GYNECARE THERMACHOICE UBT User manual

SYMBOLS USED ON LABELING
©1994, 1995, 1996, GYNECARE *Trademark
See instructions for Use
CE Mark and identification number
of Notified Body. Product conforms
to the essential requirements of the
Medical Devices Directive 93/42/EEC.
U.S.A. Distribution /Manufactured for:
GYNECARE
a Division of ETHICON, INC.
a Johnson & Johnson company
Somerville, N.J. 08876-0151 USA
Tel: 1-877-ETHICON
Authorized European Representative:
ETHICON GmbH
Robert-Koch-Strasse 1
D-22851 Norderstedt
Germany
Tel: 040/5297-01
EC
Legal Manufacturer
Gynecare
a division of ETHICON, INC.
a Johnson & Johnson company
Somerville, New Jersey 08876-0151
Somerville, New Jersey 08876-0151

GYNECARE THERMACHOICE UBT System
Operating Manual
389614
GB
US
CE0123
*
Somerville, New Jersey 08876-0151


Table of Contents
Page
Device Description ..................................................................................................1
Indications ................................................................................................................1
Contraindications ....................................................................................................6
Warnings ....................................................................................................................6
Precautions ................................................................................................................7
Adverse Events ........................................................................................................8
Other Adverse Effects ..............................................................................................8
Clinical Trial ..............................................................................................................9
Patient Population ..................................................................................................10
Patient Selection......................................................................................................14
Patient Counseling ................................................................................................14
Pretreatment Preparation of Patient....................................................................15
Directions for Use ..................................................................................................15
Set-up ........................................................................................................15
Catheter Priming ......................................................................................19
Pressure Titration ....................................................................................19
Treatment ..................................................................................................21
Post-Treatment..........................................................................................22
Operating Parameters/Alarm and Display Messages ....................................22
Error Messages........................................................................................................23
Warranty ..................................................................................................................24
