Page 1

INSTRUCTIONS FOR USE
SERVICE MANUAL
To avoid personal injury or damage to bed,
please read all sections pertaining to
your bed model before use.
ETL/UL/CSA APPROVED
UL 60601-1
IEC 60601-2-38
CSA 22.2 601.1
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com Zenith APS Service Manual 999-0831-190A
Page 2

This service manual covers the following APS Models:
76” & 80” Full Electric with Pedal Lock Caster System & Grid Decks = APS98174;
76” & 80” Full Electric with Pedal Lock Caster System, Grid Decks, and Underbed Light = APS981741;
76” & 80” Full Electric with Pedal Lock Caster System, Grid Decks, Underbed Light, and
Onboard Battery Backup System = APS981742.
,#&+#'#%+(.-,
336 Trowbridge Drive
Fond du Lac, WI 54937
For APS 9000 Bed Service Parts please contact
our Customer Service Department at
1-800-365-2338
%-"+(.-,'
2935A Northwest Parkway
Atlanta, GA 30360
www.grahamfield.com
To order an APS 9000 Bed please contact a
Graham Field Sales Representative at
1-800-554-9215
IMPORTANT NOTICE
GF Health Products, Inc. is not responsible for typographical errors.
All illustrations, specifications, packaging, and warranties contained in this Service Manual
are based on the latest product information available at the time of printing.
The most current product information can be found online at www.grahamfield.com.
Please check all parts for shipping damage and test before using.
In case of damage, DO NOT USE.
Page 3
Zenith aps seRies
TABLE OF CONTENTS
Safety & Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
ID/Warning Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 6
Entrapment/Compliance . . . . . . . . . . . . . . . . . . . . . . . . . 7
Features & Specifications . . . . . . . . . . . . . . . . . . . . . . . . 8
Unpacking Your New Bed . . . . . . . . . . . . . . . . . . . . . . . . 9
Assembly - Boards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Plugging in Your Staff Control . . . . . . . . . . . . . . . . . . . . 11
Standard Mattress Retainers . . . . . . . . . . . . . . . . . . . . . 12
Standard Wireform Wallsaver . . . . . . . . . . . . . . . . . . . . 13
Wallsaver Adapter for Trendelenberg Positions . . . . . . .14
Operations/Controllers & Staff Control . . . . . . . . . .15 - 16
Placing the Bed into the Chair Position . . . . . . . . . . . . .17
Using Trendelenberg/Reverse Trendelenberg . . . . . . . .18
Electronic Components . . . . . . . . . . . . . . . . . . . . . . . . . 19
Electrical Cabling . . . . . . . . . . . . . . . . . . . . . . . . . . 20 - 22
Optional Pivot Assist Bar . . . . . . . . . . . . . . . . . . . . . . . .23
Optional Trapeze Support Adaptor . . . . . . . . . . . . . . . . .24
Optional Battery Backup Configurations . . . . . . . . . . . . .25
Troubleshooting - No Power . . . . . . . . . . . . . . . . . . . . . 26
Troubleshooting - Staff Control & Pendant . . . . . . . 26 -27
Troubleshooting Motors/Control Box . . . . . . . . . . . . . . . 28
Replacing the Staff Control Assembly . . . . . . . . . . . . . . 29
Maintenance & Inspection . . . . . . . . . . . . . . . . . . . . . . .30
Replacement/Service Part List . . . . . . . . . . . . . . . . 31-32
Service Part Diagrams . . . . . . . . . . . . . . . . . . . . . . . 33-36
!
Consult
Accompanying
Documents
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
!
Safe
Working
Load
Double
Insulated
Protected
Grounded
Device
Type B
Equipment &
Applied Parts
3
Page 4
Zenith aps seRies
IMPORTANT SAFETY AND WARNING INFORMATION
This product is a variable height, adjustable mattress
platform, which will provide comfort and convenience
!
for residents/patients and caregivers in long term
care settings.
The MAXIMUM SAFE WORKING LOAD for the
APS 9000 Bed with weight evenly distributed
!
bedding, resident/patient, support surface, and all
accessories, is 600 lbs.
NEVER operate the bed if a Power Cord or Plug is
!
damaged or not working properly. Contact qualified
Service Personnel for examination and repair. Always
unplug the Power Cord when performing any maintenance on the bed.
DO NOT open assemblies such as the Actuators, Hand
Control, or Control Box. If unauthorized personnel
!
perform work on these components, the manufacturer’s
warranty becomes void.
DO NOT use unauthorized parts, accessories, or
!
adaptors other than those specified/authorized by
GF Health Products, Inc.
When operating the High/Low, Knee, or Back Functions
!
of the bed, ALWAYS ensure that the confined individual
is positioned properly within the confines of the bed.
DO NOT let any extremities protrude over the side or
between the bed rails when performing these functions.
DO NOT lower the bed when objects are beneath it.
!
Failure to inspect under the bed can result in damage
to property or personal injury.
The bed’s Pendant Cord MUST BE ROUTED AND
SECURED PROPERLY to ensure it does not become
!
entangled and eventually severed during use. Also
make sure all electrical cords DO NOT get tangled
around the bed, side rails, or legs during transport or
normal operation of the bed.
When using nasal- or masked-type administering
!
equipment, all oxygen or air tubing MUST BE ROUTED
AND SECURED PROPERLY to ensure that the tubing
does not become entangled and eventually severed
during the normal operation of the bed.
, including
The bed should ALWAYS be left in its lowest position
!
when unattended to reduce the risk of injury while getting
in or out of the bed.
Keep all moving parts free of obstructions (i.e. blankets/
!
sheets, heating blankets/pads, wiring, etc.)
DO NOT use the assist devices as push handles for moving
the bed. Assist devices can be deformed or broken if
!
excessive side pressure is exerted. Assist devices are not
meant for patients considered as high risks for entrapment
(i.e. patients with pre-existing conditions such as
confusion, restlessness, lack of muscle control, altered
mental status, either organic or medicinal, or a combination thereof). Additional safety measures should be
considered for such high-risk patients.
NEVER permit more than one (1) person on/in the bed
!
at any time.
Body weight should be evenly distributed over the
sleeping surface of the bed. DO NOT allow the patient
!
to lay, sit, or lean in such a way that their entire body
weight is placed only on the raised head or foot sections
of the bed. This especially applies when repositioning
or transferring a patient in or out of the bed. Increased
risk may occur when the patient’s size and/or weight are
inappropriate for the bed’s dimensions or weight capacity.
The bed is intended for use, storage, and transport within
a temperature range of -40˚C to +60˚C. It has a water-
!
resistance rating of IPX4 and IS NOT
washed or submersed.
)+-#'!('#-#(',
Operation of the bed is based on the following conditions:
Ambient Temperature of +10˚C to +40˚C; Relative Humidity
of 30% to 75% (Non-condensing); Atmospheric Pressure of
700hPa to 1060hPa; and a Splash Protection of IEC 60529.
-(+!
Storage of the bed is based on the following conditions:
Ambient Temperature of -10˚C to 70˚C; Relative Humidity
of 10% to 100%; and an Atmospheric Pressure of 500hPa
to 1060hPa.
#(+*.'1'-+ +'
RFI influences most electronic equipment. Caution should be
exercised with regard to the use of portable communications
equipment in the area around such equipment. If RFI causes
erratic behavior, shut the bed off immediately. Leave it off
while the transmission is in progress.
to be power-
4
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 5
Zenith aps seRies
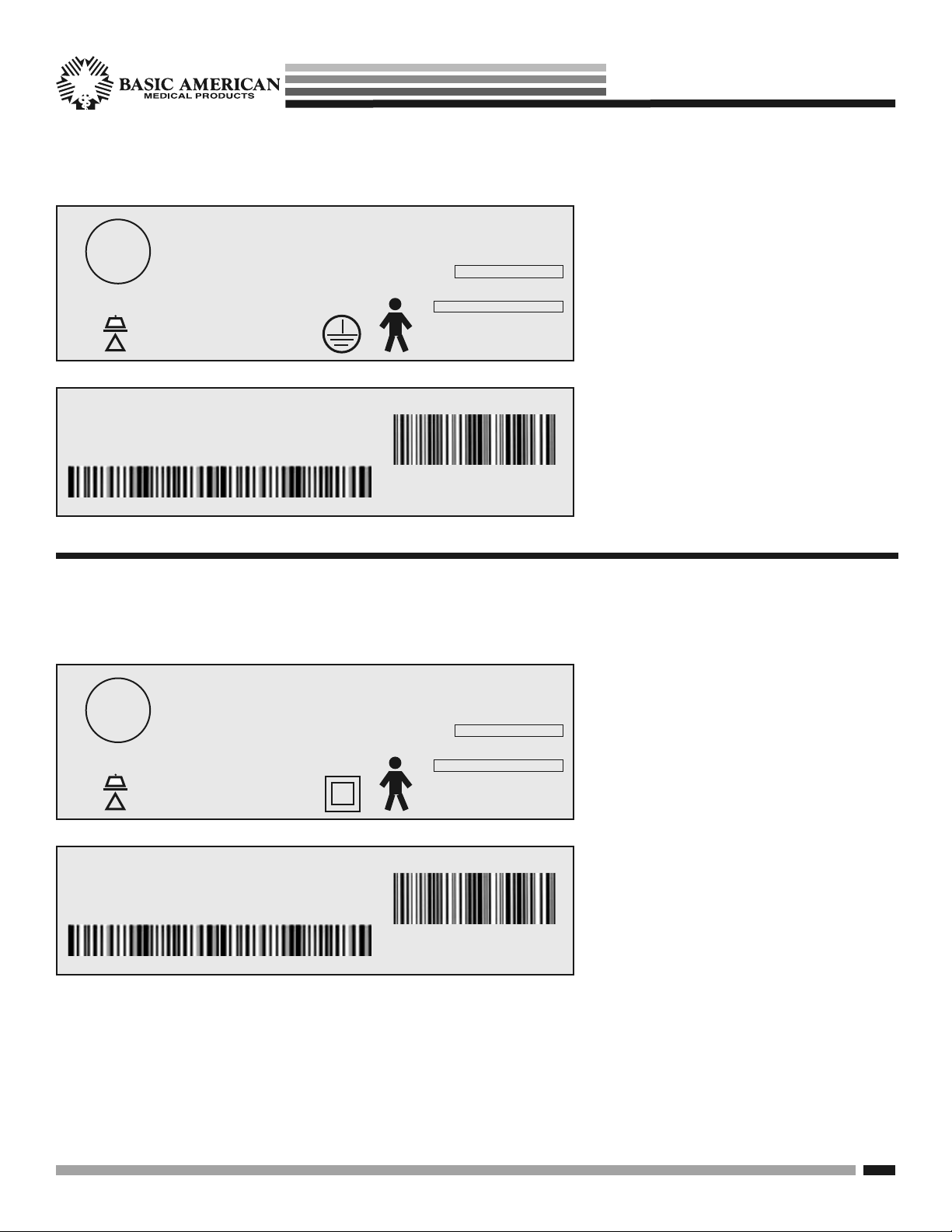
TYPICAL APS 9000 BED IDENTIFICATION LABELS
with Grounded Electrical Cable
ETL
Logo
DUTY CYCLE - 2 min Continuous/18 min
MODE OF OPERATION - Intermittent
APS98174
MODEL NO. 806-0000-000000 MFG DATE 12/23/11
GF HEALTH
PRODUCTS, INC.
600 lbs
=
SAFE WORKING LOAD
!
SERIAL APS 9
SERIAL APS98174
IPX4
ZENITH 806 SERIES
PATENT PENDING
APS9000
TYPICAL APS 9000 BED IDENTIFICATION LABELS
MODEL NO
806-0000-000000
MFG. DATE
12/23/11
VOLT HZ AMP TYPE
110-120 50/60 4.5 AC
SERIAL NO
000000000000
SERIAL APS 9
000000000000
for Class II Models
Bed labels are an important part of
identifying your bed’s make and model
when ordering replacement parts. The
Serial Number is essential if you are
claiming parts or service under warranty.
These helpful labels can be located on
the main frame rails, immediately below
the sleep decks on either side of the bed.
Please have this IMPORTANT information ready when calling our customer
service or technical support staff at
800-365-2338; it will allow us to better
assist you and quickly answer your
questions and concerns.
ETL
Logo
DUTY CYCLE - 2 min Continuous/18 min
MODE OF OPERATION - Intermittent
APS98174
MODEL NO. 806-0000-000000 MFG DATE 12/23/11
GF HEALTH
PRODUCTS, INC.
600 lbs
=
SAFE WORKING LOAD
!
SERIAL APS 9
SERIAL APS98174
IPX4
ZENITH 806 SERIES
PATENT PENDING
APS9000
MODEL NO
806-0000-000000
MFG. DATE
12/23/11
VOLT HZ AMP TYPE
110-120 50/60 4.5 AC
SERIAL NO
000000000000
SERIAL APS 9
000000000000
If you opted for an electrical system without
grounding on your electrical cord, your bed
is designated as a Class II.
The Identification bed labels to the left
represent the labels for Class II beds.
They can be located on the main frame
rails, immediately below the sleep decks
on either side of the bed.
Please have this IMPORTANT information
ready when calling our customer service
or technical support staff at 800-365-2338.
The labels at left will allow us to better
assist you in answering your questions
and concerns.
5
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 6
Zenith aps seRies
TYPICAL SAFETY/WARNING BED LABELS
The following warning labels have been placed on your bed to help protect you and your bed from
damage. Please do not remove any labels from your bed.
WARNING!
DO NOT LOWER BED WHEN OBJECTS ARE
BENEATH BED. FAILURE TO INSPECT UNDER
BED CAN RESULT IN DAMAGE TO PROPERTY
OR PERSONAL INJURY.
ATTENTION:
S’assurer de ne pas faire descendre le lit
lorsque des objets se trouvent sous le lit. Ne
pas inspecter le dessous du lit pourrait entrainer
des dommages materiels et des risques de
blessures.
WARNING!
DO NOT LOWER BED WHEN
OBJECTS ARE BENEATH BED.
FAILURE TO INSPECT UNDER
BED CAN RESULT IN DAMAGE
TO PROPERTY OR PERSONAL
INJURY.
CAUTION
THIS BED IS SUITABLE FOR USE ONLY WITH
OXYGEN ADMINISTERING EQUIPMENT OF
THE NASAL OR MASK TYPE OR A TENT
COVERING ONLY THE UPPER HALF (HEAD
END) OF THE BED. OXYGEN TENT CANOPIES
SHOULD NOT EXTEND BELOW BED SPRING
LEVEL. LOCK HAND CONTROL AT FOOT OF
BED WHEN USING OXYGEN ADMINISTERING
EQUIPMENT.
ATTENTION:
CE LIT PEUT ETRE UTILISE UNIQUEMENT
AVEC UN EQUIPMENT DESTINE A
L’ADMINISTRATION D’OXYGENE DE TYPE
NASAL OU MASQUE OU AVEC UNE TENTE
RECOUVRANT SEULEMENT LA MOTTIE
AVENT (TETE) DU LIT. LES COTES DE LAS
TENTE OXYGENE NE DOIVENT PAS SE
PROLONGER PLUS DAS QUE LA SOMMIER
DU LIT.
6
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 7
Zenith aps seRies
ENTRAPMENT & COMPLIANCE INFORMATION
On March 10, 2006, the FDA (U.S. Food and Drug Administration) released long-awaited guidelines for reducing the risk
of bed entrapment: “Hospital Bed System Dimensional and
