33 cm 3 mm Forceps 3 Pin
3301PK 33 cm 3 mm Forceps w/Trocar 3 Pin
3330PK 33 cm 5 mm Forceps 3 Pin
3345PK 45 cm 5mm Forceps 3 Pin
PlasmaKinetic Cutting Forceps 3000PK 33 cm 10mm Cutting Forceps 3 Pin
3001PK 33 cm 10mm Cutting Forceps w/cord 3 Pin
3005PK 33 cm Cutting Forceps 3 Pin
3006PK 33 cm Cutting Forceps w/cord 3 Pin
3045PK 45 cm Cutting Forceps 3 Pin
PlasmaKinetic LP Scissors 3804PK 33 cm LP Scissors 3 Pin
3844PK 45 cm LP Scissors 3 Pin
PlasmaKinetic Needle Electrode 3400PK 33 cm Needle Electrode 3 Pin
Lyons Dissecting Forceps 3700PK 33 cm Dissecting Forceps 3 Pin
3740PK 45 cm Dissecting Forceps 3 Pin
PlasmaKinetic Forceps Macro-Jaw 3600PK 33 cm Macro-Jaw Forceps 3 Pin
3640PK 45 cm Micro-Jaw Forceps 3 Pin
PlasmaKinetic Forceps Micro-Jaw 3601PK 33 cm Macro-Jaw Forceps 3 Pin
3641PK 45 cm Micro-Jaw Forceps 3 Pin
PlasmaKinetic L-Hook 3527PK 33 cm L-Hook 3 Pin
PK SEALOpen Forcep, Curved 3103PK 25 cm (9¾”) Seal Open Forcep, Curved 3 Pin
PK SEALOpen Forcep, Straight 3104PK 25 cm (9¾”) Seal Open Forcep, Straight 3 Pin
PK SEALOpen Forcep, Angle 3105PK 25 cm (9¾”) Seal Open Forcep, Angle 3 Pin
PlasmaSpatula 3220PK 24cm Plasma Spatula 5 Pin
PlasmaSpatula 3200PK 33cm Plasma Spatula 5 Pin
PlasmaSpatula 3240PK 45cm Plasma Spatula 5 Pin
1G. Connector Cable Description
The connector cable is designed and rated for 20 uses with the Generator and Instruments.
The Connector Cable is supplied non sterile and should be sterilized before use in
accordance with its instructions.
Cable types:
Model No Description
PlasmaKinetic Single Function 3900 3 Pin
PlasmaKinetic Dual Function 3905 5 Pin
1H. Dual Footswitch Connector Cable
This optional re-usable cable allows the connection of two Footswitches to the Generator.
Description Model No
Dual Footswitch Connector Cable 710003
SECTION 1 INTRODUCTION
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 2-1
The safe and effective use of electrosurgery depends to a large degree upon factors and variables
solely under the control of the operator. There is no substitute for good surgical technique and
properly trained operating room staff. It is important that the operating instructions supplied with this
or any electrosurgical equipment be read, understood, and followed.
Electrosurgery has been employed safely in numerous procedures. Before starting any surgical
procedure, the physician should be familiar with the medical literature, complications and hazards of
electrosurgery in that procedure.
2A. General
WARNING Hazardous Electrical Output: This equipment is for use only by qualified
personnel.
WARNING Do not use a monopolar generator/accessories simultaneously with the SP
generator. Activation of a monopolar generator/accessories may cause
interference with the SP generator resulting in user message changes on the
display. Before proceeding with surgery, confirm proper power settings are
displayed on the generator. Ensure the appropriate output setting is enabled for
the desired surgical outcome.
WARNING Direct contact between activated monopolar accessories and SP generator
connected accessories could damage the SP generator. If such damage is
suspected, the SP generator should be returned to Gyrus ACMI for inspection.
WARNING Use with caution in the presence of internal or external pacemakers.
