Page 1
OXYCHECK
FINGERTIP
PULSE OXIMETER
MODEL JB02017
OPERATOR MANUAL
GENERAL DESCRIPTION
The John Bunn® JB02017 OxyCheck Fingertip Pulse Oximeter
provides a simple way to spot-check users by combining the
sensor and monitor into one integrated, compact, easy to use
device. The oximeter measures pulse oxygen saturation (SpO2 )
value, pulse rate value, and pulse strength. When a nger is
inserted into the sensor’s rubber cushion, the SpO2 value automatically displays. The pulse bar graph displays the user’s pulse
beat, and the bar graph’s height shows pulse strength. The
oximeter, which is powered by two AAA batteries, features a lowbattery indicator and powers off automatically in eight seconds
when not in use.
Product Accessories (Included)
1. One lanyard
2. Two AAA batteries
3. One protective cover
4. One operator manual
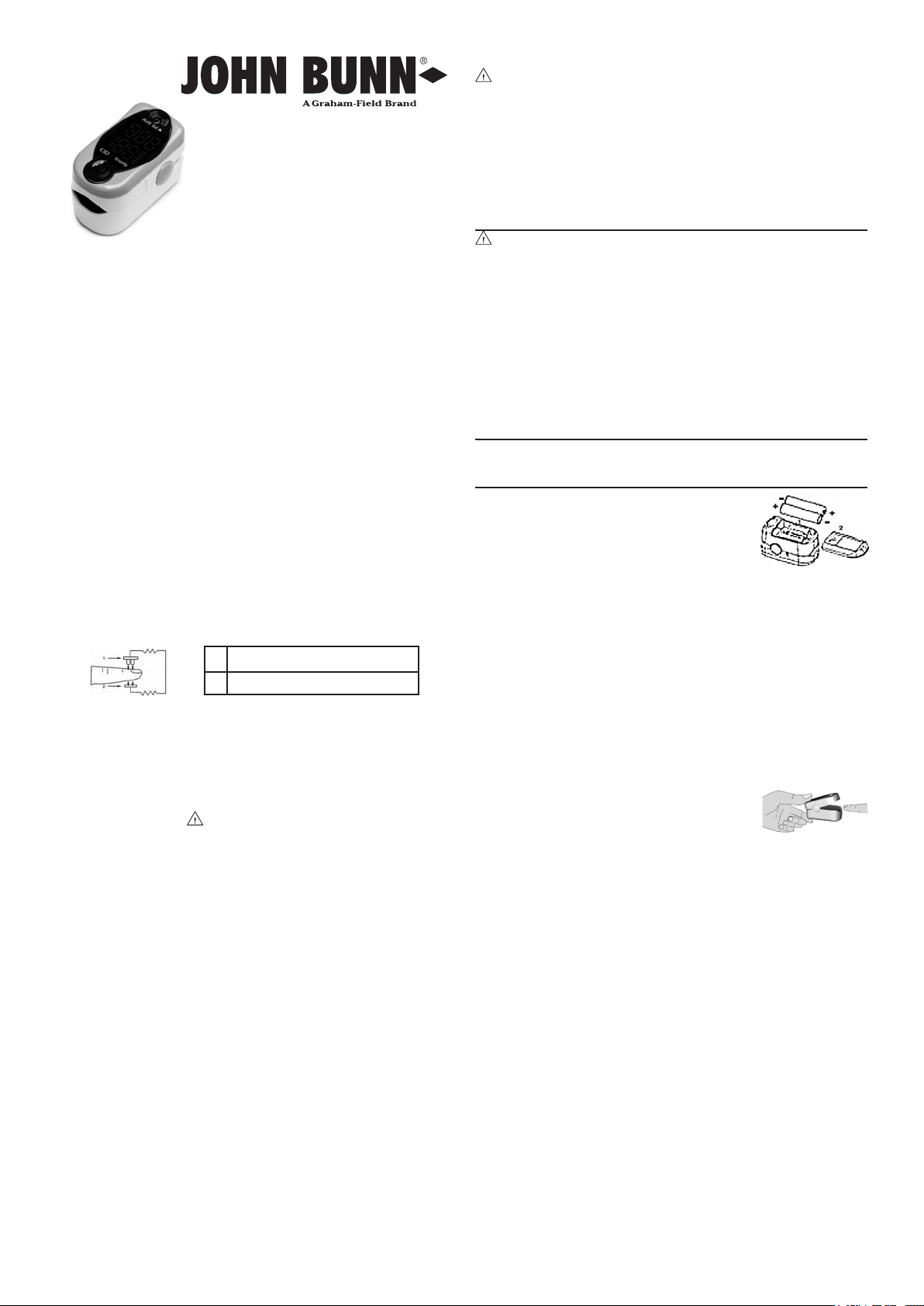
Principle of Measurement
Two beams of different wavelength (660 nm glow and 940 nm
near infrared light) are focused onto a human nail tip through a
clamping nger-type sensor. A measured signal obtained by a
photosensitive element, through processes of electronic circuits
and microprocessor, will be shown on the oximeter’s display.