Assessment Guidance to Reduce Entrapment”. The new Guidance
identifies potential entrapment areas and those body parts most
at risk for entrapment; provides design criteria for manufacturers
of new hospital/convalescent beds; recommends particular test
methods to assess the conformance of existing hospital/convalescent bed systems; and answers frequently-asked questions
about entrapment issues.
The new Guidance was a result of a long-standing collaboration
between the FDA and the Hospital Bed Safety Workgroup
(HBSW), formed in 1999. GF Health Products, Inc’s LongTerm Care Bed division: Basic American Medical Products, is
an HBSW charter member. As a result of our commitment to
product safety, %%(.+.++'-%('!-+&+,"/'
,-+#-%1-,-'(' (+&-(-"'0.#'.
The guidelines set forth by the FDA Guidance layout specific
dimensional limitations on potentially injury-threatening gaps
and spaces that can occur between bed system components,
such as rails, when not properly installed. GF Health Products,
Inc. and Basic American Medical Products have conformed
to these guidelines from a manufacturing aspect. However,
entrapment issues can often arise when a healthcare provider/
facility has not correctly assembled the components on a bed.
It is essential that the provider/facility fully understand their
responsibility in complying to the guidelines set forth by the
FDA in order to avoid injury. To that end, we have provided
the FDA’s web address at right as a resource for understanding
and following these guidelines for the safety of patients/residents.
It is also essential to have the correct bed components/accessories that correspond with the needs of your patient/resident
and the particular bed you have purchased. Matching the correct
bed component that correlates with the regulatory guidelines can
be a daunting task. Our sales team at GF Health Products, Inc.
and our friendly Customer Service Representatives at Basic
American Medical Products can help you sift through the wide
array of compliance and bed options. We will help you determine
which bed/bed part is best for your patient’s/resident’s particular
needs and help you with your compliance issues.
"',,(+#,%#,-#'-"#,
&'.%+#' .%%(&)%#'0#-"!.#%#',
(++.#'!-"+#,$( '-+)&'-2Hospital
Bed System Dimensional and Assessment Guidance
to Reduce Entrapment3
-#%,' (.'-000 !(/
7
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 8
Zenith aps seRies
ZENITH 9000 APS MECHANICS
NOTE: All dimensions are in a range of +/- .25 inches
Overall Bed Length (with brds & wallsaver) . . 82”/86”
•
Overall Bed Width (with boards) . . . . . . . . . . . 36.00”
•
High Height* . . . . . . . . . . . . . . . . . . . . . . . . . . 30.00”
•
Low Height* . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.00”
•
Maximum Head/Back Deck Angle . . . . . . . . . . . . 70°
•
Maximum Knee/Foot Deck Angle . . . . . . . . . . . . 30°
•
Maximum Safe Working Load . . . . . . . . . . . 600 lbs.
•
WITH WEIGHT EVENLY DISTRIBUTED
- includes bedding, resident, support surface,
and all accessories.
Mass of bed (without assist devices or boards) = 204 lbs.
•
* Bed height calculated from floor surface to top of
sleep deck.
ZENITH 9000 APS ELECTRICAL
• Power/Frequency . . . . . . . . . . . . . 120 Volt 50/60 Hz
• Output Rating . . . . . . . . . . . . . . . . . . . . 12/33V IPX4
• Overall Movement Draw . . . . . . . . . . . . . 4.50 Amps
• Classification . . . . . . . . . . . . . . . . . . . Class I, Type B
• Electrical Cord . . . . . . . . . . . . #18 AWG 3 Conductor
Type SJT
• Classification . . . . . . . . . . . . . . . . . . Class II, Type B
• Electrical Cord . . . . . . . . . . . . #18 AWG 2 Conductor
Type SJT
• Mode of Operations . . . . . . . . .10% Max. Duty Cycle
2 minutes on/18 minutes off
• Battery Backup Option 1 . . . . 120V External Battery
and Charger
Pack and Charger can be purchased separately
as an accessory.
• Battery Backup Option 2 . . . . 120V External Battery
Pack is mounted to the bed frame -
no charger needed.)
8
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 9
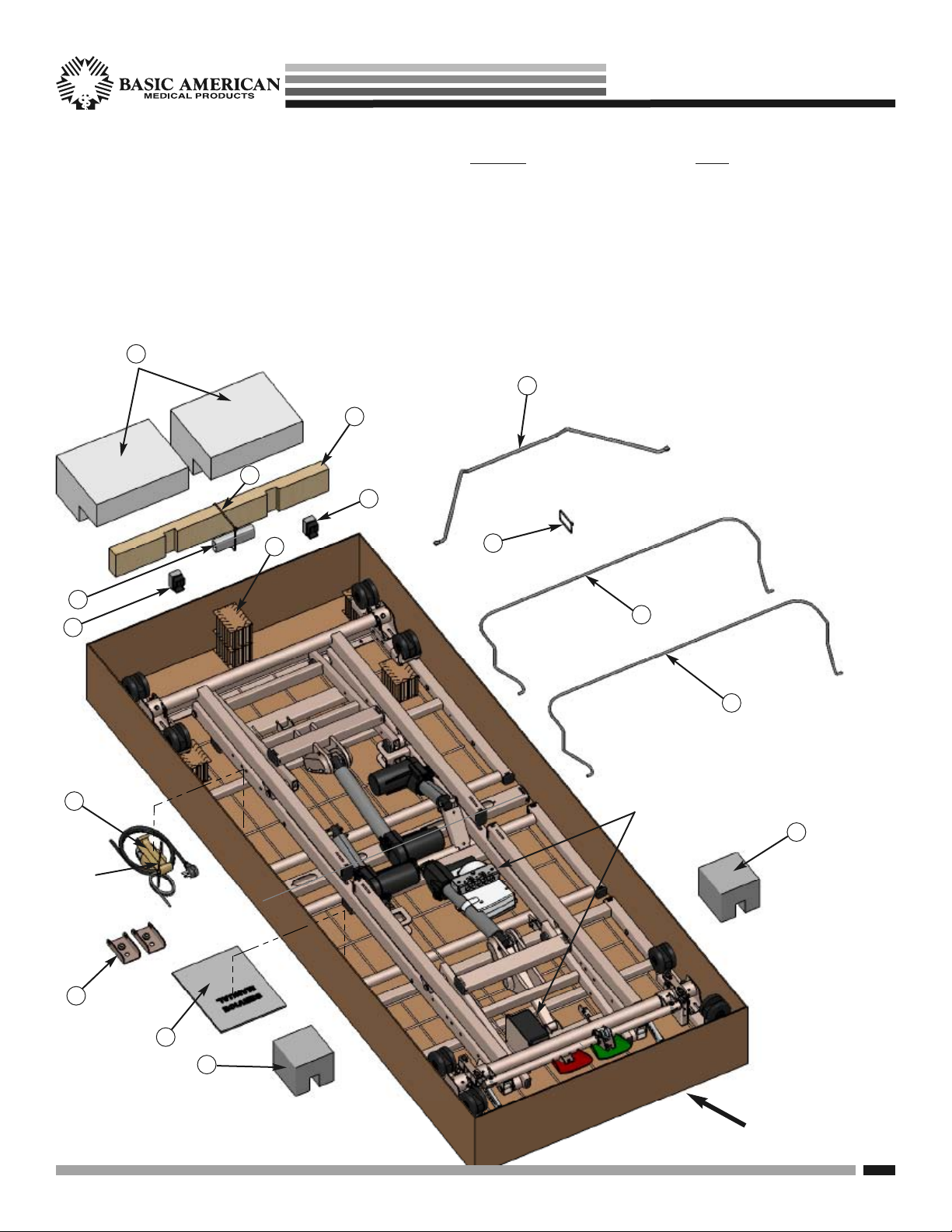
Zenith aps seRies
UNPACKING YOUR BED
Make sure all parts/components are included.
•
Check all bed components for obvious damage.
•
Inspect the Power Supply Cord for any cuts
•
and/or damage.
Check to see all actuator/motor cables are routed
•
and connected properly to the control box.
2
4
3
6
1
5
6
DISCARD
1. Cardboard Blocks
2. Large Block
3. Large Cable Tie - CUT
4. Notched 2 x 4 Board
5. Foam Wrapping
6. End Caps with Foam
7. Notched Leg Foam
8. Small Cable Tie - CUT
9
8
KEEP
9. Wireform Wallsaver
10. Two Mattress Retainers
11. Service Manual/Documents
12. Wallsaver Adaptor Brackets for
Trendelenberg and Reverse
Trendelenberg Positioning
13. Pendant Holster
(attached to pendant)
NOTE: END OF POWER CABLE
IS COILED FOR SHIPPING AND
TIED, WITH CABLE TIE, TO GRID
WIRE ALONG WITH PENDANT,
PENDANT CABLE & HOLSTER.
PLEASE CUT AND DISCARD
CABLE TIE AROUND CABLES
WHEN YOU UN-PACK YOUR BED.
10
10
NOTE:
Underbed Light is optional and available on
APS981741 Models.
Underbed Light and Mounted Battery optional
13
combination is available on APS981742 Models.
7
Cut and
discard
cable tie.
12
11
7
ALSO
DISCARD TOP
AND BOTTOM
CARTONS
9
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 10
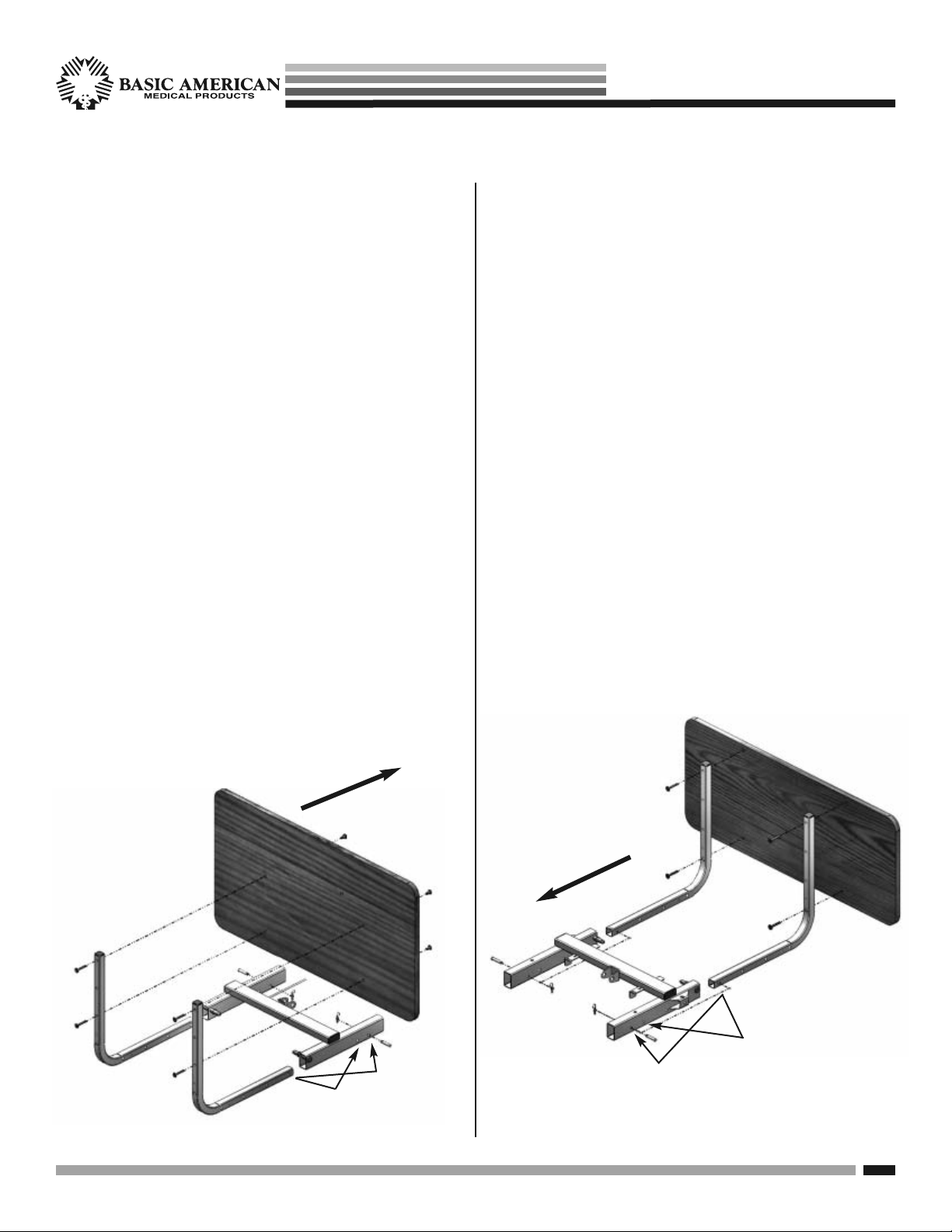
Zenith aps seRies
HEAD- AND FOOTBOARD ASSEMBLY
1. HEADBOARD INSTALLATION
• The headboard comes with four pre-installed
inserts - consider this the inside of the board.
• Position 2 mounting tubes on the outside of
the headboard with “L” facing inward.
• Align the top hole of the mounting tubes with
the top holes in the headboard.
• Insert a 40mm hex drive bolt through each of
the top holes and bottom holes and screw into
each insert. Tighten with the Hex Allen wrench
included in your kit.
• Slide the “L” portions of the Mounting tubes
into the hollow ends of the main frame rails,
at the head deck end.
• FOR 80” BEDS: Slide the mounting tubes in
until the 1ST hole in the tubes lines up with
the 1ST hole in the rails. See sample below.
• FOR 76” BEDS: Slide the mounting tubes in
until the 1ST hole in the tubes lines up with
the 2ND hole in the rails. See sample below.
2. FOOTBOARD INSTALLATION
• The footboard comes with four pre-installed
inserts - consider this the outside of the board.
• Position 2 mounting tubes on the inside of the
footboard with “L” facing outward.
• Align the top hole of the mounting tubes with
the top holes in the footboard.
• Insert a 40mm hex drive bolt through each of
the top holes and bottom holes and screw into
each insert. Tighten with the Hex Allen wrench
included in your kit.
• Slide the “L” portions of the Mounting tubes
into the hollow ends of the main frame rails,
at the foot deck end.
• FOR 80” BEDS: Slide the mounting tubes in
until the 1ST hole in the tubes lines up with
the 2ND hole in the rails. See sample below.
• FOR 76” BEDS: Slide the mounting tubes in
until the 1ST hole in the tubes lines up with
the 3RD hole in the rails. See sample below.
HEADBOARD FOOTBOARD
40mm
Bolts
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
1st
hole
CENTER
OF BED
80” hole
position
(1st hole in)
Inserts
on inside
76” hole
position
(2nd hole in)
40mm
Bolts
CENTER
OF BED
1st
hole
80” hole position
(2nd hole in)
76” hole position (3rd hole in)
NOTE: The first hole at the foot end is reserved
for attaching the Optional 4” pan extension.
Inserts
on outside
10
Page 11
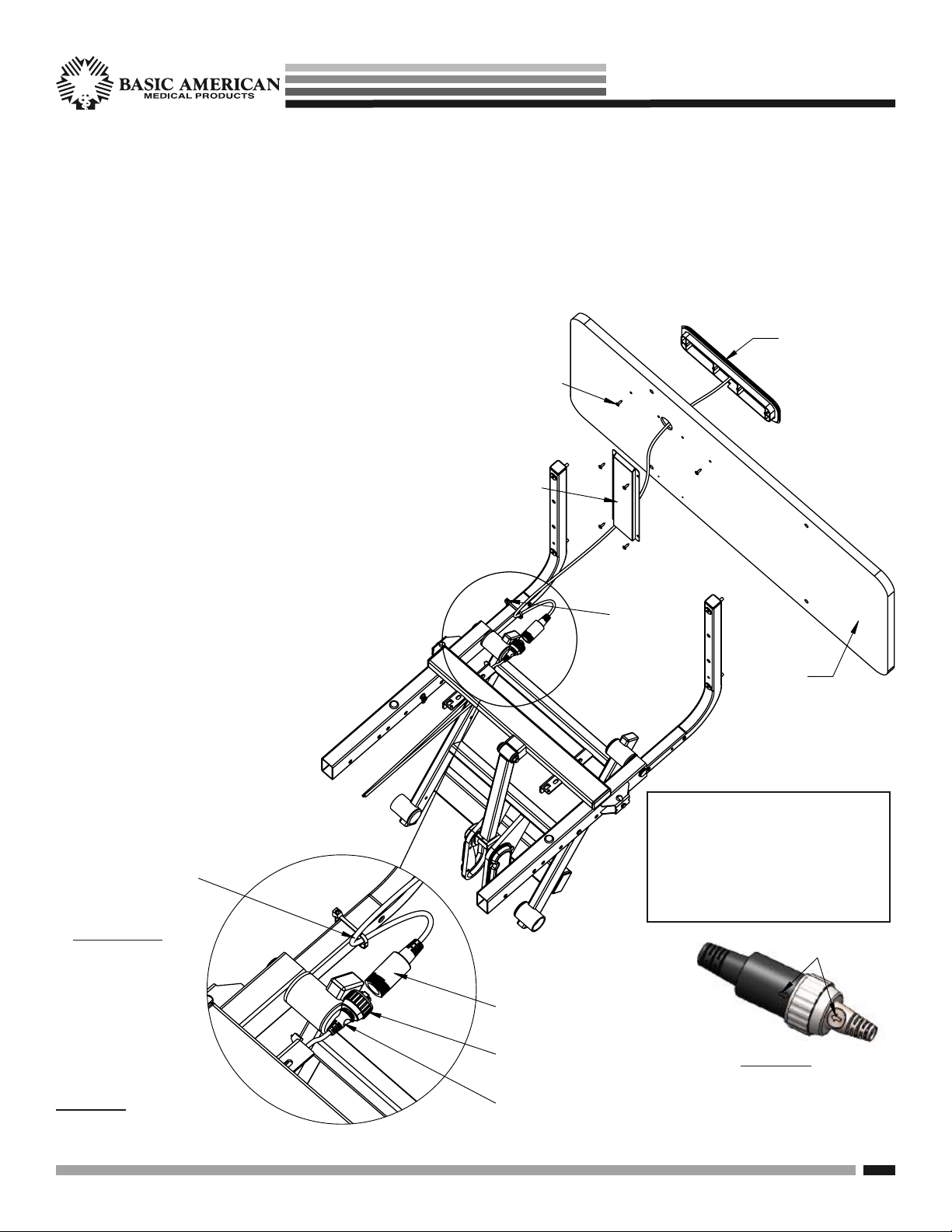
$OLJQ
$UURZV
3KLOOLSV6FUHZV
DWWDFK6KURXG
6WDII&RQWURO
$VVHPEO\IURP
LQVLGHRIERDUG
6WDII&RQWURO
$VVHPEO\
6KURXG
&DEOH&RYHU
)RRW%RDUG
ZLWKFXWRXW
&DEOH7LH
6WDII&RQWURO
$VVHPEO\
FDEOHWR
PRXQWLQJWXEH
)227
(1'
/RRS0DOH6WDII&RQURO&DEOH
DQGFDEOHWLHWKURXJKPRXQWLQJ
WXEHKROHZLWK)HPDOH6WDII
&RQWURO&DEOH
)HPDOHHQGRI6WDII&RQWURO
$VVHPEO\&DEOHSOXJVLQWR7&DEOH
(QGRI'RXEOH7&DEOHDWIRRWHQG*UH\
7UXQVRQHLWKHUVLGHRI6HDW3DQ
IRUDWWDFKLQJ\RXU+DQG&RQWUROOHU
/RFNLQJ(QG&DSRQ'RXEOH7&DEOH
Zenith aps seRies
PLUGGING IN THE FOOTBOARD NURSE/STAFF CONTROL
STEP 1 - ATTACHING THE FOOTBOARD
The APS 9000 bed features a Nurse/Staff Controller in the footboard, however the board is ordered separately with your
bed because of the variety of board styles available. If ordered at the same time as the bed, the Staff Control Assembly
and Shroud Cover will be pre-installed to the Footboard at the manufacturing factory.
STEP 2 - CONNECTING YOUR CABLES
Please refer to DETAIL A shown below.
a. If the Staff Control Assembly is not installed at the
factory you will need to first attach it to the footboard.
b. With the cutout side of the footboard facing outward
as shown, insert the staff control cable through the
large round hole.
c. With text right-side-up, insert the staff control
assembly into the cutout slot on the footboard
and attach to the board using the two outside
small holes and two screws from your staff
control hardware kit.
d. Attach the Shroud Cover over the cable on the
inside of the board using the four remaining
screws from your kit.
e. Insert the T-Cable end (extending out the
foot end with phone jack) into the round
plug, making sure the phone jack is
seated correctly inside the female plug
(arrow to arrow - see DETAIL B.)
f. Screw on the round lock cap onto
the Staff control’s female plug to
secure (see DETAIL B).
SEE PAGE 10 OF THIS
Loop Male Staff Control Cable
and cable tie through mounting
tube hole with Female Staff
Control Cable.
MANUAL FOR PROPER
ASSEMBLY OF ELBOW
MOUNTING BRACKETS
(TUBES) & FOOTBOARD
g. IMPORTANT
Make sure to tie
off the staff control
cable to the footboard mounting
tube with a cable
tie as shown.
DETAIL A
CLOSEUP OF STAFF
CONTROL CABLE CONNECTION
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
:
DETAIL B
:
MAKE SURE LOCK
END CAPS ARE
SCREWED ON
SECURELY
:
11
Page 12
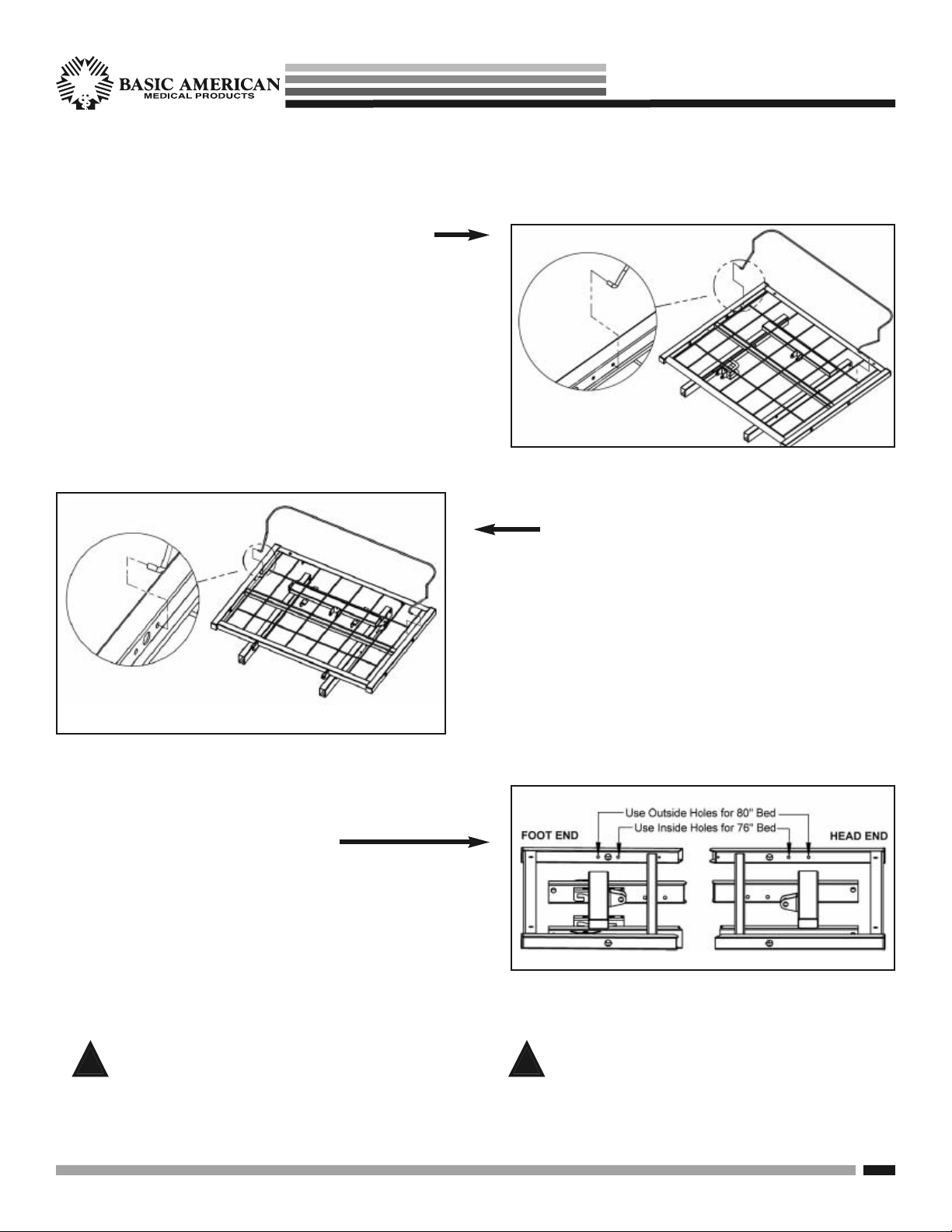
STANDARD MATTRESS RETAINER INSTALLATION
STEP 1. HEAD RETAINER INSTALLATION
1. Position retainer with curved end toward headboard.
2. Squeeze ends of the retainer toward the inside of
the bed and lower between the deck rails and first
outside grid wires.
3. Align the ends of retainer with small holes on the
inside of deck rails and slowly release.
Zenith aps seRies
STEP 2. FOOT-END RETAINER
INSTALLATION
STEP 3. RETAINER POSITIONS
• FOR 80” BEDS: Use outside small holes.
See sample at right.
• FOR 76” BEDS: Use inside small holes.
See sample at right.
1. Position retainer with curved end toward footboard.
2. Squeeze ends of the retainer toward the inside of
the bed and lower between the deck rails and first
outside grid wires.
3. Align the ends of retainer with small holes on the
inside of deck rails and slowly release.
Be sure to use a mattress that is properly sized to fit
!
the sleep deck, which will remain centered on the
deck relative to State and Federal Guidelines. Use
of an improperly fitted mattress could result in injury
or death.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Use a properly sized mattress in order to minimize
!
the gap between the side of the mattress and assist
devices. This gap must be small enough to prevent
resident/patient from getting his/her head or neck
caught in this location.
12
Page 13