Interference from an electrosurgical current can cause a pacemaker to enter an
asynchronous mode or can block the pacemaker effect entirely. For further
information, consult the pacemaker manufacturer or hospital Cardiology
Department.
WARNING Do not use electrosurgical equipment unless properly trained in its use in the
specific procedure intended.
WARNING Electrodes and probes used with monitoring, stimulation, and imaging devices
(or similar equipment) can provide a path for high frequency current even if they
are isolated. To reduce the risk of an inadvertent burn at the electrode site,
place the electrode and / or probe as far away as possible from the
electrosurgical site.
CAUTION If two accessories are connected to the SP generator, ensure the appropriate
accessory is selected prior to activation. Activation of the unintended accessory
could cause unintentional tissue effect.
CAUTION Do not activate electrodes while in contact with other instruments as unintended
tissue effect may occur.
CAUTION Do not activate the generator in an open circuit condition. To reduce the risk of
unintended effects, activate the generator only when the active accessory is
near or touching the target tissue.
CAUTION Use the lowest appropriate power setting to achieve the desired effect.
CAUTION This equipment is capable of producing a physiological effect.
CAUTION Read the instructions, cautions, and warnings provided with Gyrus ACMI
PlasmaKinetic System accessories before use. This device is an integral
system; only use Gyrus ACMI approved accessories with the Gyrus ACMI
Superpulse Generator.
SECTION 2 PATIENTS AND OPERATING ROOM SAFETY
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 2-2
CAUTION If possible, avoid the use of needle style instruments for any physiological
monitoring equipment that may be connected to the patient during
electrosurgery.
CAUTION Where practical, only use monitoring equipment that incorporates high
frequency current limiting devices during electrosurgical procedures.
CAUTION The connector cable should be positioned so that it avoids contact with the
patient and any other leads.
CAUTION Studies have shown that electrosurgical smoke generated during electrosurgical
procedures can be potentially harmful to surgical personnel.
CAUTION Examine all accessories and connections to the electrosurgical generator before
use. Improper connection may result in arcs and sparks, accessory malfunction,
or unintended surgical effects.
CAUTION Do not insert fingers or objects other than the correct cables into the socket.
Only activate the footswitch with an instrument attached.
WARNING The PK or SP system has not been cleared for tubal sterilization. Do not use
this system for these procedures.
2B. Servicing/Equipment Disposal
CAUTION Electrical Shock Hazard: Do not tamper with the generator housing or attempt to
remove the control panel. Refer to authorized personnel for service.
NOTE 1. There are no user serviceable parts within the product.
2. For maintenance of the generator, refer to the recommended periodic
equipment safety checks in Section 13.
CAUTION The generator contains electronic printed circuit assemblies. At the end of the
useful life of the equipment, it should be disposed of in accordance with any
applicable policies relating to obsolete electronic equipment.
CAUTION Dispose of any system accessories according to normal institution practice
relating to disposal of biologically contaminated items.
2C. Fire/Explosion
DANGER Explosion Hazard: Do not use in the presence of flammable anesthetics.
WARNING Explosion Hazard: The following substances will contribute to increased fire
and explosion hazards in the operating room:
Flammable substances (such as alcohol based skin prepping
agents and tinctures)
Flammable agents used for cleaning or disinfecting, or as solvents
of adhesives should be allowed to evaporate before the application
of electrosurgery. There is a risk of pooling of flammable solutions
under the patient or in body cavities during endoscopic surgery.
Any fluid pooled in these areas should be mopped up before
electrosurgery is used.
Endogenous gases.
SECTION 2 PATIENT AND OPERATING ROOM SAFETY

SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 2-3
Flammable anesthetics or oxidizing gases such as nitrous oxide
(N
2
O) and oxygen enriched atmospheres.
Some materials, for example cotton, wool and gauze, when
saturated with oxygen may be ignited by sparks produced in normal
use of electrosurgical equipment.
The sparking and heating associated with electrosurgery can provide an ignition source.
Observe fire precautions at all times.