Principle of Operation Diagram
See illustration and descriptions below.
1 Red and Infrared Emission Tube
2 Red and Infrared Receipt Tube
INTENDED USE
The intended use of the OxyCheck Fingertip Pulse Oximeter is
the measurement and display of the functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate (PR) of adults
and pediatric users in hospital, ambulatory, home, and EMS
(Emergency Medical Service) environments. The Pulse Oximeter is intended for spot-checking these levels.
Contraindications (
• If you do not understand any part of these instructions,
contact a healthcare professional for direction in the use
of this product.
• This device is not intended for continuous monitoring.
• Do not use this device in an explosive atmosphere.
• Do not use this device in an MRI or CT environment.
INACCURATE MEASUREMENTS MAY BE CAUSED BY THE FOLLOWING:
• Autoclaving, ethylene oxide sterilizing, or immersing the
device in liquid
• Signicant levels of dysfunctional hemoglobins (such as
carbonxy- hemoglobin or methemoglobin)
• Intravascular dyes such as indocyanine green or methylene blue
• High ambient light — shield the sensor area (with a surgical towel, or direct sunlight, for example) if necessary
• Excessive user movement
• High-frequency electrosurgical interference
• Venous pulsations
• Placement of a sensor on an extremity with a blood pressure cuff, arterial catheter, or intravascular line
• User hypotension, severe vasoconstriction, severe anemia, or hypothermia
• User cardiac arrest or shock
•
Improper nger placement, e.g. ngernail not facing upward
• Fingernail polish or false ngernails.
WARNINGS):
SAFETY — PRECAUTIONS FOR USE
WARNING: Indicates a potential hazard situation or
unsafe practice that, if not avoided, could result in death
or serious injury. WARNING statements follow:
Before use, carefully read the manual.
The pulse oximeter has no alarms. Do not use the pulse
oximeter in situations where alarms are required. It is not
for continuous monitoring.
The pulse oximeter is intended only as an adjunct in user
assessment. It must be used in conjunction with other
methods of assessing clinical signs and symptoms.
CAUTION: Indicates a potential hazard or unsafe prac-
tice that, if not avoided, could result in moderate or
minor personal injury. CAUTION statements follow:
Check the pulse oximeter sensor application site frequent-
ly to determine the positioning of the sensor and circulation and skin sensitivity of the user.
Do not stretch the adhesive tape while applying the pulse
oximeter sensor. This may cause inaccurate readings or
skin blisters.
Prolonged use or the user’s condition may require changing the sensor site periodically. Change sensor site and
check skin integrity, circulatory status, and correct alignment at least every 4 hours.
s NOTICE: Indicates a potential hazard or unsafe practice
that, if not avoided, could result in product/property
damage.
SETUP
Battery Installation
1. Open battery compartment cover.
2.
Install two AAA batteries in battery compart-
ment, ensuring that polarities are correct.
3. Close battery compartment cover: Push cover horizontally
along the arrow as shown at right.
s NOTICE:
• Ensure battery polarities are correct, or the device could
be damaged.
Lanyard Installation
1.
Thread the thinner end of the lanyard through the oximeter
loop.
2. Thread the thicker end of the lanyard through the threaded
end, then pull it tightly.
OPERATION INSTRUCTIONS
1. Use isopropyl alcohol to clean the test nger and the rubber
inside the oximeter that touches the nger.
2. Place clamp over ngernail as shown
at right; insert nger, ngernail up as
shown.
3. Press button on front panel once. User’s nger and body
must remain still during measurement.
4. See display: the SpO2 value automatically displays, the
pulse bar graph displays the pulse rate, and the bar graph’s
height shows the pulse strength.
MAINTENANCE AND STORAGE
s NOTICE:
• This device contains no serviceable parts. Do not disas-
semble.
• Remove the batteries if the oximeter will not be used for a
long period of time.
• Do not autoclave, sterilize with ethylene oxide, or im-
merse the device in liquid.
• See SPECIFICATIONS/Environmental Requirements for
operation and storage requirements. A wet ambience
could damage this product and shorten its lifetime.
• Recycle or dispose of this device and its used batteries in
observance of local regulations.
Info: Use isopropyl alcohol to clean the rubber (inside the
oximeter, that touches the nger) and the test nger before
and after each test. The rubber inside the oximeter is medical rubber, which has no toxins, and is not harmful to the
skin.
•
Replace the batteries when low battery indicator illuminates.