:LUHIRUP:DOOVDYHU
%HQGIDFHVXSZDUG
'(7$,/$
6WDUWZLWKWDEKHOG
KRUL]RQWDOO\
'(7$,/%
(QGZLWK
:DOOVDYHU
UHVWLQJRQ
IORRU
STANDARD WIREFORM WALLSAVER INSTALLATION
STEP 1. WALLSAVER ASSEMBLY
1. Position the Wireform Wallsaver (Part 999-0844-180) with bent
end facing upward and tab ends facing inward as shown at right.
2. Determine the position desired (See bottom of page).
3. Gently squeeze the tab ends of the wallsaver inward toward the
center of the wallsaver and, holding the tabs parallel with the slots
in the caster bases (See DETAIL A), slide the tabs into the slots
while letting the wallsaver gently expand outward.
4. Turn the wallsaver downward until it rests on the floor (See
Detail B).
Zenith aps seRies
HEAD END
STEP 2. WALLSAVER
REMOVAL
1. To remove or move the Wireform
Wallsaver to a new position, raise
the wallsaver off the floor until
the end tabs are horizontal.
STEP 2. WALLSAVER
POSITIONS
1. The APS 9000 wallsaver features
2. For 76” beds, position the wallsaver
3. If you have an optional Trapeze unit
3 positions for easy reconfiguration
- 76”, 80”, and Trapeze (if you use
Trendelenberg/Reverse Trendelenberg positions please see optional
adaptor brackets on next page).
ends in the caster base slots closest
to the FOOT end of the bed. For 80”
beds, position the wallsaver ends
in the MIDDLE slots on the bases.
on a 76” bed, position the wallsaver
ends in the MIDDLE slots on the
caster bases. For 80” beds position
wallsaver in slots closest to HEAD.
Finish with
Wallsaver
resting on
floor
76” Bed Wallsaver
Position w/o Trapeze
FOOT END
2. Squeeze the ends toward the
center of the wallsaver until the
end tabs slide out of the caster
base slots.
76” Bed Wallsaver
Position w/ Trapeze or
80” Bed w/o Trapeze
80” Bed Wallsaver
Position w/ Trapeze
HEAD END
13
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 14

