Guidant Corporation HF PARTNER 2936 Operator's Manual
Operator’s Manual
Heart Failure PARTNER™
Model 2936
Heart Failure Diagnostic
Retrieval System

The following symbol is a trademark of Guidant Corporation:
Interrogation symbol

Table of Contents (English)
Product Description 3
Intended Use 4
Contraindications 4
Warnings and Precautions 4
Federal Communications Commission (FCC) 5
Package Contents 5
Contacting Guidant 5
Inspection and Setup 6
How to Interrogate an Implanted Heart Failure Device 6
How to Print Reports 8
Qualified Printers 10
How to Delete Diagnostic Records 10
HF PARTNER Device Functions 11
Language selection 11
Speaker control 12
Table of Contents (English)
1

Voice Messages 12
Reminder Label 15
How to Care for the HF PARTNER Device 16
Cleaning 17
Operating Temperatures 17
Battery Replacement 17
Pairing the HF PARTNER Device with a Compatible Printer 19
Troubleshooting 20
Specifications 23
Explanation of Product Markings 24
Package Content Symbols 25
2
Table of Contents (English)
Product Description
The Heart Failure (HF) PARTNER™ system consists of a Model 2936 HF PARTNER
device, a printer adapter, and a printer. The HF PARTNER device is a handheld,
battery-powered product used to communicate with an implanted device. It provides
easy access to patient and device diagnostic data by interrogating the patient’s
implanted heart failure device and sending the data to a printer through the printer
adapter.
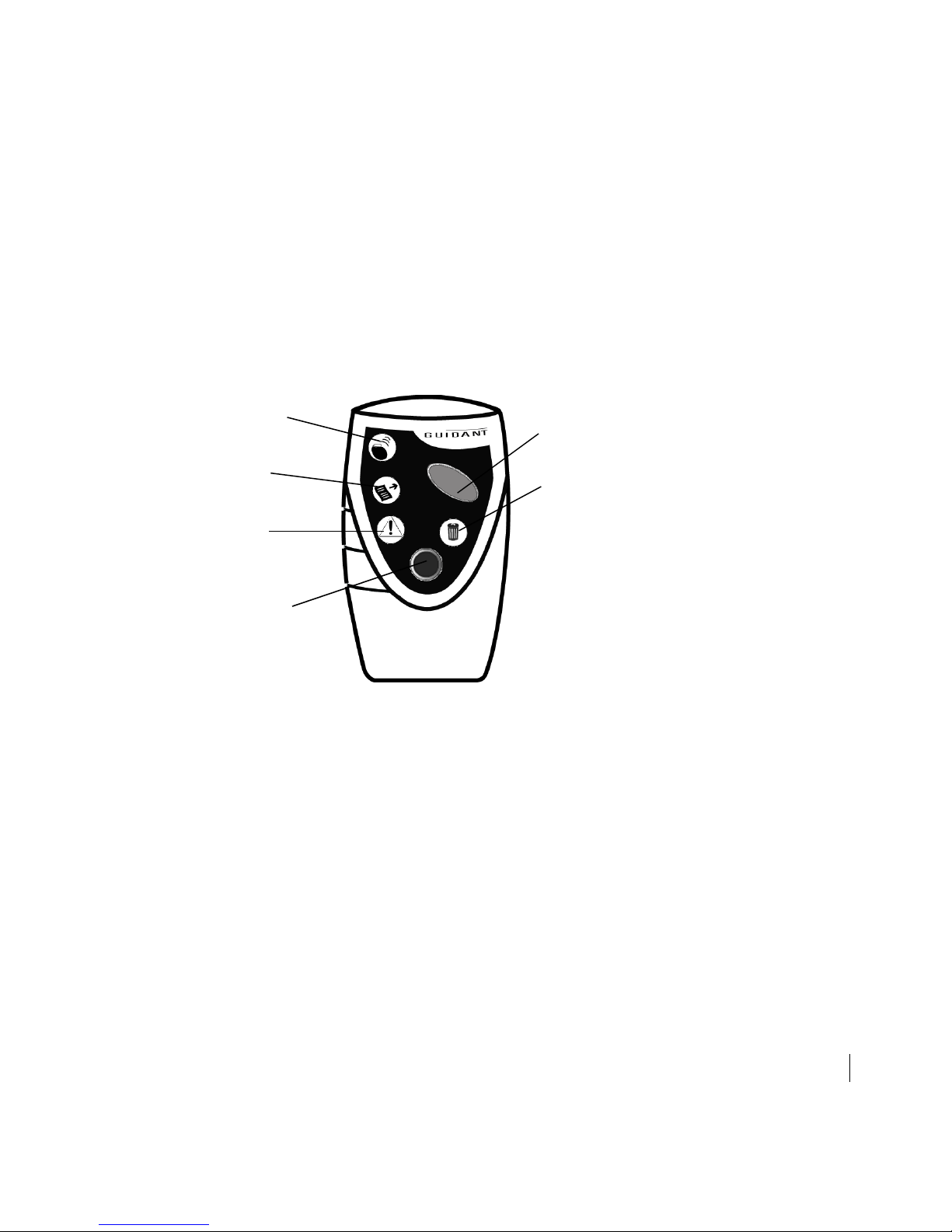
Interrogation symbol
Record Transmission
symbol
Fault Detected
symbol
Press blue PRINT
button to request
reports
Press green INTERROGATE button
to interrogate implanted device
Delete symbol
Figure 1. Model 2936 HF PARTNER device with lighted symbols and buttons
shown.
Product Description
3