WARNING Fire/Explosion Hazard: Verify that all oxygen circuit connections are leak free
before and during use of electrosurgery. When using electrosurgery in the same
room with any of the above substances or gases, prevent their accumulation or
pooling under surgical drapes, or within the area where electrosurgery is being
performed.
2D. Before Surgery
Active Accessories
WARNING Electric Shock Hazard: Do not connect wet accessories to the generator.
WARNING Electric Shock Hazard: Ensure that all accessories are correctly connected and
that no metal is exposed.
WARNING Do not attempt to re-use instruments labeled for Single Use Only. Heat or
chemical Sterilization may render the instrument mechanically or electrically
unsafe
CAUTION Read the instructions, warnings and cautions provided with the instrument
accessories before using.
CAUTION Accessories labeled as re-usable must only be processed according to the
recommended procedure and, where appropriate, recycled the specified number
of times.
CAUTION Use default power levels to test an accessory.
CAUTION Always inspect the system accessories for damage prior to use. In particular,
check the cables of any re-usable accessory for possible insulation damage.
CAUTION Use only Gyrus ACMI approved accessories supplied for use with this product.
Product damage or accessory failure may otherwise result during use.
SuperPulse Generator
WARNING Electric Shock Hazard; Connect the generator power cord to a properly
grounded receptacle. Do not use power plug adapters.
WARNING Fire Hazard; Do not use extension cords.
CAUTION Provide as much distance as possible between the generator and other
electronic equipment (such as monitors) as an activated generator may cause
interference with them.
CAUTION Non-function of the generator may cause interruption of surgery. Ensure that all
installation procedures are followed and that all connectors are correctly inserted
before use. A backup generator should be available for use.
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 2-4
CAUTION Do not stack equipment on top of the generator or place the generator on top of
electrical equipment.
CAUTION Do not set the activation tone down to an inaudible level. The activation tone
alerts surgical personnel when an accessory is active.
2E. During Surgery
Contact With Metal Objects
WARNING Use extreme caution when using electrosurgery in close proximity to or in direct
contact with any metal objects. Do not activate in contact with another metal
object. Localized heating of the instrument and the adjacent metal object may
result in product damage or inadvertent injury.
WARNING While using electrosurgery during a surgical procedure, the patient should not
be allowed to come into direct contact with grounded metal objects (e.g.,
surgical table frame, instrument table, etc.). If this is not possible, use extreme
caution to maximize patient safety. The use of antistatic sheeting is
recommended for this purpose
SuperPulse Generator Power Settings
WARNING Do not simultaneously activate the SuperPulse Generator whilst activating with
any other electrosurgical equipment (on the same patient). Failure to observe
this may result in the attached instrument being unrecognized by the system.
CAUTION Upon reconnection of an instrument to the SuperPulse Generator, or after
navigation using the Mode / Menu button, the power settings for cutting and
coagulation may be changed from previously selected values.
WARNING Confirm proper power settings are displayed on the generator before proceeding
with surgery. Ensure the appropriate output setting is enabled for the desired
surgical outcome before activating the instrument and ensure that activation is
for the minimum time to achieve the desired surgical effect.
CAUTION Failure of the HF SURGICAL EQUIPMENT could result in an unintended
increase or decrease in output power.
CAUTION Use caution when overriding the default power settings.
CAUTION Should a power supply interruption occur, the generator will revert to its Standby
state. The user should press the standby/on button to restart the generator and
then press the Mode / Menu button on the front panel to accept the default
instrument settings.
Instrument Accessories
WARNING When not in use, place instruments in a clean, dry, non-conductive, and highly
visible area not in contact with the patient. Inadvertent activation while in contact
with the patient may result in burns.
WARNING Do not wrap accessory cords around metal objects. This may induce currents
that could lead to injury to the patient or surgical personnel.
WARNING Fire Hazard: Do not place active accessories near or in contact with flammable
materials (such as gauze or surgical drapes). Electrosurgical accessories, which
are activated or hot from use, can cause a fire.