Zenith aps seRies
:LUHIRUP:DOOVDYHU
%HQGIDFHVXSZDUG
'(7$,/$
6WDUWZLWK
WDEKHOG
KRUL]RQWDOO\
'(7$,/%
(QGZLWK
:DOOVDYHU
UHVWLQJRQ
IORRU
OPTIONAL WALLSAVER ADAPTOR BRACKET INSTALLATION TO
USE WITH TRENDELENBERG/REVERSE TRENDELENBERG FEATURE
3
STEP 1. BRACKET
INSTALLATION
1. Remove the tall Lock Nut (#1)
from the head end of both the
right and left-hand caster bases.
2. Position the two saddle
adaptor brackets (#2
- 999-0831-002) so
that the ends with
slots face away from
the bases as shown.
4. Secure brackets to caster bases
using short Nylon Lock Nuts (#3
- 100-6744-002) included in your
adaptor bracket bag/kit.
3
2
2
1
3. Lower the brackets
onto the caster bases,
aligning holes in brackets
with caster stems.
1
STEP 2. WALLSAVER
ASSEMBLY
1. Position the Wireform Wallsaver
(Part 999-0844-180) with bent
end facing upward and tab ends
facing inward as shown at right.
2. Gently squeeze the tab ends of
the wallsaver inward toward the
center of the wallsaver and, hold
-ing the tabs parallel with the
slots in the caster bases (See
DETAIL A), slide the tabs into the
slots while letting the wallsaver gently expand outward.
4. Turn the wallsaver downward until it rests on the floor (See Detail B).
STEP 3. WALLSAVER REMOVAL
NOTE: MOUNTING OF THE ADAPTOR
BRACKETS IS ONLY NECESSARY IF YOU
PLAN ON USING THE TRENDELENBERG/
REVERSE TRENDELENBERG FEATURE.
Finish with
Wallsaver
resting on
floor
1. To remove or move the Wireform Wallsaver to a new position, raise
the wallsaver off the floor until the end tabs are horizontal.
2. Squeeze the ends toward the center of the wallsaver until the
end tabs slide out of the caster base slots.
IMPORTANT:
WALLSAVER END NEAREST THE WALL MUST FACE UPWARD
AS SHOWN FOR WALLSAVER TO SEAT PROPERLY IN SLOTS.
14
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 15

