Page 1

Electrical Neuromuscular
Stimulation
(EMS), Digital
Model GF-TX5EMS
Operation Manual
Read this manual before operating your GF-TX5EMS.
Save this manual for future use.
The most current version of this manual can be found online at
www.grahamfield.com
GF-TX5EMS-INS-LAB-RevC11
Page 2

CONTENTS
GENERAL DESCRIPTION ................................................................................................... 3
WHAT IS EMS?
INDICATIONS AND CONTRAINDICATIONS
SAFETY
ABOUT THE DEVICE.......................................................................................................... 7
EXPLANATION OF KEY / KNOB CONTROL FUNCTIONS
ATTACHING THE LEAD WIRES
ELECTRODE SELECTION AND CARE
TIPS FOR SKIN CARE
CONNECTING THE DEVICE
BATTERY INFORMATION
CARING FOR YOUR DEVICE
TROUBLESHOOTING
TECHNICAL SPECIFICATIONS
LIMITED WARRANTY
MANUAL DE OPERACIÓN EN ESPAÑOL ............................................................................ 17
................................................................................................................ 3
........................................................................... 3
........................................................................................................................... 4
........................................................ 8
........................................................................................... 9
................................................................................... 9
.......................................................................................................9
.............................................................................................. 10
................................................................................................. 11
.............................................................................................12
....................................................................................................... 12
.......................................................................................... 13
...................................................................................................... 15
GF Health Products, Inc. (“Graham-Field”) is not responsible for typographical errors. Packaging, warranties, products and specifications are subject to
change without notice.
Graham-Field and Grafco are registered trademarks of GF Health Products, Inc.
2
Page 3

GENERAL DESCRIPTION
EMS, Electrical Neuromuscular Stimulation (Electrical Myostimulation), is a
battery-operated pulse generator that sends electrical impulses to electrodes
attached to the body to stimulate motor nerves and cause contraction and
relaxation of muscles. It has proven valuable as a method of pain therapy
and is of great assistance to the experienced therapist.
With some indications, physicians / clinicians can prescribe EMS units to
patients for their use at home.
This unit is a dual-channel digital stimulator for active treatment application,
which has a Liquid Crystal Display indicating operation modes and output, as
well as an 8-bit microcomputer for controlling the system.
The electronics of the unit create electric impulses; the intensity, duration,
frequency per second and modulation of these impulses can be adjusted.
WHAT IS EMS?
An injured muscle usually experiences little if any movement. EMS therapy
remedies this by using low voltages to stimulate motor nerves to cause involuntary muscular contractions.
Like exercise, EMS helps strengthen the injured area, and has been found to
effectively treat a variety of musculoskeletal and vascular conditions. Common candidates for EMS therapy are patients recovering from orthopedic
surgery, muscle strains or tears, or athletes who have undergone cartilage or
tendon repair. EMS is non-invasive and does not use pharmaceuticals.
INDICATIONS AND CONTRAINDICATIONS
Read the operation manual before using this EMS device.
Federal law (USA) restricts this device to sale by or on the order of a physi-
cian.
Observe your physician’s / clinician’s precise instructions and allow them to
show you where to apply the electrodes. For a successful therapy, the correct
application of the electrodes is an important factor. Carefully write down the
settings your physician / clinician recommends.
3
Page 4

Indications
• Relaxation of muscle spasm
• Prevention or retardation of disuse atrophy
• Increasing local blood circulation
• Muscle re-education
• Immediate postsurgical stimulation of calf muscles to prevent venous
thrombosis
• Maintaining or increasing range of motion
Powered muscle stimulators should only be used under medical supervision
for adjunctive therapy for the treatment of medical diseases and conditions.
Contraindications
WARNING: This device should not be used by patients with cardiac demand
pacemakers.
SAFETY
Always follow basic safety precautions, including the following:
WARNING: Indicates a potential hazard situation or unsafe practice that, if not
avoided, could result in death or serious personal injury.
CAUTION: Indicates a potential hazard or unsafe practice that, if not avoided,
could result in minor or moderate personal injury.
s NOTICE: Indicates a potential hazard or unsafe practice that, if not avoided,
could result in product / property damage.
Warnings
WARNING: This device does not have AAP/APG protection.
Explosion hazard is possible if used in the presence of explosives, flammable
materials or flammable anesthetics.
WARNING: The long-term effects of chronic electrical stimulation are un-
known.
4
Page 5

WARNING: Do not place electrodes over the carotid sinus nerves, particularly
in patients with a known sensitivity to the carotid sinus reflex.
WARNING: Do not place electrodes over the neck or mouth. This may result in
spasms of the laryngeal and pharyngeal muscles, and the contraction may be
strong enough to close the airway or cause difficulty in breathing.
WARNING: Do not apply stimulation transthoracically, because the introduction
of electrical current into the heart may cause cardiac arrhythmia.
WARNING: Do not apply stimulation transcerebrally.
WARNING: Do not apply stimulation over swollen, infected or inflamed areas or
skin eruptions; e.g. phlebitis, thrombophlebitis, varicose veins, etc.
WARNING: Do not apply stimulation over or in proximity to cancerous lesions.
WARNING: The safety of the device during pregnancy or delivery has not been
established.
WARNING: Caution should be used for patients with suspected or diagnosed
heart problems.
WARNING: Caution should be used when applying this device to patients sus-
pected of having heart disease. Further clinical data is needed to show if there
are adverse side effects on individuals with heart disease.
WARNING: Caution should be used for patients with suspected or diagnosed
epilepsy.
WARNING: Caution should be used in the presence of the following:
When there is a tendency to hemorrhage following acute trauma or fracture;
Following recent surgical procedures when muscle contraction may disrupt the
healing process;
Over the menstruating or pregnant uterus; and
Over areas of the skin which lack normal sensation.
5
Page 6