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 2-5
Endoscopic Procedures
WARNING As visualization may be impaired during endoscopy for a number of reasons, be
particularly alert to these potential hazards:
Ensure the tip of the instrument is visible before activation.
The instrument tip may remain hot enough to cause burns after the
electrosurgical current is deactivated.
Inadvertent activation or movement of activated electrodes outside of the
field of vision may result in injury to the patient.
Localized burns to the patient or physician may result from electrical
currents carried through conductive objects. Electrical current may be
generated in conductive objects by direct contact with the active
instrument, or by the active or return instrument being in close proximity to
the conductive object while activated.
Carefully insert and withdraw instruments from trocars to avoid the
possibility of damage to the devices and/or injury to the patient.
2F. After Surgery
WARNING Electric Shock Hazard. Always unplug the generator before cleaning.
CAUTION Do not reuse or resterilize accessories labeled “disposable” or “single use only.”
2G EMC Classification
The SuperPulse System has been manufactured and tested to the following requirements:
Group 2 Class A as per IEC60601-1-2 (2001)
EMC PRECAUTIONS
Medical electrical equipment needs special precautions regarding EMC and needs to be
installed and put into service according to the EMC information in this document.
EMC WARNINGS
• The generator should not be used adjacent to or stacked with other electrical equipment.
If adjacent or stacked use is necessary both the generator and other equipment should be
observed to verify normal operation in the configuration in which it will be used.
• The EMC classification of the SuperPulse system (class A) is suitable for use on
dedicated supply systems not connected to the public mains network, such as hospitals.
NOTE: Although class A limits have been derived for industrial and commercial
establishments, administrations may allow, with whatever additional measures
necessary, the installation and use of class A ISM equipment in a domestic
establishment or establishment connected directly to domestic electricity power
supplies.
• Portable and mobile RF communications equipment can affect medical electrical
equipment.
• The use of accessories and cables other than those for which the system was designed
can significantly degrade emissions and immunity performance.
SECTION 2 PATIENT AND OPERATING ROOM SAFETY

SECTION 2 PATIENT AND OPERATING ROOM SAFETY
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
• Keep the accessory cables away from cables from other electrical equipment. Electrical
currents may be induced in the other equipment causing unintended effects.
• Do not use a monopolar generator/accessories simultaneously with the SuperPulse
generator. Activation of a monopolar generator/accessories may cause interference with
the SuperPulse generator resulting in user message changes on the display. Before
proceeding with surgery, confirm proper power settings are displayed on the generator.
Ensure the appropriate output setting is enabled for the desired surgical outcome.
• Provide as much separation as possible between the generator and other electronic
equipment (such as monitors). When activating the generator, unintended electromagnetic
coupling may cause interference with the other equipment.
• Should any unintentional effects appear upon other equipment when using the generator,
repositioning the generator, the connecting leads or other equipment may alleviate the
problem. It may also help to use different mains supply sockets for any affected
equipment
Page 2-6
SECTION 2 PATIENT AND OPERATING ROOM SAFETY
SECTION 3 INSTALLATION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 3-1
The electrosurgical generator described in this manual, in conjunction with the Gyrus ACMI PK
Instruments, is designed to provide advanced electrosurgical effects during endoscopic surgery.
3A. Responsibility of the Manufacturer
The manufacturer is responsible for safety, reliability and performance of the equipment
only if:
Installation procedures in this manual are followed.
Assembly operations, extensions, re-adjustments, modifications or repairs are
carried out by persons authorized by the manufacturer and the electrical
installation of the relevant operating room complies with local codes and
regulatory requirements.
The equipment is used in accordance with this User Manual and the Instructions
For Use that accompany all system accessories.
3B. Generator Power Requirements
Please refer to section 10-1 for full voltage details.
Check the Generator Power Connection
The power connector meets all requirements for safe grounding. Its purpose should not be
defeated by using extension cords or any form of adaptor. When disconnecting from the
mains socket or from the generator, cords should always be grasped by the plug. Do not
pull on the cord itself.