Servicing/Equipment Disposal............................................................................25
Ordering Information ............................................................................................25
Specifications (Controller & Umbilical Cable) ..................................................25
Environmental Conditions....................................................................................26
Electromagnetic Interference ................................................................................26
Maintenance ............................................................................................................26
Calibration ................................................................................................26
Fuse Replacement ....................................................................................28
Cleaning: Controller System ..................................................................28
Disinfection: Umbilical Cable ................................................................29
Power Cord ..............................................................................................29
GYNECARE THERMACHOICE UBT System Operating Manual

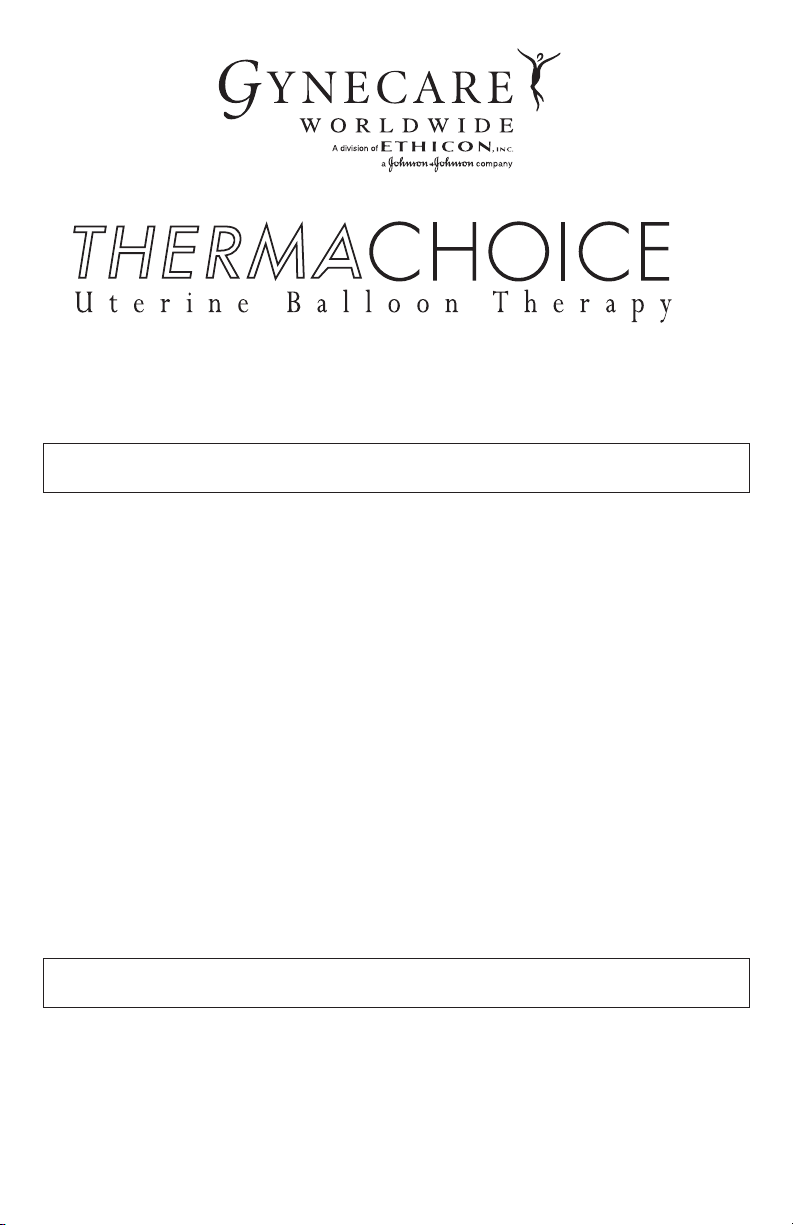
Thermal Balloon Ablation System
Read all directions, precautions and warnings prior to use.
This manual provides directions for using the GYNECARE THERMACHOICE Uterine
Balloon Therapy (UBT) System.
DEVICE DESCRIPTION
The GYNECARE THERMACHOICE UBT System is a software-controlled device
designed to ablate uterine tissue by thermal energy. The system is comprised of a
single-use balloon catheter, a reusable controller, umbilical cable, and power cord. The
GYNECARE THERMACHOICE UBT catheters are designed for use only with
GYNECARE THERMACHOICE UBT controllers.
The balloon catheter is 1) connected to the controller, 2) inserted through the cervix
into the uterus, 3) filled with sterile, injectable fluid (plain 5% dextrose in water- D
5W)
carefully stabilizing the pressure to 160-180mmHg pressure, and 4) activated to
thermally ablate endometrial tissue by maintaining a temperature of approximately
87°C (188°F) for 8 minutes.
The GYNECARE THERMACHOICE UBT controller is designed to work with
3 different versions of the balloon catheter. They are:
a) GYNECARE THERMACHOICE IIIC silicone balloon catheter (version 3.0): This
balloon catheter has a fluid circulation mechanism inside the balloon (See Diagram 1).
b) GYNECARE THERMACHOICE IIC silicone balloon catheter (version 2.0): This
balloon catheter has a fluid circulation mechanism inside the balloon (See Diagram 2).
c) GYNECARE THERMACHOICE balloon catheter (version 1.2): This version does
not have a fluid circulation mechanism inside the balloon (See Diagram 3).
INDICATIONS
The GYNECARE THERMACHOICE UBT System is a thermal ablation device intended
to ablate the endometrial lining of the uterus in women with menorrhagia (excessive
uterine bleeding) due to benign causes for whom childbearing is complete.
GYNECARE THERMACHOICE UBT System Operating Manual Page 1
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician
with appropriate training.
Caution: The GYNECARE THERMACHOICE balloon catheter version 1.2 (only)
contains natural rubber latex which may cause allergic reactions.