WARNING: Electrode placement and stimulation settings should be based on
the guidance of the prescribing practitioner.
WARNING: Keep this device out of the reach of children.
WARNING: Use this device only with the leads and electrodes recommended
for use by GF Health Products, Inc.
WARNING: Do not use this device while driving, operating machinery, or dur-
ing any activity in which involuntary muscle contractions may put the user at
undue risk of injury.
WARNING: EMS devices should be used only under the continued supervision
of a physician / clinician.
Precautions / Adverse Reactions
CAUTION: Some patients may experience skin irritation or hypersensitivity due
to the electrical stimulation or electrical conductive medium. Using an alter-
nate conductive medium or alternate electrode placement can usually reduce
the irritation. Consult your physician / clinician before using an alternative
conductive medium or electrode placement.
CAUTION: Isolated cases of skin irritation may occur at the site of electrode
placement following long-term application.
CAUTION: If skin irritation occurs EMS treatment should be stopped and elec-
trodes removed until the cause of the irritation can be determined.
CAUTION: Effectiveness is highly dependent upon patient selection of a doctor
qualified in the management of pain patients.
CAUTION: If the device treatment becomes ineffective or unpleasant, stimula-
tion should be discontinued until reevaluation by a physician / clinician.
CAUTION: Always turn the device OFF before applying or removing electrodes.
6
Page 7
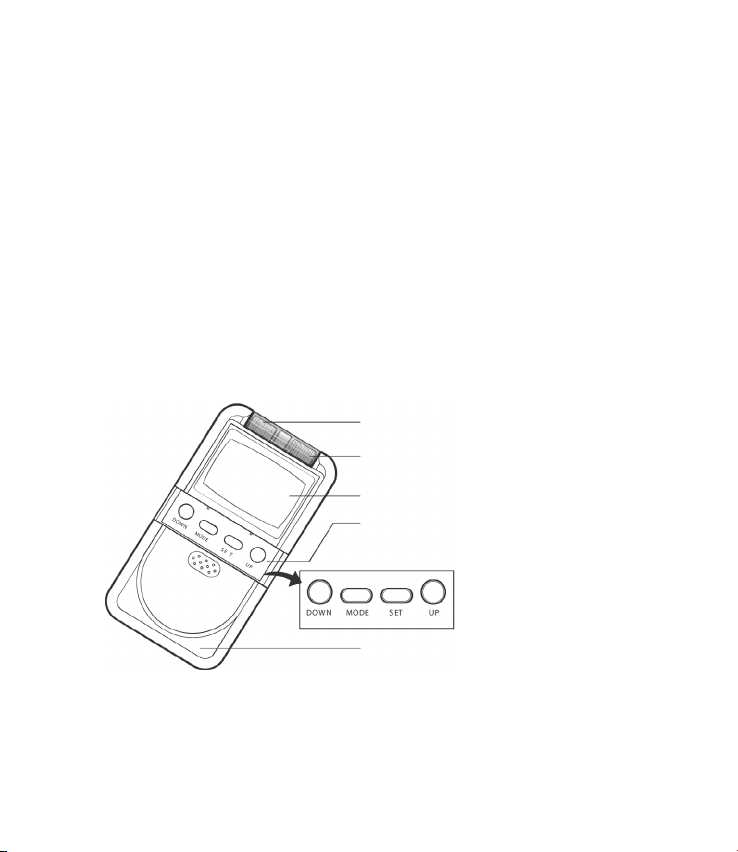
ABOUT THE DEVICE
This device is a battery-operated device that includes two controllable output
channels. This device creates electrical impulses whose intensity, duration,
and modulation can be altered. The device controls are easy to use and the
slide cover protects accidental changes in settings.
System Components
Your device will include the following components or accessories:
• EMS Unit
• Carrying case
• Lead wires
• 9-Volt battery
• Operation Manual
• Electrodes
Device Controls
Ch1 ON/OFF and Intensity Control Knob
Ch2 ON/OFF and Intensity Control Knob
LCD Panel
Key Control (Push Buttons)
Slide Cover
Slide Cover
This cover located on the front of the unit conceals the controls for DOWN,
MODE, SET, and UP. Press the top portion of the cover and pull down in order
to open the cover.
7
Page 8

EXPLANATION OF KEY / KNOB CONTROL FUNCTIONS
DOWN Key This key decreases the setting
MODE Key
SET Key
UP Key This key increases the setting
Ch1 / Ch2
Knobs
of treatment parameters. *
*Decrease by pressing the DOWN key: The width can be adjusted by 10μ/step.
The rate can be adjusted in 1Hz~20Hz by 1Hz/step, 20Hz~150Hz by 5Hz/step.
The cycle on/ramp/off time can be adjusted by 1 second/step.
This key selects Timer Mode,
Stimulation Mode,
Pulse Width Mode,
Pulse Rate Mode, or
Cycle On/Ramp/Off Time
This key switches between
the different settings within
Timer Mode and
Stimulation Mode.
of treatment parameters. *
*Increase by pressing the UP key: The width can be adjusted by 10μs/step.
The rate can be adjusted in 1Hz~20Hz by 1Hz/step, 20Hz~150Hz by 5Hz/step.
The cycle on/ramp/off time can be adjusted by 1 second/step.
Channel 1 and Channel 2
Intensity Control Knobs
This key regulates the number of pulse width,
pulse rate, and cycle on/ramp/off time.
This key changes the treatment parameter.
Each time the MODE key is pressed, the next
parameter will display. The selected treatment
parameter in the current mode will ash.
Each time the SET key is pressed, the
parameter will change to the next setting
within the parameter. The selected setting will
ash. When the desired setting is ashing,
press the MODE key to conrm the choice.
This key regulates the number of pulse width,
pulse rate, and cycle on/ramp/off time.
These knobs control the strength of the
stimulation and also function as ON / OFF
controls.
8
Page 9