3C. Grounding of the Generator
To ensure user safety, the generator must be properly grounded through the inlet plug and
power cord. Use only hospital grade power cords.
IMPORTANT Ensure that the electrical installation of the relevant room complies with
local codes and regulatory requirements.
3D. Routine Maintenance of the Superpulse Generator
It is recommended that the generator be inspected by qualified service personnel in
accordance with Section 13, Periodic Equipment Safety Checks.
3E. Medical Electrical Systems
When the Superpulse generator forms part of a medical electrical system (as defined in EN
60601-1 2.201) or is used with an endoscope that is compliant with EN 60601 2-18 the
following applies:
The Superpulse generator is to be placed outside the patient environment.
The Superpulse generator footswitch accessory is to be placed outside the sterile field.
The instruments intended for use with the Superpulse generator are suitable for use
inside the patient environment.
WARNING Multiple portable socket outlets shall not be placed on the floor.
WARNING Additional multiple portable socket outlets or extension cords shall NOT be
connected to the system.
SECTION 3 INSTALLATION
SECTION 4 GENERAL INFORMATION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
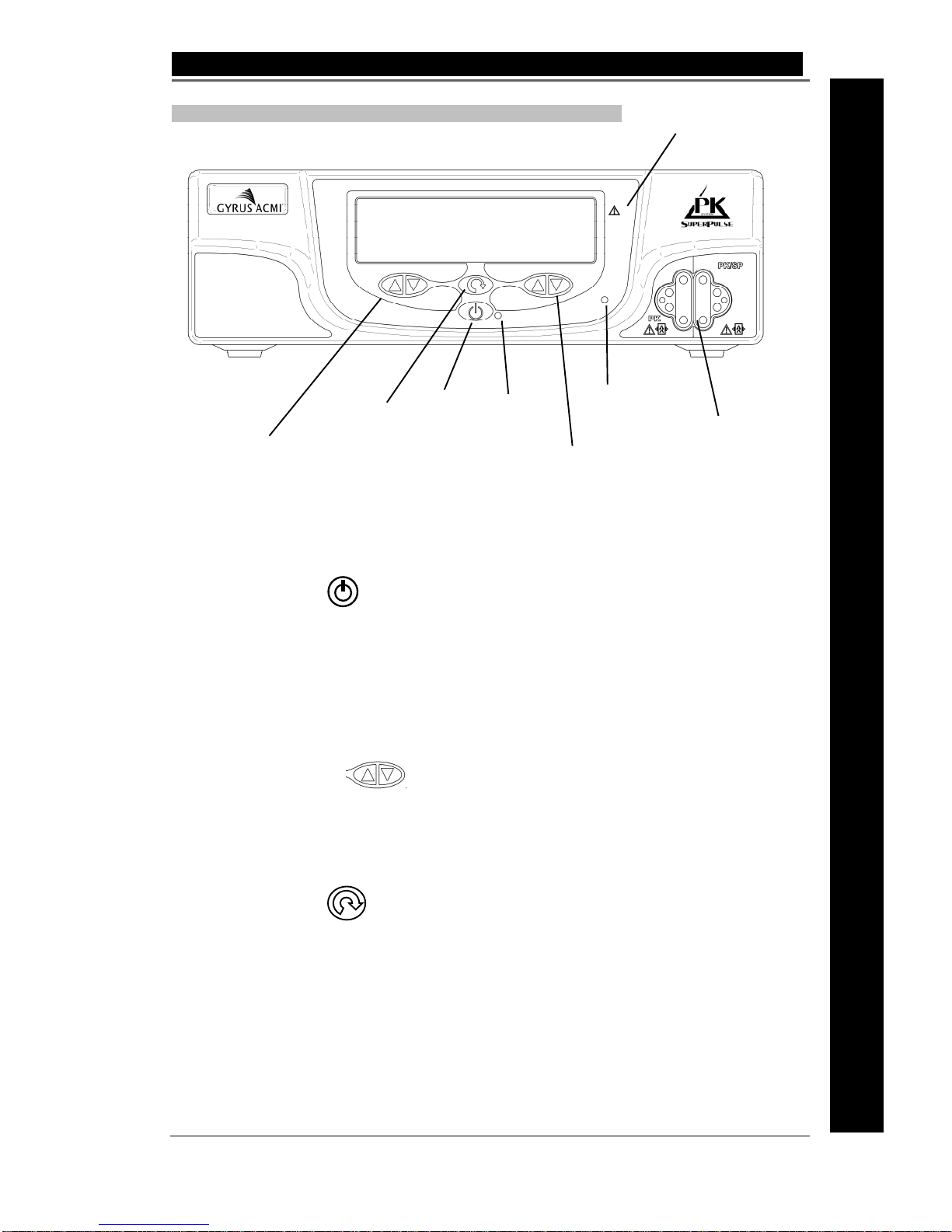
4A. Gyrus ACMI SuperPulse Generator Indicators and Displays
Fig 4.1
Keypad: Standby/On, Up, Down Arrows and Mode / Menu Button
Standby / On
The Standby/On button switches the Generator back and forth between the Standby and