Somerville, New Jersey 08876-0151
*
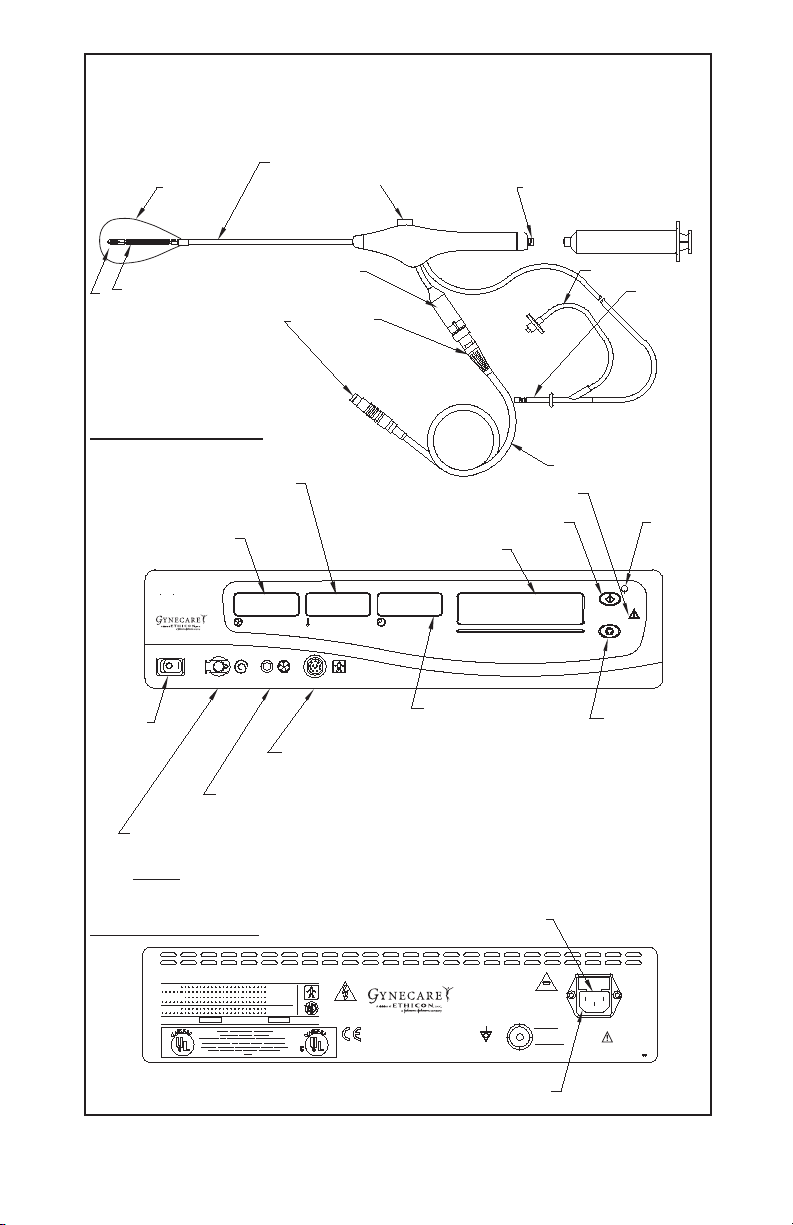
Page 2
Diagram 1
GYNECARE THERMACHOICE IIIC Single Use Silicone Balloon Catheter
with Fluid Circulation (Version 3.0) and Umbilical Cable
Depth, sound measurement (cm)
Silicone
balloon
Fluid fill valve
(Trumpet valve)
Fluid fill port for syringe
Catheter umbilical
Heater
Circulating element
cable connection
Connection
plugs
Front Panel of Controller
Catheter heater
temperature display (°C)
Intra-balloon
pressure display
(mmHg)
CHOICE
THERMA
Uterine Balloon Therapy
PRESSURE (mmHg)
PRESSURE LINE
TEMPERATURE (°C)
UMBILICAL CABLECIRCULATION
Power
switch
Connection port for
reuseable umbilical cable
Connection port for pressure line
(pre-attached to single use balloon catheter)
Connection port for circulation catheter
(pre-attached to single use catheter)
NOTE: Remove the plug before connecting
the circulation catheter.