ATTACHING THE LEAD WIRES
WARNING: Ensure the device is OFF before connecting the lead wires.
WARNING: Never insert the lead wire plug into an AC power supply socket.
Personal injury and damage to the EMS unit could occur.
s NOTICE: Use care when you plug and unplug the wires. Pulling on the lead wire
instead of its insulated connector may cause wire breakage.
The lead wires provided with the device insert into the ports located on the
top of the unit. If only one lead will be used, plug it into the channel 1 port.
After connecting the wires to the unit, attach each wire to an electrode.
Lead wires provided with the device are compliant with mandatory compliance standards set forth by the FDA.
ELECTRODE SELECTION AND CARE
Using Electrodes
Use the electrodes as prescribed. Follow application procedures outlined in
electrode packaging to maintain stimulation and prevent skin irritation. The
electrode packaging provides instructions for care, maintenance, and proper
storage of electrodes.
TIPS FOR SKIN CARE
Good skin preparation is important for effective and comfortable use of your
EMS device.
• Always clean the electrode site with mild soap and water solution, rinse
well, and dry thoroughly prior to any electrode application.
• Any excess hair should be clipped, not shaved, to ensure good electrode
contact with the skin.
• If a skin treatment or preparation is recommended by your physician /
clinician, apply the skin treatment as recommended, let dry, and apply
electrodes as directed. Following these recommendations will both reduce the chance of skin irritation and extend the life of your electrodes.
• Avoid excessive stretching of the skin when applying electrodes. Proper
9
Page 10

application is best accomplished by applying the electrode, then smoothly pressing it in place from the center outward.
• When removing electrodes, always remove by pulling in the direction of
hair growth.
• It may be helpful to rub skin lotion on electrode placement area when not
wearing electrodes.
CONNECTING THE DEVICE
Inserting the Battery
Turn the device to the OFF position before inserting or removing the battery.
When inserting the battery, ensure the battery polarity (+ and –) markings
match the markings on the device.
Preparing the Skin
Prepare the skin as previously described and according to the instructions
provided with your electrodes. Before attaching the electrodes, identify the
area that your physician / clinician has recommended for electrode placement.
1. Connect the Lead Wires to the electrodes: Connect the lead wires to the
electrodes before applying the electrodes to the skin.
CAUTION: Ensure both intensity controls for Channel 1 and 2 are turned to the
“OFF” Position (counterclockwise) before applying the electrodes.
2. Place Electrodes on the Skin: Place the electrodes on the skin as recommended by your physician / clinician.
3. Insert Lead Wire Connector into the Device: Plug end of lead wire into the
channel output port (jack) to be used; push the plug in as far as it will go.
4. Select Treatment Settings: Ensure your unit is still set to the proper settings recommended by your physician / clinician.
5. Adjusting Channel Intensity Control: Locate the intensity control knob
(Channel 1 or 2) at the top of the unit. Slowly turn the intensity control
knob clockwise until the stimulation is at the level recommended by your
physician / clinician. (If you don’t feel anything, turn the knob OFF then
10
Page 11

ON again and carefully turn the control knob until you feel a tingling or
slight twitch under or around the electrodes.) Always start with the lowest setting and increase the intensity slowly.
If the stimulation levels are uncomfortable or become uncomfortable,
reduce the stimulation intensity to a comfortable level; or cease stimulation and contact your physician.
6. Setting the Patient Compliance Counter:
a) To turn on the Patient Compliance Counter: While the unit is ON,
hold down the UP key and press the MODE key simultaneously.
b) To reset the Patient Compliance Counter: While the unit is ON, press
the UP key and the MODE key simultaneously (this will take you into
the Compliance Counter), then push the DOWN key and press the
MODE key simultaneously.
c) Press the UP and MODE keys simultaneously to return to the treat-
ment status.
BATTERY INFORMATION
A 9-volt battery is provided with your unit. When the LCD (Liquid Crystal
Display) low battery mark illuminates, the battery has become too weak to
power the unit, and the existing battery should be replaced with a new battery. At this point, the unit will turn OFF until a new battery is inserted.
Replacing the Battery
When the LCD low battery mark illuminates, and the unit does not remain illuminated once turned on, the battery should be replaced.
1. Turn unit OFF.
2. Remove the front panel cover by pressing on the top of the panel and
pressing down in order to slide the panel down. Continue sliding the
panel downwards until the panel is completely removed from the unit.
This will reveal the battery compartment.
3. Remove the discharged battery from the device.
4. Place new battery in the compartment. Note: Be sure the proper polarity
(+ and –) markings match the markings in the device.
11
Page 12

5. Replace the front panel cover.
6. Dispose of the old battery according to local guidelines and regulations.
CARING FOR YOUR DEVICE
Your device may be cleaned by wiping gently with a damp cloth moistened
with mild soap and water. Do not immerse the device in water or other liquids.
Wipe lead wires with damp cloth moistened with soap and water. Do not immerse the lead wires.
To properly store the device for an extended period of time, remove the
battery from the unit. Place the unit and accessories in the carrying case
provided and store in a cool, dry location.
TROUBLESHOOTING
If the device does not function properly:
1. Ensure the battery is properly installed or replace the battery. Be sure to
observe proper polarity markings when replacing the battery. If the LCD
low battery mark illuminates when the unit is turned on, replace the battery and check again.
2. If the intensity has been adjusted and no stimulation is felt, check to
ensure the lead wires are properly connected and the electrodes are
properly applied to the skin. If the unit appears to be functioning and no
stimulation is felt, the lead wires or electrodes may need to be replaced.
3. If the battery appears to be charged and the unit is not functioning, turn
both intensity control knobs to the OFF position (counterclockwise). Then
gradually turn the intensity Control Knob (clockwise) until stimulation
is felt. If device still is not working, turn the unit off and contact your
authorized GF Health Products, Inc. distributor.
If there is any other problem, please contact an authorized GF Health Products, Inc. distributor. Do not try to repair a defective device.
12
Page 13