Figure 2. Reminder instructions on back of the HF PARTNER device.
Intended Use
The HF PARTNER device is intended for use in a clinical setting. Physicians and
clinicians can use the HF PARTNER device to collect device and patient diagnostic
data from an implanted heart failure device and send the data to a paired printer only.
The HF PARTNER device has no programming abilities, or ability to provide therapy
of any kind.
The HF PARTNER device can be used with any compatible Guidant cardiac
resynchronization therapy (CRT) device except CONTAK CD. To verify compatibility,
please call Guidant Technical Services. See “Contacting Guidant” on page 5.
Contraindications
There are no contraindications for the HF PARTNER device.
Warnings and Precautions
• Use of the HF PARTNER device is for medical professionals only and should be
used within a clinical setting.
• Only a clinician/medical professional can make diagnostic or interpretive
decisions regarding the health of a patient based on the data presented or
acquired by the HF PARTNER device.
4
Intended Use

• Only Guidant-recommended accessories, such as the Bluetooth enabled printer
and Bluetooth accessory should be used with the HF PARTNER device.
• The HF PARTNER device is not intended to sound any physiological alarms.
Federal Communications Commission (FCC)
This device complies with Title 47, Part 15 of the FCC rules. Operation is subject to
the following two conditions:
1. This device may not cause harmful interference, and
2. This device must accept any interference received, including interference that
may cause undesired operation of the device.
CAUTION: Changes or modifications not expressly approved by Guidant could void
the user’s authority to operate the equipment.
Package Contents
The following items are included in the HF PARTNER device package:
• Model 2936 HF PARTNER device with batteries installed
• Operator’s manual
• Warranty
• Blank notebook
• IFU poster
• Guidant brand label
• Declaration letter
Contacting Guidant
Use the following methods to contact Guidant Technical Services:
• US 1.800.CARDIAC (1.800.227.3422)
• European +32 2 416 93 57 or eurtechservice@guidant.com
Federal Communications Commission (FCC)
5
Inspection and Setup
Inspect the HF PARTNER device, printer and printer adapter package contents for
any signs of damage or defects. If any defect or damage is visible, contact your
Guidant sales representative or call Guidant Technical Services. See “Contacting
Guidant” on page 5.
The following steps explain how to setup your HF PARTNER system:
1. Connect the USB cable to the printer adapter and the USB port on the printer.