Rear Panel of Controller
Start button
Message display
TIME (min: sec)
To tal time display
(minutes : seconds)
Pressure line
Circulation
connection
Umbilical cable
Hazard light
Start
button
light
Stop button
Fuse
CAUTION
ATTENTION
CAUTION
MODEL NUMBER
SERIAL NUMBER
CAUTION
0123
Manufactured For:
GYNECARE
a Division of ETHICON, INC.
a Johnson & Johnson Company
Somerville, NJ 08876-0151
1-877-ETHICON
01511 Rev C
Connection port for AC power cord
WARNING: RISK OF
FIRE, REPLACE
FAST BLOW FUSE
AS MARKED.
2X
1.6A@250V FAST BLOW
100-240V~
50/60 Hz
1.1A@120V~
0.55A@240V~
See instruction
for use
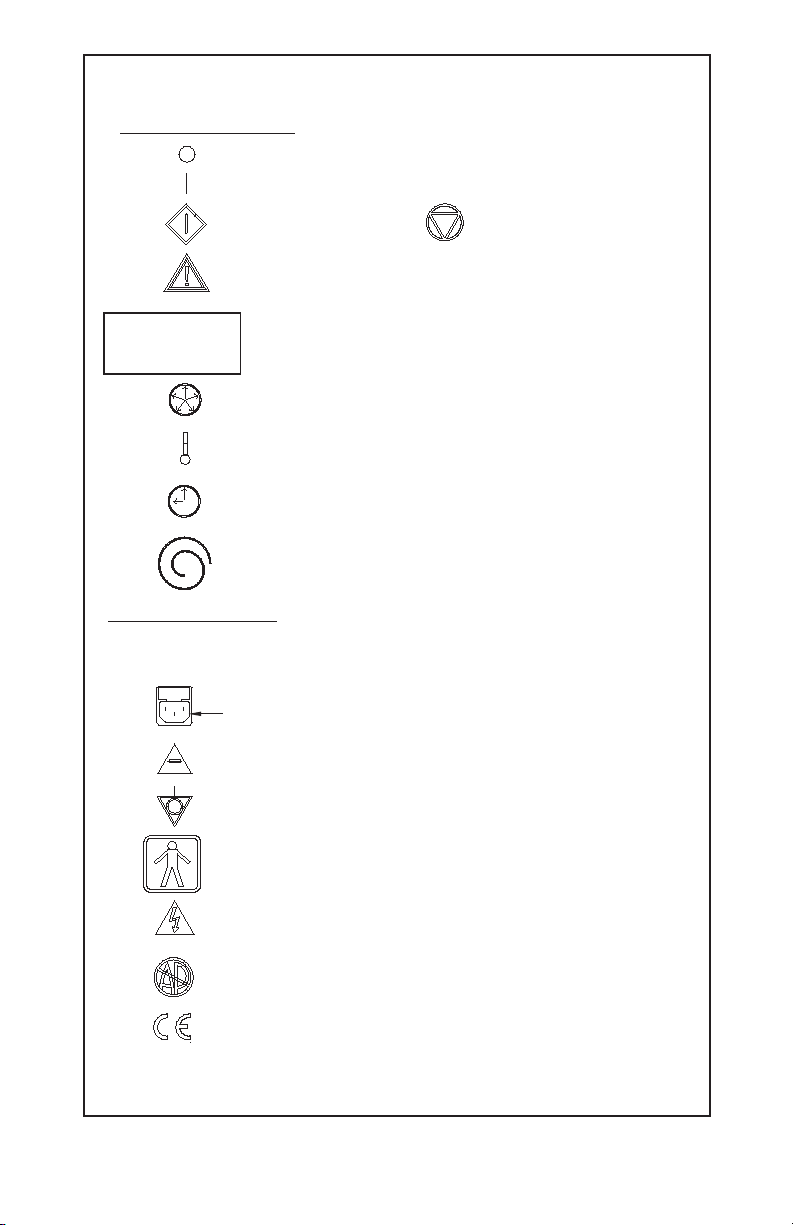
GYNECARE THERMACHOICE UBT System Operating Manual Page 3
DESCRIPTION OF SYMBOLS
Front Panel of Controller
Off (power: disconnection from the mains)
On (power: connection to the mains)
Start Button
When symbol is illuminated, a hazard condition exists which
willautomatically terminate procedure (see ERROR MESSAGES
section)—consult Operator ’s Manual for further instruction. An
unilluminated symbol indicates normal operating conditions.