TECHNICAL SPECIFICATIONS
Channel Dual, isolated between channels
Pulse Intensity Adjustable 0 mA – 100 mA peak into 500 ohm load each channel,
Pulse Width 30μs~300μs, adjustable by 10μs/step
Pulse Rate 1Hz-150Hz,
Timer 10, 20, 30, 40, 50, 60 minutes and continuous
Patient Compliance
Counter
Patient Lock System To prevent changes in any set parameter by physician / clinician. To
Wave Form Symmetrical bi-phasic square pulse
LCD Displays Timer, Function Mode, Pulse Width, Pulse Rate, Cycle ON/
Function Mode C Constant Mode
Voltage 50 volt (peak to peak), at 500 ohm load
Power Source 9-volt battery (alkaline or nickel-cadmium rechargeable)
Battery Life Approximately 70 hours at normal setting
Note: All electrical specifications are ± 10% 500 ohm load.
constant current
adjustable
To show the usage time by user, counted per each 30 minutes treatment
time
unlock or lock the unit, press the UP and DOWN keys simultaneously for
2-3 seconds
Ramp/OFF Time, Low Battery, Patient Compliance Counter, and Patient
Lock System
S Synchronous Mode
A Alternating Mode
Cycle ON Time
(Contraction)
ON Ramp Adjustable 1-10 second, 1 second/step
Cycle OFF Time
(Relaxation)
1Hz~20Hz adjustable by 1Hz/step
20Hz~150Hz adjustable by 5Hz/step
Adjustable 1-30 second, 1 second/step
Adjustable 1-30 second, 1 second/step
13
Page 14

LCD (Liquid Crystal Display)
The LCD displays timer (10, 20, 30, 40, 50, 60 minutes and C continuous),
function modes (S, C, A, Cycle ON / Ramp / OFF time), pulse width, pulse
rate, low battery, and patient compliance counter.
1. To check the LCD function, turn device ON and all the parameters will be
displayed for 1~2 seconds.
2. After 1~2 seconds, the LCD will go to previous operation parameter.
Changing the LCD Parameters
• To set the treatment parameters on the EMS device, the unit must be
in the UNLOCK position. If the device is in the LOCK position it, can be
unlocked by pressing the UP and DOWN keys simultaneously for 2~3
seconds.
• To switch between the different treatment parameters, press the MODE
key.
• Once in the desired mode, press the UP, DOWN, or SET key until the
desired treatment parameter is obtained.
• Once all of the desired treatment settings are displayed, the unit may
be locked by pressing the UP and DOWN keys simultaneously for 2-3
seconds while in the timer function.
14
Page 15

LIMITED WARRANTY
GF Health Products, Inc. warrants the Electrical Neuromuscular Stimulation
(EMS), Model GF-TX5EMS, against manufacturer’s defects for one year.
The warranty does not apply to damage resulting from failure to follow the
operating instructions, accidents, abuse, alterations or disassembly by unauthorized individuals.
During the warranty period, defective items will be repaired or replaced at
GF Health Products, Inc. option. The warranty does not include any labor
charges incurred in replacement part(s) installation or any associated freight
or shipping charges to GF Health Products, Inc.
The warranties contained herein contain all the representations and warranties with respect to the subject matter of this document, and supersede all
prior negotiations, agreements and understandings with respect thereto. The
recipient of this document hereby acknowledges and represents that it has
not relied on any representation, assertion, guarantee, warranty, collateral
contract or other assurance, except those set out in this document.
15
Page 16

USA Corporate Headquarters:
GF Health Products, Inc.
2935 Northeast Parkway
Atlanta, Georgia 30360 USA
tel: 770-368-4700
www.grahamfield.com
© 2010 GF Health Products, Inc.
Page 17

Estimulación Eléctrica
Neuromuscular
(EMS), Digital
Modelo GF-TX5EMS
Manual de Operación
Lea este manual antes de usar su GF-TX5EMS.
Guarde este manual para referencia futura.
La versión más reciente de este manual se podrá encontrar
en línea en www.grahamfield.com
GF-TX5EMS-INS-LAB-RevC11
Page 18

CONTENIDOS
DESCRIPCIÓN GENERAL ................................................................................................... 3
¿QUÉ ES EMS?
INDICACIONES Y CONTRAINDICACIONES
SEGURIDAD
ACERCA DEL APARATO
EXPLICACIÓN DE CLAVE / PERILLA FUNCIONES DE CONTROL
COLOCACIÓN DE LOS CABLES CONDUCTORES................................................................... 9
SELECCIÓN Y CUIDADO DE ELECTRODOS
CONSEJOS PARA LA ATENCIÓN DE LA PIEL
CONECTAR EL APARATO
INFORMACIÓN DE BATERÍA
CUIDADO DE SU APARATO
SOLUCIÓN DE PROBLEMAS
ESPECIFICACIONES TÉCNICAS........................................................................................ 13
GARANTÍA LIMITADA
................................................................................................................. 3
........................................................................... 3
..................................................................................................................... 4
..................................................................................................... 7
............................................ 8
.......................................................................... 9
.......................................................................9
................................................................................................. 10
............................................................................................ 11
.............................................................................................. 12
............................................................................................ 12
...................................................................................................... 15
GF Health Products, Inc. (“Graham-Field”) no es responsable por errores
tipográficos. El empaquetado, garantías, productos y especificaciones están
sujetos a cambiar sin aviso previo.
Graham-Field y Grafco son marcas registradas de GF Health Products, Inc.
2
Page 19