Zenith aps seRies
BED OPERATIONS - APS PENDANT/HAND CONTROLLER
BACKLIT PENDANT
1 HEAD DECK ANGLE UP BUTTON
2 HEAD DECK ANGLE DOWN BUTTON
3 HI/LO UP BUTTON (RAISE ENTIRE BED)
4 HI/LO DOWN BUTTON (LOWER ENTIRE BED)
5 KNEE & FOOT DECK ANGLE UP BUTTON
6 KNEE & FOOT DECK ANGLE DOWN BUTTON
7 SET TO CHAIR POSITION BUTTON
8 UNDO CHAIR POSITION BUTTON
9 OPTIONAL UNDERBED LIGHT BUTTON
NON-BACKLIT PENDANT
SAME FEATURES BUT WITHOUT
UNDERBED LIGHT BUTTON
OPERATION:
1. To angle the Head Deck upward, PRESS 1
2. To angle the Head Deck downward, PRESS 2
3. To raise the Bed Up horizontally, PRESS 3
4. To lower the Bed Down horizontally, PRESS 4
5. To angle the Knee/Foot Decks upward, PRESS 5
6. To angle the Knee/Foot Decks downward, PRESS 6
7. To put the Bed in the Chair position, PRESS 7
8. To release the Bed from the Chair position, PRESS 8
9. To turn the underbed light on or off (*optional pendant only)
PRESS THE LIGHT BULB ICON near the bottom of the
hand controller (not shown on sample at left).
Back of both
Pendants
9
BACKLIT PENDANT
Model #00HB851D-003
Part # 999-0806-301
Backlit and includes
Underbed Light Button
(button #9)
Non-Backlit Pendant
Model #00HB841A-001
Part # 999-0806-305
(without Underbed
Light Button)
include
attachment
hook.
A Pendant
holster also
comes
standard
with the
APS bed.
NOTE:
THE ZENITH 9000 APS BED COMES STANDARD WITH A FOOTBOARD NURSE/STAFF CONTROL
(PLEASE MAKE SURE TO ORDER WITH APPROPRIATE BOARD STYLE). THE BACKLIT PENDANT
WITH UNDERBED LIGHT BUTTON AND THE UNDERBED LIGHT CAN BE PURCHASED AS OPTIONS..
PLEASE SEE NEXT PAGE FOR NURSE/STAFF CONTROL OPERATING INSTRUCTIONS.
15
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 16