INSERT CATHETER
FILL CATHETER
WITH D
W
5
Message Display—displays prompts and error messages
Displays pressure inside balloon in mmHg
Displays heater temperature inside balloon in °C
Displays total running time for preheat and therapy
in MINUTES : SECONDS
Circulation catheter connection port
Rear Panel of Controller
(See SPECIFICATIONS for additional information.)
Stop Button
Connection port for power cord
Location of fuses; type and value rating
Equipotentiality
Class I Type BF Equipment
Cover to be removed by qualified service personnel only
CAUTION
Danger: Risk of explosion if used in the presence of flammable
anesthetics!
CE-Mark and identification number of Notified Body.
0123
Product conforms to the essential requirements of the
Medical Devices Directive 93/42/EEC.
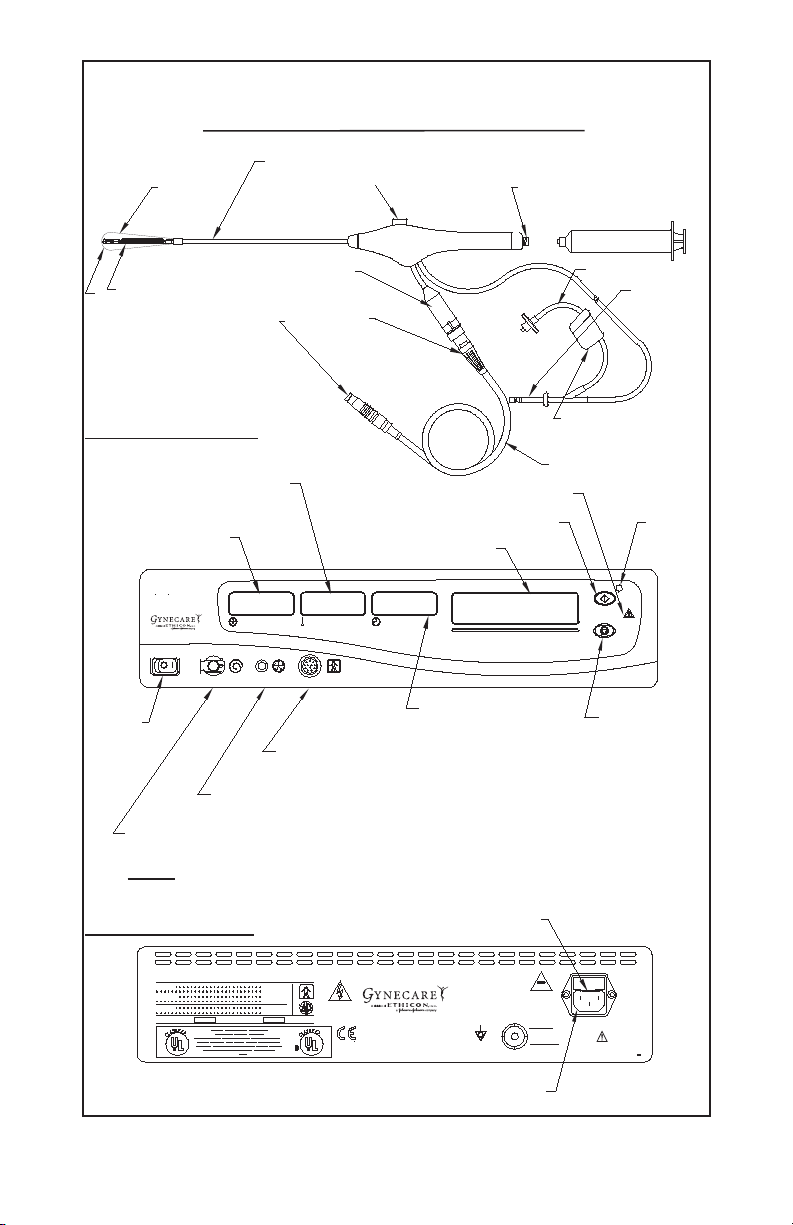
Page 4
Diagram 2
GYNECARE THERMACHOICE IIC Single Use Silicone Balloon Catheter
with Fluid Circulation (Version 2.0) and Umbilical Cable
Depth, sound measurement (cm)
Silicone
balloon
Fluid fill valve
(Trumpet valve)
Fluid fill port for syringe
Catheter umbilical
Heater
Circulating element
cable connection
Connection
plugs
Front Panel of Controller
Catheter heater
temperature display (°C)
Intra-balloon
pressure display
(mmHg)
CHOICE
THERMA
Uterine Balloon Therapy
PRESSURE (mmHg)
PRESSURE LINE
TEMPERATURE (°C)
UMBILICAL CABLECIRCULATION
Power
switch
Connection port for
reuseable umbilical cable
Connection port for pressure line
(pre-attached to single use balloon catheter)
Connection port for circulation catheter
(pre-attached to single use catheter)
NOTE: Remove the plug before connecting
the circulation catheter.