DESCRIPCIÓN GENERAL
EMS, Estimulación Eléctrica Neuromuscular, es un generador de pulsos a pilas
que envía impulsos eléctricos a los electrodos colocados en el cuerpo para estimular los nervios motores y provocar la contracción y relajación de los músculos.
Se ha demostrado útil como método de tratamiento del dolor y es de gran ayuda
para el terapeuta experimentado.
Con algunas indicaciones, los médicos / clínicos pueden prescribir unidades de
EMS a los pacientes para su uso en el hogar.
Esta unidad es un estimulador de doble canal digital para aplicaciones de tratamiento activo, que tiene una pantalla de cristal líquido que indica los modos de
operación y producción, así como un microordenador de 8 bits para controlar el
sistema.
La electrónica de la unidad crean impulsos eléctricos; la intensidad, duración,
frecuencia por segundo y la modulación de estos impulsos se pueden ajustar.
¿QUÉ ES EMS?
Un músculo lesionado normalmente experimenta poco o ningún movimiento. EMS
recursos mediante el uso de esta terapia de bajo voltaje para estimular los nervios
motores de causar contracciones musculares involuntarias.
Como el ejercicio, EMS ayuda a fortalecer el área lesionada, y se ha encontrado
para tratar con eficacia una variedad de enfermedades reumáticas y vasculares.
Candidatos comunes para la terapia de EMS son pacientes que se recuperan de
la cirugía ortopédica, lesiones musculares o de las lágrimas, o atletas que hayan
sido objeto de reparación de cartílago o tendón. EMS no es invasiva y no utiliza
productos farmacéuticos.
INDICACIONES Y CONTRAINDICACIONES
Lea el manual de instrucciones antes de usar este aparato EMS.
La ley federal (USA) restringe la venta de este aparato por o en el orden de un
médico.
Observe a su médico / clínico instrucciones precisas y permitir que le demuestren
dónde aplicar los electrodos. Para una terapia exitosa, la aplicación correcta de
los electrodos es un factor importante. Con cuidado, escriba la configuración de
su médico / clínico recomienda.
3
Page 20

Indicaciones
• Relajación del espasmo muscular
• Prevención o retraso de atrofia por desuso
• El aumento de la circulación sanguínea local
• Reeducación muscular
• La estimulación postquirúrgica inmediata de los músculos de la pantorrilla
para prevenir la trombosis venosa
• Mantener o aumentar rango de movimiento
Estimulación eléctrica neuromuscular sólo se debe utilizar bajo supervisión médica para la terapia adyuvante para el tratamiento de enfermedades y condiciones
médicas.
Contraindicaciones
ADVERTENCIA: Este aparato no debe ser utilizado por pacientes con marcapasos car-ADVERTENCIA: Este aparato no debe ser utilizado por pacientes con marcapasos car-
díacos demanda.
SEGURIDAD
Siempre siga las precauciones básicas de seguridad, incluyendo las siguientes:
ADVERTENCIA: Indica una situación de peligro o práctica insegura que, si no se evita,
podría resultar en muerte o daños corporales serios.
PRECAUCIÓN: Indica un peligro o una practica insegura que, si no se evita, podría
resultar en daños corporales de menor importancia.
s AVISO: Indica un peligro o una practica insegura que, si no se evita, podría resultar en
daños a producto / daños materiales.
Advertencias
ADVERTENCIA: Este aparato no tiene protección de AAP/APG.
Peligro de explosión es posible si se utiliza en la presencia de explosivos, materiales
inflamables o anestésicos inflamables.
ADVERTENCIA: Los efectos a largo plazo de la estimulación eléctrica crónica son des-ADVERTENCIA: Los efectos a largo plazo de la estimulación eléctrica crónica son des-
conocidos.
4
Page 21

ADVERTENCIA: No coloque los electrodos en los nervios del seno carotídeo, en particu-ADVERTENCIA: No coloque los electrodos en los nervios del seno carotídeo, en particu-
lar en pacientes con hipersensibilidad conocida al reflejo del seno carotídeo.
ADVERTENCIA: No coloque los electrodos sobre el cuello o la boca. Esto puede resultar
en espasmos de los músculos laríngeos y faríngeos, y la contracción puede ser lo
suficientemente fuertes como para cerrar las vías respiratorias o causar dificultad para
respirar.
ADVERTENCIA: No aplique el estímulo transtorácicamente, porque la introducción de la
corriente eléctrica al corazón puede causar arritmia cardiaca.
ADVERTENCIA: No aplique el estímulo transcerebralmente (a través de la cabeza).
ADVERTENCIA: No aplique el estímulo sobre las áreas hinchadas, infectadas o infl ama-ADVERTENCIA: No aplique el estímulo sobre las áreas hinchadas, infectadas o inflama-
das o sobre erupciones en la piel, por ejemplo, flebitis, tromboflebitis, venas varicosas,
etc.
ADVERTENCIA: No aplique el estímulo sobre o en la proximidad de las lesiones cancero-ADVERTENCIA: No aplique el estímulo sobre o en la proximidad de las lesiones cancero-
sas.
ADVERTENCIA: La seguridad del aparato durante el embarazo o el parto no ha sido
establecida.
ADVERTENCIA: Se debe tener precaución en pacientes con sospecha o diagnóstico de
los problemas del corazón.
ADVERTENCIA: Se debe tener precaución al aplicar este aparato a pacientes con sos-ADVERTENCIA: Se debe tener precaución al aplicar este aparato a pacientes con sos-
pecha de enfermedad cardíaca. Otros datos clínicos que se necesita para demostrar si
hay efectos secundarios adversos en individuos con enfermedades del corazón.
ADVERTENCIA: Se debe tener precaución en pacientes con sospecha o diagnóstico de
la epilepsia.
ADVERTENCIA: Se debe tener precaución en presencia de los siguientes:
Cuando hay una tendencia a la hemorragia después de un traumatismo agudo o fractura
Después de los procedimientos quirúrgicos recientes, cuando la contracción muscular
puede interrumpir el proceso de curación;
Sobre el útero menstruando o embarazadas, y
En las zonas de la piel que carecen de sensibilidad normal.
5
;
Page 22