BED OPERATIONS - NURSE/STAFF CONTROL PANEL
Press “Underbed Light” button (#14) to turn underbed light on or off.
Press “CPR Heart” button (#7) to Level/Flatten the bed horizontally for CPR position.
“Safe Mode” (#15) LED light will glow green automatically once power cord is plugged in.
Green light will blink intermittently for approximately 10 minutes. After 10 minutes of nonactivity Hi/Lo Lock icons on the nurse/staff control panel will show orange (locked).
LOCK SYMBOLS LOCK SYMBOL
Press “Head”, “Knee”, or “Chair” and “Key” at the same time to unlock individual
lock out functions.
LOCK SYMBOLS
Zenith aps seRies
1 ALL LOCKED BUTTON
2 HEAD DECK UP BUTTON
3 HEAD DECK DOWN BUTTON
4 KNEE & FOOT DECK UP BUTTON
5 KNEE & FOOT DECK DOWN BUTTON
6 CHAIR POSITION BUTTON
7 CPR/UNDO CHAIR POSITION BUTTON
8 KEY LOCK/UNLOCK BUTTON
9 HI/LO (T/TR) LOCKED BUTTON
10 HI/LO UP BUTTON
11 HI/LO DOWN BUTTON
12 TRENDELENBERG
13 REVERSE TRENDELENBERG
14 UNDERBED LIGHT BUTTON
15 SAFE MODE RELEASE BUTTON
LOCK OUT SINGLE FUNCTIONS
To individually lock out the “Head”, “Knee”, “Chair”,
and “Hi/Lo” functions, press the appropriate top
icon (#2, 3, 4, 10, or 12) button and the “Key”
button at the same time. An orange LED lock
symbol will appear under the related icon.
To “Unlock” any of the individual functions, press
the top icon (#2, 3, 4, 10, or 12) and the “Key” Button
(#8) simultaneously. LED lights will not
show up.
Press “Hi/Lo Locked” to lock out “Hi/Lo”, “Trendelenberg”,
and “Reverse Trendelenberg” functions.
Press “Hi/Lo Locked” and “Key” at the same time
to unlock all Hi/Lo lock out functions.
Press “All Locked” and “Key” at
the same time to unlock all functions.
LOCK SYMBOLS
LOCK SYMBOLSLOCK SYMBOLS LOCK SYMBOL
LOCK OUT HI/LO FUNCTIONS
To lockout the functions for raising and lowering
the entire bed and tilting the bed for Trendelenberg
positions, press the “Hi/Lo Locked” (#9) button. Orange
LED lock symbols will appear under the icons.
To “Unlock” all Hi/Lo functions, press the “Hi/Lo
Loc ked” bu tton (# 9) and the “Ke y” butt on (#8)
simultaneously. Orange LED lights will not
LOCK OUT EVERYTHING
To lock out all functions, press the “All Locked”
button (#1). Orange LED lock symbols will appear
under the “Head”, “Knee”, “Chair”, “Hi/Lo”, and
“Trendelenberg/Reverse Trendelenberg” icons.
To “Unlock” all functions, press the “All Locked”
button (#1) and the “Key” button (#8) simultaneously.
Orange LED lights will not show up.
SAFE MODE:
Green light will show automatically when bed is
plugged in and will blink intermittently during 10
minute “sleep mode”. After 10 minutes of lapsed
time since the last function was used, the LED light
shows green and Hi/Lo Lock icons will show orange.
show up.
The LED will be lit green when Safe Mode automatically engages
when plugging in the bed. Press “Safe Mode” and “Key” to disable
the Safe Mode feature.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
To manually disable the function, hold the “Safe
Mode” button (#15) and “Key” button (#8) at the
same time. Green LED light will go out.
16
Page 17

Zenith aps seRies
BED OPERATIONS - CHAIR POSITION
3
2
HEAD
END
1
1. Make sure the Chair position on the staff
control panel is not locked out (orange
lock icon). To unlock, press the Key icon #1
and Chair icon #2) (or All Locked button
#3) at the same time.
4
2. To move the bed to the Chair position
(foot & knee decks angled up, head deck
angled up, and head of bed tilted upward),
press on the chair icon button (#2).
To release the chair feature and return
the bed back into a horizontal position,
press the CPR icon (#4). The Chair feature
can also be operated using the Pendant.
(See page 15.)
BED OPERATIONS - CPR/HEART POSITION
3
(The CPR button places the bed in a horizontal position)
2
This illustration shows bed all the way down as a reference only -
the CPR button will not lower the bed.)
1
CPR POSITION
FOOT
END
1. Make sure the bed positions on the staff
control panel are not locked out (orange
lock icon). To unlock, press the Key icon (#1)
and the All Locked button (#3) at the same
time.
2. To smoothly move the bed into a prone,
horizontal position for CPR access, press
and hold the CPR/Heart icon button (#2).
17
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 18

75(1'(/(1%(5*
)227
(1'
5(9(56(75(1'(/(1%(5*
+($'
(1'
)227
(1'
+($'
(1'
Zenith aps seRies
BED OPERATIONS - TRENDELENBERG/REVERSE TRENDELENBERG
1. Make sure the Hi/Lo function on the staff
control panel is not locked out (orange
lock icon). To unlock, press the Key icon #1
and Hi/LO Locked button #2 at the same
time.
2. To move the bed to the Trendelenberg
position (foot end up), press on button #3.
3. To move the APS bed into the Reverse
Trendelenberg position (head end up),
press on button #4.
1 2 3
4
NOTE: THE TRENDELENBERG AND REVERSE
TRENDELENBERG FEATURE CAN ONLY BE
ACCESSED USING THE STAFF CONTROL. IT
CANNOT
BE ACCESSED USING THE HAND
CONTROLLER/PENDANT.
18
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 19

Zenith aps seRies
APS 9000 SERIES ELECTRICAL COMPONENTS (LINAK)
Control/Junction Box
Part # 999-0831-300
Quantity = 1
Power Cable plugs
into side port (part
listed at bottom)
Head Motor/Actuator
Part # 999-0822-052
Quantity = 1
Foot Motor/Actuator
Part # 999-0822-053
Quantity = 1
STANDARD:
Pendant/Hand Controller
with Underbed Light Button
Part # 999-0806-301
Quantity = 1
Bed also includes
Pendant Holster
Part # 999-0791-000
Quantity = 1
Double “T” Cable
Part # 999-0806-200
Quantity = 1
Motor Cable (Hd & Hi/Lo)
Part # 999-0806-203
Quantity = 3
Hi/Lo Motor/Actuator
Part # 999-0831-051
Quantity = 2
STANDARD:
3 Prong Power Cable
Part # 999-0775-208
Quantity = 1
OPTIONAL:
2 Prong Power Cable
Part # 999-0775-206
Quantity = 1
OPTIONAL UNDERBED LIGHT
Underbed Light Adaptor Cable
Part # 999-0806-212
Quantity = 1
DO NOT use unauthorized parts, accessories, or adaptors other than those specified/authorized
!
by GF Health Products, Inc. DO NOT open assemblies such as the Actuators, Hand Control, or
Control Box. If unauthorized personnel perform work on these components, the manufacturer’s
Motor Cable (Ft.)
Part # 999-0806-202
Quantity = 1
Battery Backup Cable
Part # 999-0831-200
Quantity = 1
Underbed Light
Part # 999-0806-220
Quantity = 1
warranty becomes void. NEVER operate the bed if a Power Cord or Plug is damaged or not working
properly. Contact qualified Service Personnel for examination and repair. Always unplug the Power
Cord when performing any maintenance on the bed.
19
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 20