Rear Panel of Controller
Start button
Message display
TIME (min: sec)
To tal time display
(minutes : seconds)
Pressure line
Circulation
connection
Over pressure
relief valve
Umbilical cable
Hazard light
Start
button
light
Stop button
Fuse
CAUTION
ATTENTION
CAUTION
MODEL NUMBER
SERIAL NUMBER
CAUTION
0123
Manufactured For:
GYNECARE
a Division of ETHICON, INC.
a Johnson & Johnson Company
Somerville, NJ 08876-0151
1-877-ETHICON
01511 Rev C
Connection port for AC power cord
WARNING: RISK OF
FIRE, REPLACE
FAST BLOW FUSE
AS MARKED.
2X
1.6A@250V FAST BLOW
100-240V~
50/60 Hz
1.1A@120V~
0.55A@240V~
See instruction
for use
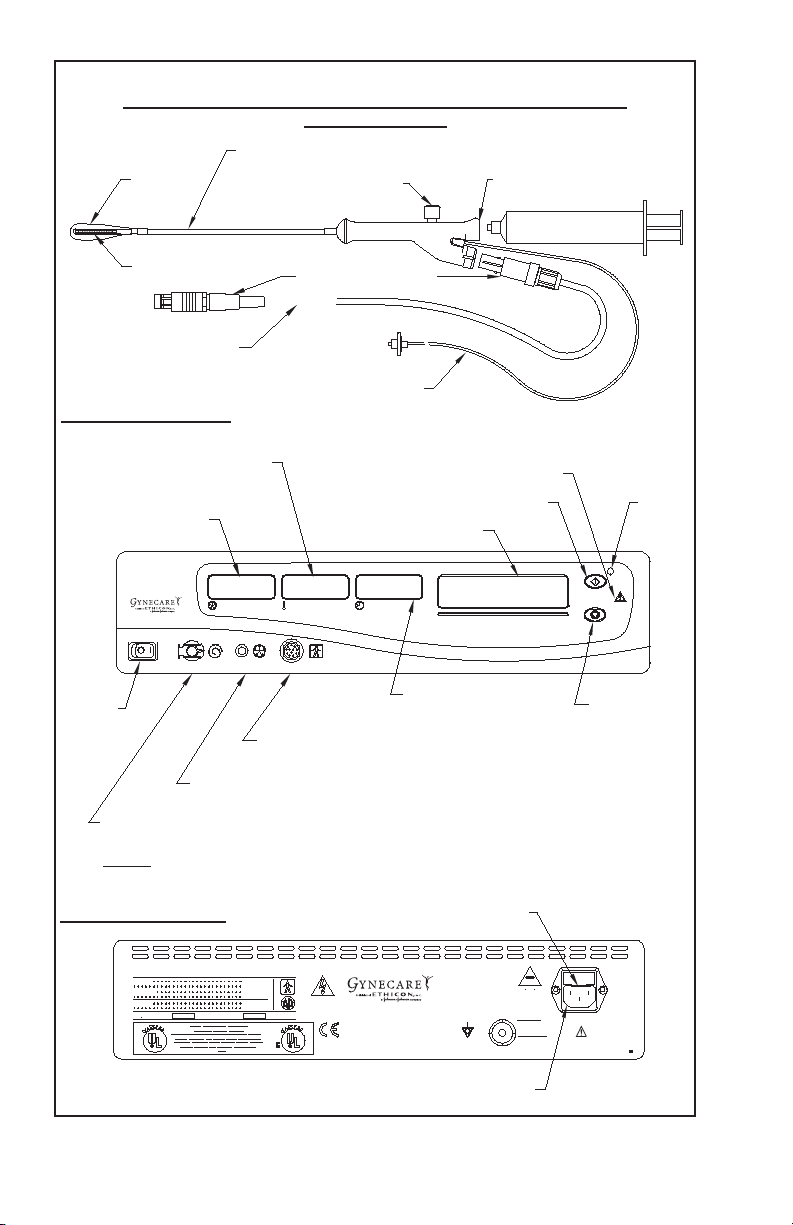
GYNECARE THERMACHOICE UBT System Operating Manual Page 5
Diagram 3
GYNECARE THERMACHOICE Single Use Balloon Catheter (Version 1.2)
and Umbilical Cable
Depth, sound measurement (cm)
Latex
balloon
Fluid fill valve
(Trumpet valve)
Fluid fill port for syringe
(Soft luer)
Heater
Connection plugs
Umbilical cable
Pressure line
Front Panel of Controller
Catheter heater
temperature display (°C)
Intra-balloon
pressure display
(mmHg)
THERMA
CHOICE
Uterine Balloon Therapy
CIRCULATION
PRESSURE (mmHg)
PRESSURE LINE
TEMPERATURE (°C)
UMBILICAL CABLE
Message display
TIME (min: sec)
Power
switch
Connection port for
reusable umbilical cable
Connection port for pressure line
(pre-attached to single use balloon catheter)
Connection port for circulation catheter
(pre-attached to single use catheter)
NOTE: Do not remove the plug. The circulation port is
not used for this version of the catheter.
Hazard light
Start button
To tal time display
(minutes : seconds)
Start
button
light
Stop button
WARNING: RISK OF
FIRE, REPLACE
FAST BLOW FUSE
AS MARKED.
Fuse
2X
1.6A@250V FAST BLOW