ADVERTENCIA: Configuración de la colocación del electrodo y la estimulación debe
basarse en la orientación del médico que prescribe.
ADVERTENCIA: Mantenga este aparato fuera del alcance de los niños.
ADVERTENCIA: Utilice este aparato sólo con los cables y los electrodos se recomienda
su uso por GF Health Products, Inc.
ADVERTENCIA: No utilice este aparato mientras se conduce, al operar maquinaria,
o durante cualquier actividad en la que las contracciones musculares involuntarias
pueden poner al usuario en riesgo indebido de la lesión.
ADVERTENCIA: El aparato EMS sólo debe utilizarse bajo la supervisión continua de un
médico / clínico.
Precauciones / Reacciones Adversas
PRECAUCIÓN: Algunos pacientes pueden experimentar irritación de la piel o hipersen-
sibilidad debido a la estimulación eléctrica o medio conductor eléctrico. El uso de un
medio alterno de conducción o colocación de los electrodos alternativa por lo general
pueden reducir la irritación. Consulte a su médico / clínico antes de usar un medio
alternativo de conducción o colocación de los electrodos.
PRECAUCIÓN: Casos aislados de irritación de la piel puede ocurrir en el lugar de colo-
cación de los electrodos después de la aplicación a largo plazo.
PRECAUCIÓN: Si la piel se irrita tratamiento EMS debe ser detenido y los electrodos
removidos hasta que la causa de la irritación puede ser determinada.
PRECAUCIÓN: La eficacia depende en gran medida la selección del paciente de un
médico cualificado en el manejo de pacientes con dolor.
PRECAUCIÓN: Si el tratamiento con el aparato se convierte en ineficaz o desagra-
dables, la estimulación se debe suspender hasta nueva evaluación por un médico /
clínico.
PRECAUCIÓN: Siempre apague el aparato antes de aplicar o retirar los electrodos.
6
Page 23

ACERCA DEL APARATO
Este aparato es un aparato operado con baterías que incluye dos canales controlables de salida. Este aparato genera impulsos eléctricos que se pueden alterados
en intensidad, duración y modulación. Los controles del aparato son fáciles de
usar y la cubierta deslizante protege los cambios accidentales en la configuración.
Componentes del Sistema
El aparato incluirá los siguientes elementos o accesorios:
• Unidad EMS
• Estuche de transporte
• Cables conductores
• Batería de 9 voltios
• Manual de Operación
• Electrodos
Controles de Aparato
Canal 1 Encendido/Apagado y Control de Intensidad
Canal 2 Encendido/Apagado y Control de Intensidad
LCD (Pantalla de Cristal Líquido)
Control Clave (Teclas)
(ABAJO) (MODO) (FIJAR) (ARRIBA)
Cubierta Deslizante
Cubierta Deslizante
Esta cubierta se encuentra en la parte delantera de la unidad oculta los controles para DOWN, MODE, SET, y UP. Presione la parte superior de la cubierta y tire
hacia abajo para abrirla.
7
Page 24

EXPLICACIÓN DE CLAVE / PERILLA FUNCIONES DE CONTROL
La Tecla
DOWN
(ABAJO)
La Tecla
MODE
(MODO)
Esta tecla disminuye el ajuste cuando en
Pulse Width (Ancho de Pulso) Mode o
Pulse Rate (Frecuencia de Pulso) Mode. *
* Para disminuir, pulse la tecla ABAJO: El ancho se puede ajustar por 10μs/paso.
La frecuencia se puede ajustar en 1Hz~20Hz por 1Hz/paso, 20Hz~150Hz por
5Hz/paso. El ciclo de encendido / rampa / tiempo apagado se puede ajustar
por 1 segundo/paso.
Esta tecla selecciona
Timer (Temporizador) Mode,
Stimulation (Estimulación) Mode,
Pulse Width (Ancho de Pulso) Mode, o
Pulse Rate (Frecuencia de Pulso) Mode, o
Ciclo de Encendido / Rampa / Ciclo de Apagado
Esta tecla regula el número de ancho de
pulso, frecuencia de pulso, y el ciclo de
encendido / rampa / ciclo de apagado
se puede ajustar por 1 segundo/paso.
Esta tecla cambia el parámetro de
tratamiento. Cada vez que se pulsa la
tecla MODO, el parámetro de tratamiento
próxima mostrará. El parámetro de
tratamiento seleccionado en el modo
.
actual parpadeará.
La Tecla SET
(FIJAR)
La Tecla UP
(ARRIBA)
Las Perillas
de Canal 1 &
Canal 2
Esta tecla cambia entre los ajustes distintos en
los Timer (Temporizador) Mode y Stimulation
(Estimulación) Mode.
Esta tecla aumenta la conguración cuando
en Pulse Width (Ancho de Pulso) Mode o
Pulse Rate (Frecuencia de Pulso Mode. *
* Para aumentar, pulse la tecla ARRIBA: El ancho se puede ajustar por 10μs/paso.
La frecuencia se puede ajustar en 1Hz~20Hz por 1Hz/paso, 20Hz~150Hz por
5Hz/paso. El ciclo de encendido / rampa / tiempo apagado se puede ajustar
por 1 segundo/paso.
Canal 1 y Canal 2 Perillas de Control de
Intensidad
8
Cada vez que se pulsa la tecla FIJAR,
el parámetro cambia a la conguración
próxima en el parámetro. La
conguración seleccionado parpadeará.
Cuando la conguración seleccionado
deseado esté parpadeando, pulse la
tecla MODO para conrmar la elección.
Esta tecla regula el número de ancho
de pulso o frecuencia de pulso de los
pulsos corrientes individuales.
Estas perillas controlan la fuerza
de la estimulación y funcionan
también como controles ON / OFF
(ENCENDIDO / APAGADO).
Page 25