APS9000 SERIES ELECTRICAL CABLING - STANDARD
u
POWER CABLE
v
T-CABLE
w
HEAD CABLE
x
FOOT CABLE
y
HI/LO CABLE (FOOT)
z
HI/LO CABLE (HEAD)
{
BATTERY BACKUP CABLE
|
GROUND WIRE
Portable
Battery
Cable
Extension
v
Zenith aps seRies
u
u
x
|
v
Foot
Head
T-Cable
Hi/Lo (Ft)
Hi/Lo (Hd)
T-Cable (HB)
Hi/Lo (Ft) - Port 4
Foot - Port 3
Hi/Lo (Hd) - Port 2
Head - Port 1
Power Cable &
Battery Cable Side of Control Box
Ground
Wire
Power Cable
w
z
y
{
u
{
v
20
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 21

Zenith aps seRies
APS9000 SERIES ELECTRICAL CABLING - WITH UNDERBED LIGHT
NOTE: Cabling for Beds with Underbed Light feature
u
POWER CABLE
v
T-CABLE
w
HEAD CABLE
x
FOOT CABLE
y
HI/LO CABLE (FOOT)
z
HI/LO CABLE (HEAD)
{
BATTERY BACKUP CABLE
|
GROUND WIRE
}
UNDERBED LIGHT CABLE (UBL)
Portable
Battery
Cable
Extension
is identical to Standard APS beds, except that the
T-Cable plugs into Port 2 in the Underbed Light Box
and an extra Extension Cable is plugged into Port 1
on the Underbed Light Box and the HB Port in the
Control Box.
v
u
|
x
z
w
y
{
v
Ground
Wire
Power Cable
Foot
Head
Hi/Lo (Ft)
UBL-Cable
T-Cable - Port 2 in Underbed Light Box
Hi/Lo (Ft) - Port 4 in Control Box
Foot - Port 3 in Control Box
Hi/Lo (Hd) - Port 2 in Control Box
Head - Port 1 in Control Box
Power Cable &
Battery Cable Side of Control Box
Hi/Lo (Hd)
Light
UNDERBED
LIGHT (UBL)
v
{
v
T-Cable
}
}
UBL Cable
21
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 22

Zenith aps seRies
APS9000 SERIES ELECTRICAL CABLING - WITH
UNDERBED LIGHT & BATTERY
u
POWER CABLE
v
T-CABLE
w
HEAD CABLE
x
FOOT CABLE
y
HI/LO CABLE (FOOT)
z
HI/LO CABLE (HEAD)
{
BATTERY BACKUP CABLE
|
GROUND WIRE
}
UNDERBED LIGHT CABLE (UBL)
Mounted
Battery
Cable
w
u
v
|
{
{
z
y
x
v
}
Ground
Wire
Power Cable
Foot
Head
Hi/Lo (Ft)
UBL-Cable
NOTE: Cabling for Beds with Underbed Light and Mounted
Battery Backup features are identical to Standard APS beds,
except that the T-Cable plugs into Port 2 in the Underbed
Light Box and an extra Extension Cable is plugged into Port 1
on the Underbed Light Box and the HB Port in the Control Box.
A Battery Cable runs from the Control Box directly to an
external Battery Pack at the foot end.
Hi/Lo (Hd)
Underbed Light (UBL)
{
Attached
Battery
Cable
MOUNTED
BATTERY
OPTION
22
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 23

+($''(&.
6/((3685)$&(
+($'
'(&.
(1'
+($'
'(&.
(1'
+($'
(1'
5HFRPPHQG
3LYRW7RZDUG
+HDGERDUG
%ODFN.QREWR
/RFNRU5HOHDVH
+($'
(1'
E
E
D
D
OPTIONAL PIVOT ASSIST BAR INSTALLATION & OPERATION
INSTALLATION
Zenith aps seRies
1. At the head end of the bed, position the Pivoting Assist Bar (#2)
bracket over the Head Deck (#1) frame and align the two holes
in the Assist Bar bracket with the two holes in the frame rail on
either side of the head deck.
2. From the outside, insert the Clevis Pins (#3) through the
holes in the pivot bracket and deck frame.
3. From the inside, insert a Hair Pin Clip (#4) through
each of the small holes in the clevis pins.
PLEASE ORDER KIT #ZA85400. KIT INCLUDES ONE PIVOTING
ASSIST BAR ASSEMBLY AND TWO LANYARD ASSEMBLIES
WITH CLEVIS PINS AND HAIR PIN CLIPS. (A STATIONARY ASSIST
BAR IS ALSO AVAILABLE AS AN OPTION - KIT # ZA85000).
THE ASSIST BAR CAN BE POSITIONED ON EITHER
THE RIGHT SIDE OR LEFT SIDE OF THE HEAD DECK.
IT IS NOT OFFERED FOR THE FOOT END OF THE BED.
PIVOTING ASSIST BAR OPERATION
Position the Assist Bar
Bracket ON TOP of the
Deck Frame, aligning
holes in the bracket with
holes in the frame.
1
1. To release the pivoting assist bar from its
vertical lock position, hold onto the top of
the bar with one hand and slightly pull out
the black knob on the outside of the pivot
assembly with the other hand to release
the locking mechanism. For ease of patient
access, it is recommended that you pivot
the rail toward the headboard until it stops
and rests in place.
2
3
3
4
4
SAMPLE SHOWS PIVOTING
ASSIST BAR POSITIONED ON
THE LEFT SIDE OF THE BED.
2. To set the assist bar back to its upright
locked position, grab the top of the assist
bar with one hand and pivot the assembly
upward until the black knob mechanism
snaps into place, locking the assist bar
vertically.
FOR YOUR SAFETY, MAKE SURE YOUR FINGERS
! !
ARE CLEAR OF THE SIDES OR UNDERNEATH THE
PIVOTING ASSEMBLY WHEN PIVOTING THE
ASSIST BAR UP OR DOWN.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
PLEASE MAKE SURE THE PIVOT ASSIST BAR
IS ALWAYS IN THE FULL, VERTICAL, LOCKED
POSITION WHEN RESIDENT/PATIENT IS LEFT
UNATTENDED.
23
Page 24

Zenith aps seRies
0DLQ)UDPH
+($'
(1'
OPTIONAL TRAPEZE SUPPORT INSTALLATION
(The Trapeze Support is attached to the head section of the bed.)
Please Order Kit Number ZA79000
Trapeze Support vertical
support tube. (TMI Bar
Sleeve would be inserted
inside the tube.)
Use first holes in
Trapeze Support
to mount to main
frame tubes.
Use third hole
from the end of the
main frame tubes.
USE SAME CLEVIS PINS AND HAIR PIN CLIPS
THAT WERE USED TO CONNECT HEAD BOARD
TO MOUNTING TUBES.
1. Begin by removing the headboard panel assembly (#2),
if previously assembled to the bed, by pulling out the
two Clevis Pins and two Hair Pin Clips that attach the
main frame tubes to the headboard’s mounting tubes.
Set all pins aside for later use.
2. Detach the headboard panel from the mounting tubes
by unscrewing the four 40mm Allen Head Bolts that
hold the board to the tubes (these will be replaced
with the longer Phillips Head Pan Screws from your
trapeze adaptor hardware bag). Do not remove the
four 1/4-20 inserts (#3) from the board. They will be
used again to mount the board to the trapeze support.
3. Assemble the Trapeze Support (#1) to the main frame
tubes using the two Clevis Pins and Hair Pin Clips you
removed in Step 1. The first holes in the Trapeze tubes
must line up with the third hole from the end in the main
frame tubes as shown.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
4. Insert the 1/4-20 x 2.50” Carriage Bolt (#5) into the vertical
support as shown and fasten with a 1/4-20 Hex Head Lock
Nut (#6). Both parts should be in your hardware bag.
5. Assemble the headboard to the inside of the Trapeze
Support so that the side of the headboard with the inserts
is facing away from the Trapeze Support. From the outside,
insert the four 1/4-20 x 1.75” long Machine Screws (#4)
from your hardware bag through the holes in the Trapeze
Support, screwing them into the inserts in the headboard.
6. OPTIONAL - FOR TMI TRAPEZE BAR WITH SLEEVE:
Insert the Sleeve into the vertical support with the slot on
the bottom. Rotate the sleeve until it falls into place over
the carriage bolt you inserted in Step 4. This will lock it into
the proper position. (NOTE: The top of the Sleeve should
sit flush with the top of the vertical support on the
Trapeze Support Adaptor.)
24
Page 25