1
Page 2
SPECIFICATIONS
Display Type LED (Light Emitting Diode)
SpO
2
Measurement
range
70-99%
Accuracy 80%-99%: ±2%
70%-79%: ±3%
≤69% : no denition
Pulse Rate Measurement
30-235 BPM
range
Accuracy 30~99 BPM: ±2 BPM
100~235 BPM: ±2%
Pulse Intensity Bargraph Indicator
Power Requirement Two AAA alkaline Batteries
Power Consumption <40 mA
Low Power Indicator
Battery Life ~ 30 hours of continuous operation
Dimension
(L x W x H)
2.20″ ~ 2.44″ x 1.26″ ~ 1.50″ x 1.34″ ~ 1.50″
(56 mm ~ 62 mm x 32 mm ~ 38 mm x 34 mm ~ 38 mm)
Weight 1.59 oz ~ 2.12 oz (0.10 lb ~ 0.13 lb) (45 g ~ 60 g)
including two AAA batteries
Environmental
Requirements
Temperature Operation 41°F ~ 104°F
(5°C ~ 40°C)
Storage 68°F ~ 131°F
(20°C ~ 55°C)
Interference
Resistance Capacity
Humidity
(non-condensing)
Device works normally when mixed noise produced
by BIO-TEK INDEX Pulse Oximeter tester
Operation ≤80% RH
Storage ≤93% RH
against Ambient Light
DECLARATION
This product’s EMC complies with IEC60601-1-2 standard.
The materials with which the user can come into contact have
no toxicity, no action on tissues, and comply with ISO10993-1,
ISO10993-5 and ISO10993-10.
GUIDANCE AND MANUFACTURER’S DECLARATION:
ELECTROMAGNETIC EMISSIONS FOR ALL EQUIPMENT
AND SYSTEMS
Guidance and manufacturer’s declaration – electromagnetic emission
The Pulse Oximeter is intended for use in the electromagnetic
environment specied below. The customer or the user of the Pulse
Oximeter should assure that it is used in such an environment.
Emission Test
RF emission
CISPR 11
Compliance
Group 1
Electromagnetic environment – guidance
The Pulse Oximeter uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emission
CISPR 11
Class B
The Pulse Oximeter is suitable for use
in all establishments, including domestic
establishments and those directly connected to
the public low-voltage power supply network that
supplies buildings used for domestic purposes.
TROUBLESHOOTING
Problem Possible reason Solution
SpO2 or PR
cannot be
displayed
normally
SpO2 or PR
display is
unstable
1. User's nger is incorrectly
inserted
2. User’s Oxyhemoglobin
value is too low to be
measured
1. Finger may not be
inserted deeply enough
2. Finger trembling or user
1. Reinsert user's nger
2.
Attempt several times to
obtain a reading; If sure that
no problem exists, obtain
further clinical examination
1. Reinsert nger
2. Ask user to remain still
moving
Troubleshooting continued
Problem Possible reason Solution
Oximeter
cannot be
powered on
1. Battery power may be
inadequate or batteries
may not be installed
2. Batteries may be installed
1. Replace batteries
2. Reinstall batteries
3. Contact GF distributor
incorrectly
3.
Oximeter may be damaged
Indicator
lamps are
suddenly
off
1. Device automatically
powers off when no signal
is detected for longer than
8 seconds
1. Normal
2. Replace the batteries
2. Batteries too weak to
power device
“Error3” or
“Error4”
displays
1. Low power
2. Receiving tube and/or
connector may be
shielded or damaged
3. Mechanical misplace for
1. Replace batteries
2. Contact GF distributor
3. Contact GF distributor
4. Contact GF distributor
receive-emission tube
4. Amp circuit malfunction
“Error7”
displays
1. Low power
2. Emission diode damaged
3. Current control circuit
1. Replace batteries
2. Contact GF distributor
3. Contact GF distributor
malfunction
SYMBOL DEFINITIONS
Symbol Denition Symbol Denition
﹪ SpO
❤
BPM
Type BF applied part
Attention, consult
accompanying documents
Oxygen saturation
2
Heart rate (BPM)
SN
Low power indicator
No SpO2 Alarm
Power switch
Serial Number
Info: The illustration used in this manual may differ slightly
from the appearance of the actual product.
LIMITED WARRANTY
GF Health Products, Inc. warrants the John Bunn® JB0217 OxyCheck Fingertip Pulse Oximeter to be free from defects in workmanship and materials for one year.
The warranty does not apply to damage resulting from failure to
follow the operating instructions, accidents, abuse, alterations or
disassembly by unauthorized individuals.
If a product is deemed to be under warranty, GF Health Products,
Inc. shall provide, at its option, (1) replacement of any defective
part or product or (2) a credit of the original selling price made to
GF Health Products, Inc.’s initial customer. The warranty does not
include any labor charges incurred in replacement part(s) installation or any associated freight or shipping charges to GF Health
Products, Inc.
The warranties contained herein contain all the representations
and warranties with respect to the subject matter of this document,
and supersede all prior negotiations, agreements and understandings with respect thereto. The recipient of this document hereby
acknowledges and represents that it has not relied on any representation, assertion, guarantee, warranty, collateral contract or
other assurance, except those set out in this document.
Graham-Field and John Bunn are registered trademarks of GF Health Products, Inc.
Packaging, warranties, products, and specications are subject to change without notice.
GF Health Products, Inc. is not responsible for typographical errors.
© 2010 GF Health Products, Inc., Atlanta GA 30360, 770-368-4700 JB02017-INS-LAB-RevA10
2
www.grahamfield.com
 Loading...
Loading...