COLOCACIÓN DE LOS CABLES CONDUCTORES
ADVERTENCIA: Asegúrese de que el aparato está apagado antes de conectar los cables.
ADVERTENCIA: Nunca inserte el enchufe del cable conductor en una fuerza de alimen-
tación CA. Herida personal o/a daños a la unidad EMS podría ocurrir.
s AVISO: Tenga cuidado al conectar y desconectar los cables. Tirando del cable conduc-
tor en lugar de su conector aislado puede causar la rotura del alambre.
Los cables conductores suministrado con el aparato se inserten en las puertas
situada en la parte superior de la unidad. Si sólo un cable se usa, el enchufe en
el puerto de canal 1. Después de conectar los cables a la unidad, conecte cada
cable a un electrodo.
Cables conductores suministrado con el aparato son compatibles con las normas
de cumplimiento obligatorio establecidas por la FDA.
SELECCIÓN Y CUIDADO DE ELECTRODOS
Uso de Electrodos
Utilice los electrodos según lo prescrito. Siga los procedimientos de aplicación
previstos en empaquetado de los electrodos para cuidado, mantenimiento y almacenaje adecuado de los electrodos.
CONSEJOS PARA LA ATENCIÓN DE LA PIEL
Buena preparación de la piel es importante para utilización eficaz y cómodo de su
aparato de EMS.
• Siempre limpie el sitio del electrodo con jabón suave y agua, enjuague bien, y
seque bien antes de la aplicación de los electrodos.
• Cualquier exceso de pelo se debe cortar, no afeitar, para asegurar el contacto
bueno del electrodo con la piel.
• Si un tratamiento de la piel o el preparado está recomendado por su
médico / clínico, aplique el tratamiento de la piel como se recomienda, dejar
secar, y aplique los electrodos como se indica. Siguiendo estas recomendaciones tanto a reducir la posibilidad de irritación de la piel y prolongar la vida
de sus electrodos.
9
Page 26

• Evite el exceso de estiramiento de la piel cuando está aplicando electrodos.
La aplicación adecuada se logra mejor mediante la aplicación del electrodo, a
continuación, pues presionándola en lugar del centro hacia fuera.
• Al retirar los electrodos, retire siempre tirando en la dirección del crecimiento
del vello.
• Puede ser útil para frotar loción para la piel sobre la zona de colocación de
los electrodos cuando no esté usando electrodos.
CONECTAR EL APARATO
Insertar la Batería
Gire el aparato a la posición de apagado antes de insertar o extraer la batería.
Al insertar la batería, asegúrese de que las marcas de la polaridad de la batería
(+ y -) coincidan con las marcas en el aparato.
Preparar la Piel
Prepare la piel como se describió previamente y de acuerdo con las instrucciones
proporcionadas con sus electrodos. Antes de colocar los electrodos, identificar el
área que su médico / clínico ha recomendado para la colocación de los electrodos.
1. Conecte los cables conductores a los electrodos: Conecte los cables a los
electrodos antes de aplicar los electrodos a la piel.
PRECAUCIÓN: Asegúrese de que los controles de intensidad para el canal 1 y 2 se
volvió hacia la posición "OFF" (izquierda) antes de aplicar los electrodos.
2. Coloque los electrodos sobre la piel: Coloque los electrodos sobre la piel
según lo recomendado por su médico / clínico.
3. Inser te el enchufe del cable conductor en el conector del aparato: Enchufe
el extremo del cable en el puerto del canal de salida (jack) para ser utilizado;
introduzca el enchufe en la medida de lo que pueda.
4. Seleccione Ajustes de Tratamiento: Asegúrese de que su unidad está aún
establecida en la configuración adecuada recomendados por su médico /
clínico.
5. Ajuste el control de intensidad del canal: Busque la perilla de control de
intensidad (Canal 1 o 2) en la parte superior de la unidad. Gire lentamente
hacia la derecha la perilla del control de intensidad hasta que la estimulación
10
Page 27

es en el nivel recomendado por su médico / clínico. (Si usted no se siente
nada, apagar la perilla y encenderlo de nuevo y apretar la perilla de control
hasta que sienta una ligera sensación de hormigueo o de contracción debajo
o alrededor de los electrodos.) Comience siempre con la posición más baja y
aumentar la intensidad poco a poco.
Si los niveles de estimulación son incómodos o se sienten incómodas, reduz-
ca la intensidad de la estimulación a un nivel cómodo, o cese la estimulación
y contacte a su médico.
6. Establecer el Contador de la Conformidad del Paciente:
a) Para activar el Contador de la Conformidad del Paciente: Mientras la
unidad está encendida, pulse las teclas UP y MODE simultáneamente.
b) Para reinicializar el contador: Mientras la unidad está encendida, pulse
las teclas UP y MODE simultáneamente (esto le llevará en el contador), a
continuación, pulse las teclas DOWN y MODE simultáneamente.
c) Pulse las teclas UP y MODE simultáneamente para volver a la condición
del tratamiento.
INFORMACIÓN DE BATERÍA
Una batería de 9 voltios se proporciona con la unidad. Cuando la marca de
batería baja de LCD (pantalla de cristal líquido) se lumina, la batería se ha vuelto
demasiado débil para alimentar la unidad, y la batería existente debe ser reemplazado por una batería nueva. En este punto, la unidad se apagará hasta que
una nueva batería se inserta.
Sustitución de la Batería
Cuando la marca de batería baja de LCD se lumina y la unidad no se queda iluminada, una vez encendida, la batería debe ser reemplazada.
1. Apague la unidad.
2. Presione la parte superior de la cubierta y tire hacia abajo para abrirla.
Continúe deslizando el panel hacia abajo hasta que el panel se elimina por
completo de la unidad. Esto revelará el compartimiento de la batería.
3. Quite la batería descargada del aparato.
4. Coloque la nueva batería en el compartimiento. Nota: Asegúrese de que las
marcas de la polaridad de la batería (+ y -) coincidan con las marcas en el
aparato.
11
Page 28