100-240V~
50/60 Hz
1.1A@120V~
0.55A@240V~
See instructions
for use
Rear Panel of Controller
CAUTION
ATTENTION
CAUTION
MODEL NUMBER
SERIAL NUMBER
CAUTION
0123
Manufactured For:
GYNECARE
a Division of ETHICON, INC.
a Johnson & Johnson Company
Somerville, NJ 08876-0151
1-877-ETHICON
01511 Rev C
Connection port for AC power cord

Page 6
CONTRAINDICATIONS
The device is contraindicated for use in:
• A patient who is pregnant or who wants to become pregnant in the future.
• A patient with a history of latex allergy or who has demonstrated a sensitivity
to latex material (for catheter version 1.2 only).
• A patient with known or suspected endometrial carcinoma (uterine cancer)
or pre-malignant change of the endometrium such as unresolved adenomatous
hyperplasia.
• A patient with any anatomic or pathologic condition in which weakness of the
myometrium could exist, such as history of previous classical cesarean sections
or transmural myomectomy.
• A patient with active genital or urinary tract infection at the time of procedure
(e.g., cervicitis, vaginitis, endometritis, salpingitis, or cystitis).
• A patient with an intrauterine device (IUD) currently in place.
WARNINGS
Failure to follow all instructions or to heed any warnings or precautions could result
in serious patient injury.
• The device is intended for use only in women who do not desire to bear children
because the likelihood of pregnancy is significantly decreased following this
procedure. There have been reports of women becoming pregnant following this
procedure. Pregnancies after ablation can be dangerous for both mother and fetus.
• Endometrial ablation using the GYNECARE THERMACHOICE UBT System is not a
sterilization procedure.