2. Attach the printer adapter power cord to the printer adapter and plug the printer
adapter power cord into a standard wall outlet.
3. Attach the printer power cord to the printer power input and plug the other end of
the power cord into a standard wall outlet.
4. Press the printer ON button.
Press the green INTERROGATE button to ensure that the HF PARTNER device
is operational; the symbols on the front will light up and a voice message will be
heard.
How to Interrogate an Implanted Heart Failure
Device
Use the following steps to interrogate an implanted heart failure device using the
HF PARTNER device:
1. Press the green INTERROGATE button . The “Interrogation requested” voice
message will be heard, followed by “Locate implanted device”.
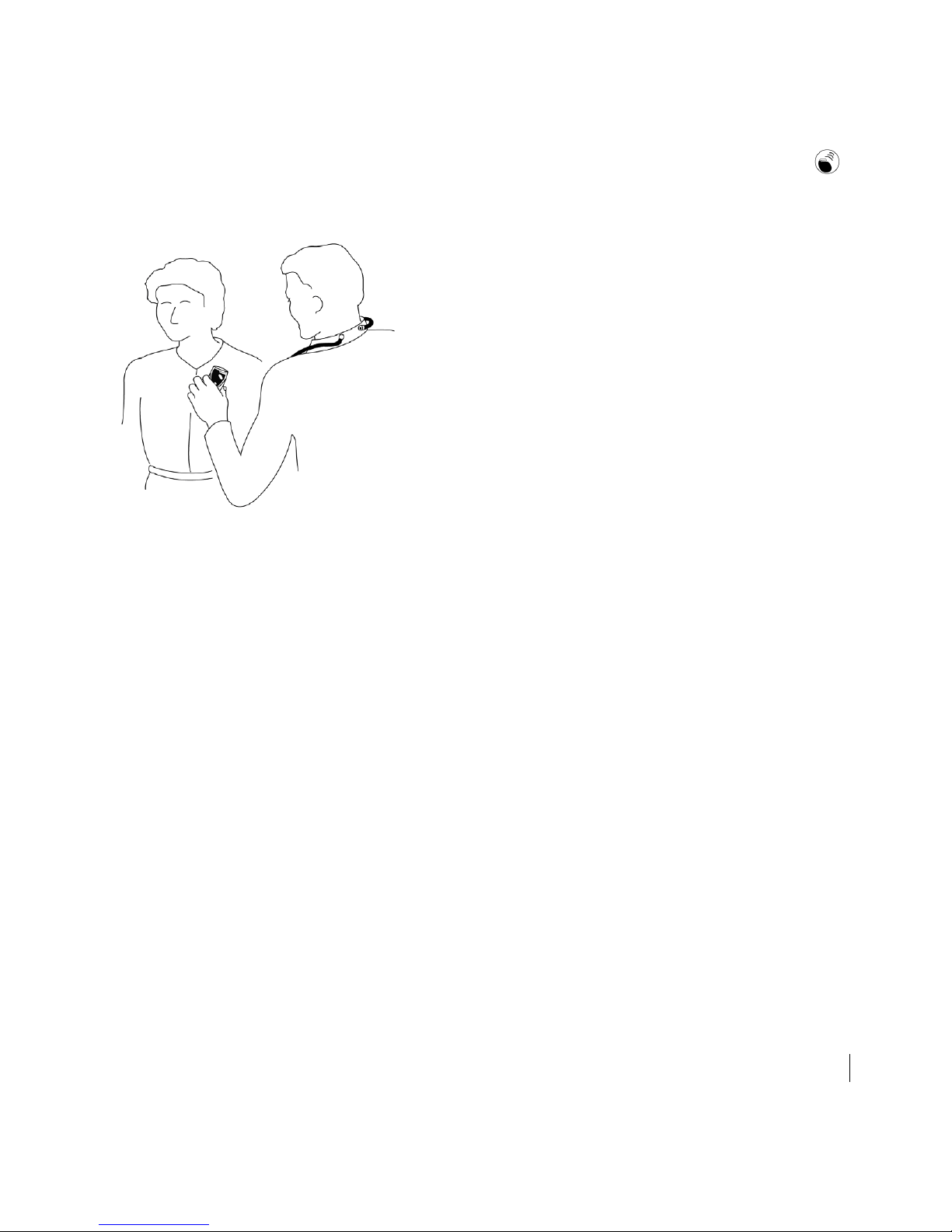
2. Move the HF PARTNER device over the patient’s implanted device (Figure 3).
The visual indicators on the front of the HF PARTNER device will light in
consecutive sequence until the communication with the implanted device is
established. During interrogation, the Interrogation light will blink while data
is transmitting. At the end of a successful interrogation, the HF PARTNER device
6
Inspection and Setup
will emit the “Interrogation complete” message. The Interrogation light will
stay on for five seconds after successful interrogation.
Figure 3. Hold the HF PARTNER device over the patient’s implanted device, with
buttons and lights toward you.
NOTES:
• You have about 10 seconds from the time you press a button until you locate the
implanted device. If the HF PARTNER device is unable to locate the device, the
voice command “Locate implanted device” will be repeated. Position the top half
of the HF PARTNER device over the implanted device and move the
HF PARTNER device slowly. If the interrogation is unsuccessful, hold the
HF PARTNER device over the patient's implanted devices with the button and
lights AWAY from you.
• If the interrogation was unsuccessful, the HF PARTNER device will notify you by
communicating “Interrogation unsuccessful, please interrogate again”. Repeat
the interrogation process until you receive the “Interrogation complete” message.
• If you hear the “Locate implanted device” message (NOT followed by the
“Interrogation unsuccessful, please interrogate again” message), you have
requested the interrogation of an incompatible device. Ensure that you have a
compatible Guidant device.
• If communication is lost during interrogation, the visual indicators on the front of
the HF PARTNER device will light in consecutive sequence. Reposition the
How to Interrogate an Implanted Heart Failure Device
7
 Loading...
Loading...