5. Reemplace la cubierta.
6. Deseche la batería usada de acuerdo a las directrices y regulaciones locales.
CUIDADO DE SU APARATO
El aparato se puede limpiar frotando suavemente con un paño humedecido con
jabón suave y agua. No sumerja el aparato en agua u otros líquidos.
Limpie los cables conductores con un paño humedecido con agua y jabón. No
sumerja los cables conductores.
Para almacenar correctamente el aparato durante un largo periodo de tiempo,
retire la batería de la unidad. Coloque la unidad y los accesorios en el estuche de
transporte suministrado y guárdelo en un lugar fresco y seco.
SOLUCIÓN DE PROBLEMAS
Si el aparato no funciona correctamente:
1. Asegúrese de que la batería está correctamente instalado o cambiar la batería. Asegúrese de observar las marcas de la polaridad adecuada al reemplazar la batería. Si la marca de batería baja de LCD se ilumina cuando la
unidad se enciende, reemplace la batería y vuelva a comprobarlo.
2. Si la intensidad ha sido ajustada y no se sentía la estimulación, fijarse que
los cables estén bien conectados y los electrodos se aplican correctamente a
la piel. Si la unidad parece estar funcionando y no se sentía la estimulación,
los cables o electrodos pueden necesitar ser reemplazadas.
3. Si la batería parece estar cargado y la unidad no está funcionando, apague
las dos perillas de control de intensidad (gire las perillas a la izquierda). Luego, gradualmente, gire la perilla de control de intensidad (a la derecha) hasta
que la estimulación se hace sentir. Si el aparato sigue sin funcionar, apague
la unidad y contacte a su distribuidor autorizado de GF Health Products, Inc.
Si hay cualquier otro problema, por favor póngase en contacto con un distribuidor
autorizado de GF Health Products, Inc. No trate de reparar un aparato defectuoso.
12
Page 29

ESPECIFICACIONES TÉCNICAS
Canal
Intensidad del Pulso
Ancho del Pulso
Frecuencia del Pulso
Temporizador
Contador de la
Conformidad del
Paciente
Sistema de Bloqueo
del Paciente
Forma de Onda
LCD (Pantalla de
Cristal Líquido)
Modo de Función C
Voltaje
Fuente de Energía
Duración de la Batería
Nota: Todas las especificaciones eléctricas son ± 10% de carga de 500 ohmios.
Dual, aislado entre canales
Ajustable desde 0 mA hasta 100 mA pico en la carga de 500 ohm cada canal,
de corriente constante
30μs~300μs, ajustable por 10μs/paso
1Hz-150Hz,
ajustable
10, 20, 30, 40, 50, 60 minutos y continua
Para mostrar el tiempo de uso por usuario, cuentan por cada 30 minutos el
tiempo de tratamiento
Para evitar un cambio por el paciente en cualquier conjunto de parámetros por
el médico / clínico. Para desbloquear o bloquear la unidad, pulse las teclas UP y
DOWN (ARRIBA y ABAJO) simultáneamente durante 2-3 segundos
Simétrica bifásica cuadro
Muestra el Temporizador, Modo de Función, Ancho de Pulso, Frecuencia de Pulso,
Marca de Batería Baja, Contador de la Conformidad del Paciente, y
Sistema de Bloqueo del Paciente
S
A
Ciclo de ENCENDIDO
1Hz~20Hz ajustable por 1Hz/paso
20Hz~150Hz ajustable por 5Hz/paso
Constant Mode (Modo Constante)
Synchronous Mode (Modo Sincrónico)
Alternating Mode (Modo Alternar)
Ajustable 1-30 segundos, por 1 segundo/paso
(contracción)
Rampa de ENCENDIDO
Ciclo de APAGADO
Ajustable 1-10 segundos, por 1 segundo/paso
Ajustable 1-30 segundos, por 1 segundo/paso
(relajación)
0 a 100 voltios (circuito abierto)
Batería de 9 voltios (alcalinas o recargables de níquel-cadmio)
Aproximadamente 70 horas a los ajustes nominales
13
Page 30

LCD (Pantalla de Cristal Líquido)
La pantalla LCD muestra el temporizador (10, 20, 30, 40, 50, 60 minutos y C
continua), los modos de funcionamiento (S, C, A, ciclo de encendido / rampa /
tiempo apagado), el ancho de pulso, la frecuencia de pulso, la marca de batería
baja, y el contador de la conformidad del paciente.
1. Para comprobar la función LCD, a su vez aparato de encendido y todos los
parámetros se mostrarán durante 1-2 segundos.
2. Después de 1-2 segundos, la pantalla LCD se destinará a los parámetros de
operación anterior.
Cambiar los Parámetros LCD
• Para establecer los parámetros de tratamiento en el aparato EMS, la unidad
debe estar en la posición UNLOCK (desbloqueo). Si el aparato está en la posición LOCK (bloqueo), se puede abrir pulsando las teclas UP y DOWN simultáneamente durante 2-3 segundos.
• Para cambiar entre los parámetros diferentes de tratamiento, pulse la tecla
MODE.
• Una vez en el modo deseado, presione la tecla UP, DOWN, o SET hasta que el
parámetro de tratamiento deseado se obtiene.
• Una vez que todos los ajustes deseados del tratamiento se muestran, la
unidad se puede bloquear pulsando las teclas UP y DOWN simultáneamente
durante 2-3 segundos, mientras que en la función de temporizador.
14
Page 31

GARANTÍA LIMITADA
GF Health Products, Inc. garantiza que la Estimulación Eléctrica Neuromuscular
(EMS), Modelo GF-TX5EMS, contra defectos de fabricación por un año.
La garantía no cubre los daños ocasionados por no seguir las instrucciones de
uso, accidentes, abuso, alteraciones o el desmontaje por personas no autorizadas.
Durante el período de garantía, los artículos defectuosos serán reparados o reemplazados a GF Health Products, Inc. opción. La garantía no incluye ningún cargo
de labor, incurrido en la instalación de piezas reemplazas, o el flete o los gastos
de envío asociados a GF Health Products, Inc.
Las garantías que figuran en este documento contiene todas las declaraciones y
garantías en relación con la materia objeto de este documento, y sustituyen todas
las negociaciones previas, acuerdos y entendimientos con respecto a la misma.
El receptor del presente documento reconoce y declara que no ha basado en
ninguna declaración, afirmación, garantía, contrato de garantía u otra promesa,
excepto los establecidos en este documento.
15
Page 32

USA Ocinas Centrales:
GF Health Products, Inc.
2935 Northeast Parkway
Atlanta, Georgia 30360 USA
tel: 770-368-4700
www.grahamfield.com
© 2010 GF Health Products, Inc.
 Loading...
Loading...