Idle / Ready states. The green indicator will change from flashing to continuous when the
equipment state changes from Standby to Idle / Ready by pressing the button. To place
the generator into Standby press the standby button. When prompted press again to
confirm entry to Standby is required.
Following an error condition the generator may be reset by pressing the Standby/On
button twice.
Up/Down Arrows
Depressing the up or down arrow when parameter change is permitted increases or
decreases the parameter step-wise. Holding the button down will increase or decrease
the value in preset steps
Mode / Menu
This button provides access into the waveform selection and setup menus.
Repeated short presses will give access to the frequently used functions, listed below: -
Cut waveform selection (PK)
Coagulation waveform selection (VP/DES)
Volume
Page 4-1
Standby
/ On
Button
Red warning
lamp
Connector Cable
sockets
PlasmaKinetic and
ThermoKinetic Up/Down
Mode /
Menu
Button
Vapor Pulse and Des
Up/Down
Standby / On
indicator
Active instrument
indicator
Gyrus Medical Ltd
Superpulse System
(c) 2003
Version V2.22
SECTION 4 GENE
R
AL INFORMATION
SECTION 4 GENERAL INFORMATION
Gyrus ACMI PlasmaKinetic SuperPulse Generator
USER MANUAL
Part Number: 144012-MB
Page 4-2
A long press will give access to the setup menu, giving access to the following functions
below with repeated short presses: -
Display intensity
Key click on/off
Select language
Enter PIN Code
NOTE If there is no user activity for a short period, the generator will exit the menu
and return to the previous state.