• Patients who undergo endometrial ablation procedures who have previously
undergone tubal ligation are at increased risk of developing post ablation tubal
sterilization syndrome which can require hysterectomy. This can occur as late as 10
years post-procedure.
• Endometrial ablation procedures using the GYNECARE THERMACHOICE UBT
System should be performed only by medical professionals who have experience in
performing procedures within the uterine cavity, such as IUD insertion or dilation and
curettage (D&C), and who have adequate training and familiarity with the
GYNECARE THERMACHOICE UBT System.
• Endometrial ablation procedures do not eliminate the potential for endometrial
hyperplasia, or adenocarcinoma of the endometrium and may mask the physician’s
ability to detect or make a diagnosis of such pathology.
• The GYNECARE THERMACHOICE III UBT Balloon Catheter is for single use only –
do not reuse or resterilize.
• Do not treat patients for more than one therapy cycle in a given treatment session
because of the potential for transmural injury to the uterus or injury to adjacent
viscera.
• UTERINE PERFORATION
• Uterine perforation can occur during any procedure in which the uterus is
instrumented. Use caution not to perforate the uterine wall when sounding the
uterus, dilating the cervix or inserting the catheter.
• Any of the following indicates possible uterine perforation.

1. If the catheter can be inserted to a greater depth than was determined by the
uterine sound
2. If the pressure cannot be stabilized at 160 – 180 mmHg with a maximum of
30ml of fluid
3. If the pressure drops quickly at any point during the procedure
• If a perforation is suspected, THE PROCEDURE SHOULD BE TERMINATED
IMMEDIATELY. The physician may elect to perform a diagnostic procedure to
confirm perforation. If the physician cannot absolutely rule out perforation, the
procedure should be abandoned.
• For patients in whom the procedure was aborted due to a suspected uterine wall
perforation, a work-up for perforation should be considered prior to discharge.
• If a perforation is present, and the procedure is not terminated, thermal injury to
adjacent tissue may occur if the heater is activated.
•After completing the procedure it is important not to touch the GYNECARE
THERMACHOICE Uterine Balloon for the following reasons:
- The balloon is covered with blood and body fluids
- There are mechanical and electrical parts that could puncture the balloon
• Proper care should be taken in disposing of the catheter.
PRECAUTIONS
• The GYNECARE THERMACHOICE III UBT catheter, controller, and umbilical cable are
designed as a system. To ensure proper function, never use other components with the
GYNECARE THERMACHOICE UBT System.
•Astarting pressure of 160 – 180 mmHg is recommended and typically requires
6 – 15 ml of fluid and may require as much as 30 ml. Titration to achieve a stable
pressure (no fluctuations greater that ±10 mmHg for at least 30 seconds) prior to
activating the heating element is critical to proper functioning of the device. When
inserting fluid, do not exceed a pressure of 200 mmHg. Typically, pressure levels
decline slowly during the course of the procedure as the uterus relaxes. If a pressure of
160 – 180 mmHg cannot be reached with 30 ml or less of fluid, or if there is a rapid
drop in pressure, it is likely there is a uterine perforation.
• Rapid loss of pressure during a therapy cycle may indicate a uterine wall defect.
Adding additional fluid to the balloon may create (or exacerbate if already present) a
uterine wall defect such as a perforation.
• Those patients who have undergone endometrial ablation and are later placed on
hormone replacement therapy should have progestin included in their regimen in
order to avoid the increased risk of endometrial adenocarcinoma associated with
unopposed estrogen replacement therapy.
• Never add additional fluid during a therapy cycle.
• The safety and effectiveness of the GYNECARE THERMACHOICE UBT System has
not been fully evaluated in patients:
- with large uterine cavities (>30 ml in volume or uterine sound >12 cm)
- with small uterine cavities (<2 ml in volume or uterine sound <4 cm)
- with submucosal myomas, bicornuate or septate uteri or previous endometrial
resection/ablation
- undergoing repeat endometrial ablation procedures
GYNECARE THERMACHOICE UBT System Operating Manual Page 7
 Loading...
Loading...