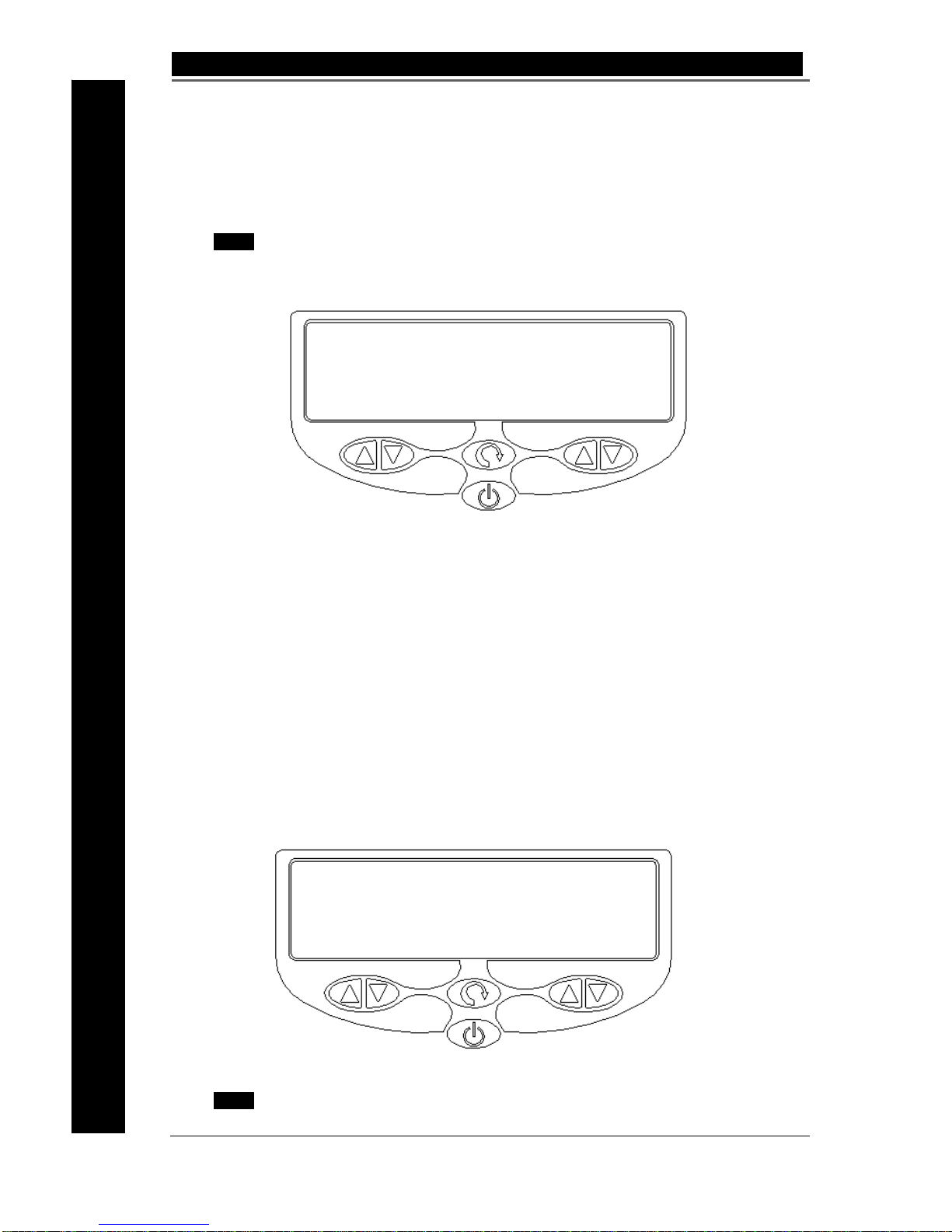
When a PK connector cable is attached, the symbol below appears on the display.
Fig 4.2 Screen for one 3-Way PK cable installed on the selected socket.
Output Displays for PK Instruments
The display is split into two halves; the upper portion of the display is used to indicate the
type of instrument active, that is the instrument that will provide an output when the Cut or
Coag pedal is pressed. The lower half of the display indicates the output waveform type
and power selected.
The left lower portion displays Plasmakinetic (PK) mode selection PK1, PK2, PK3, and
ThermoKinetic mode selection T1 or T2, with the default power setting from 10 to 200
dependent on the type of Gyrus ACMI PK Instrument attached.
The right portion displays Vapor Puls e Coagulation (VPC) mode selection VP1, VP2, VP3 and
standard desiccate (DES), with the default pow er setting fr om 10 to 120 depen dent on the type
of Gyrus ACMI PK instrument attached. The VPC mode is only available with Gyrus ACMI PK
instruments.
The appropriate display will flash and an audible alarm will sound when an output is
activated.
Fig 4.3 Screen for Needle or Hook selected
NOTE The PK output is not available for some PK instruments. The lower left
hand portion of the display remains blank in this case.
3 Way Cable attached
Insert Device
Needle / Hook
PK1 VP1
40 20
SECTION 4 GENERAL INFORMATION
 Loading...
Loading...