Zenith aps seRies
OPTIONAL BATTERY BACKUP REPLACEMENT CONFIGURATIONS
Configuration 1:
Replacing Single Battery
Cable for a “Portable”
Battery Backup Unit
1. Replacing your existing battery backup
cable (Part 999-0711-001) is fairly simple.
First, if your external battery unit is hooked
up, unplug it from the foot-end battery
cable plug (#1).
2. Carefully cut all cable ties (#2).
3. Unplug the existing cable from the Control
Box (#3). Plug in the new cable and run
along same path as previous cable.
4. Use cable ties to re-secure to tie rod and
mounting brackets (#2).
Configuration 2
:
Replacing Mounted Battery Backup Unit (includes attached battery cable)
1. If your APS bed came with a mounted Battery
Backup Pack (Part 999-0711-003) you must
first unplug the power cord from the wall unit,
then unplug the attached battery cable from
the Control Box. Carefully cut all cable ties
along the battery cable.
2. Using a 3/8” socket wrench, remove the two Nylon Lock Nuts
(#6 - Part 100-6725-003) that secure the Battery Mounting
Bracket (#5 - Part 999-0831-001) to the welded Main Frame
Bracket (#4). Remove the bolts (#7 - Part 100-5225-009) and
set aside.
3. Once the Bracket and Battery Pack (#9) are free, remove the
pack from the bracket by unscrewing the four Phillips Head
Screws (#8 - Part 100-5414-001). Place the new battery pack
on the bracket and reverse the process to reattach bracket
and cable.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
25
Page 26

TROUBLESHOOTING - NOTHING WORKS - NO POWER
1. NOTHING WORKS - NO POWER
a. Check to make sure you have power coming from your
outlet. Unplug the power cable from the outlet and test
the outlet by plugging in a lamp or similar portable device.
OUTLET WORKS = move to Step b.
b. Replug the power cord into the outlet -
NOTHING WORKS - UNPLUG THE POWER CORD
and check the power cord from the head end to the control
box, making sure it is not pinched, frayed, or damaged
in any way.
Zenith aps seRies
Test Outlet for Power.
Unplug Power Cord
and check entire cord
to make sure it is not
pinched, frayed, or
damaged.
- POWER CORD IS PINCHED = With cord unplugged,
try to move the bed part slightly to release the pinched
cord. If you can release the cord, re-plug the power
cord into the wall outlet and test the bed.
- BED WORKS = Make sure the cord is not frayed or
exposed. If it is OK, you should not have to replace.
- POWER CORD IS DAMAGED = Cut cable ties and
immediately replace Power Cord.
ALWAYS UNPLUG THE POWER CORD BEFORE DOING
ANY ELECTRICAL WORK ON YOUR BED.
!
TROUBLESHOOTING STAFF CONTROL - BLINKING LIGHTS
1. STAFF CONTROL LIGHTS ARE BLINKING
NOTHING ON STAFF CONTROL WORKS
BUT PENDANT WORKS
a. This could mean that your staff control is not getting
enough power from the Control Box.
b. Unplug the power cord from the wall outlet. Unplug
the staff control cable from the T-Cable at the foot
end of the bed.
3-Prong Power Cable
999-0775-208
2-Prong Power Cable
999-0775-206
IF 3-PRONG (OR OPTIONAL 2 PRONG) POWER CORD
IS DAMAGED, REPLACE IMMEDIATELY!
c. If you have a spare staff control assembly, plug the
cable into the T-Cable at the foot end and test.
- WORKS AND LIGHTS NO LONGER BLINK =
Replace the Staff Control Assembly in your foot-
board. (See page 29 for dis-assembly. Please
see page 32 for order number.)
- DOESN’T WORK & LIGHTS STILL BLINKING =
Replace the Control Box. (Please see Page 31
for order number).
26
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 27

Zenith aps seRies
NOTHING ON STAFF CONTROL WORKS BUT PENDANT WORKS
a. Check the Staff Control connections at the foot end. Is the
Staff Control Cable plugged securely into the T-Cable? Also
check if the other end of the T-Cable is plugged securely
into the Control Box (or Underbed Light). If the STAFF
CONTROL STILL DOESN’T WORK = go to step b.
b. Unplug the Power Cord from your wall outlet. Remove the
Cable Cover (Shroud) on the inside of the footboard and
locate the single terminal end that plugs directly into the
back of the Staff Control Panel. Make sure it is properly
seated into the panel. Plug in the power cord and test the
staff control. If the STAFF CONTROL STILL DOESN’T
WORK = Replace the Staff Control Assembly. (See page
29 for dis-assembly. See page 32 for order information.)
TROUBLESHOOTING PENDANT & DOUBLE T-CABLE
1. STAFF CONTROL WORKS BUT PENDANT/
HAND CONTROLLER FUNCTION DOESN’T
a. Check to see if the functions are locked out on the Staff
Control panel - if orange lock icons show on panel then
that function is locked out. Press the Key and Function
(i.e. Head) button at the same time until the orange lock
icon goes out. Test the function. If the PENDANT STILL
DOESN’T WORK = go to step b.
b. Check the connections at the seat pan (Pendant/Hand
Controller to T-Cable) and Staff Control Cable connection
to the T-Cable at the foot end making sure plugs are fully
engaged. Also check the T-Cable connection at the Control
Box. If you have an Underbed Light feature, make sure all
cables are secure. If the PENDANT STILL DOESN’T
WORK = move to step c.
c. UNPLUG THE POWER CORD FROM THE WALL OUTLET.
d. Unplug the Pendant/Hand Controller plug from the end of
the T-Cable on either side of the beds Seat Pan. Unplug
the Staff Control Cable from the end of the T-Cable at the
foot end of the bed and unplug the other end from the
Control Box.
If orange lock icon lit, press key symbol and function to unlock.
Make sure plugs at foot
B
Make sure plugs
are fully seated
(O-ring does
not show).
end are fully seated
and cap locked on.
Unplug Staff Control
Cable from T-Cable
at foot end.
C
Unplug
Power
Plug
before
working
on bed
electonics.
A
B
D
e. Plug the Pendant/Hand Controller plug directly into the HB
port on the Control Box, making sure it is fully seated.
Plug in the Power Cord and test the Pendant.
- ALL BUTTONS WORK = Replace the T-Cable
(See page 32 for order information.)
- NOTHING WORKS = Replace the Hand Controller.
(See page 31 for order information.)
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Plug Pendant Cable directly into HB port on Control Box
E
(or into port 2 in Underbed Light Box) and test.
E
27
Page 28

TROUBLESHOOTING MOTOR CABLES & CONTROL BOX
1. HEAD, FOOT, OR HI/LO FUNCTION
NOT WORKING
a. Check plugs to make sure they are firmly seated in all ports
on your Control Box and are plugged into the correct ports
(See diagram at right).
b. Check all cables to make sure they are not frayed, pinched,
or damaged in any way. If any cable is damaged, UNPLUG
YOUR BED’S POWER CORD FROM THE WALL OUTLET
and REPLACE THE CABLE AND/OR THE ELECTRONIC
COMPONENT.
c. HEAD DECK NOT RAISING - Unplug the power cord. Switch
the head and foot plugs (ports 1 and 3). Re-plug the power
cord and press the FOOT button to test.
- HEAD WORKS = Control Box head port bad.
Replace box (See page 31 for order information.)
Zenith aps seRies
POWER PLUG
BATTERY
HB43
2
1
- DOESN’T WORK = Replace the Head Motor.
(See page 31 for order information.)
d. FOOT AND KNEE DECKS NOT RAISING - Unplug the
power cord. Switch the foot and head plugs (ports 3 and 1).
Re-plug the power cord and press the HEAD button to test.
- FOOT WORKS = Control Box foot port bad.
Replace box (See page 31 for order information.)
- DOESN’T WORK = Replace the Foot Motor.
(See page 31 for order information.)
e. BED DOES NOT GO UP AND DOWN IN HORIZONTAL
POSITION - If you press the Up or Down Hi/Lo button and
you hear a click or buzz but nothing happens = Check your
Hi/Lo Motors by touching them & pushing the Hi/Lo buttons.
- YOU FEEL NOTHING = possible bad motor - go to the
third step below.
- YOU FEEL SLIGHT VIBRATION = Motor OK.
- Unplug the Power Cord = Switch the two Hi/Lo plugs
(ports 4 and 2) in the Control Box. Re-plug the power cord
and press the same Hi/Lo button, then touch the same
non-vibrating motor.
FOOT
T-CABLE
FOOT HILO
HEAD
HEAD HILO
2. BED LOOKS UNLEVEL - HILO MOTORS
ARE NOT IN SYNC.
a. Press the Hi/Lo “DOWN” button repeatedly on either
the Staff Control or Pendant/Hand Controller until
the bed goes completely down and rests in its full
horizontal position. The two HI/LO motor shafts
should be completely retracted inside the motors.
QUICK REFERENCE
- HI/LO & FOOT WORKS, BUT HEAD DOES NOT:
Switch Head & Foot Motor Cable at the Control Box
and test (See Step C in left column).
- HI/LO & HEAD WORKS, BUT FOOT DOES NOT:
Switch Foot & Head Motor Cable at the Control Box
and test (See Step D in left column).
i. MOTOR NOW VIBRATES = Bad port in the
Control Box - Replace box. (See page 31 for
order information.)
ii. MOTOR DOES NOT VIBRATE = Replace
the bad Hi/lo Motor. (See page 31.)
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
- HEAD & FOOT WORKS, BUT HI/LO DOES NOT:
a. Switch Head and Foot Hi/Lo Motor Cables at the
Control Box and test (See Step E in left column).
b. Test to see if Hi/Lo Motors need to be re-aligned
(See Step F above).
28
Page 29

Zenith aps seRies
6WDII&RQWURO
%HYHO
6FUHZV
6WDII&RQWURO
$VVHPEO\
)RRW%RDUG
ZLWKFXWRXW
6KURXG6FUHZV
6WDII&RQWURO
%HYHO6FUHZV
,03257$175XQD&DEOH
7LHWKURXJKWKHHQGKROHLQ
WKHPRXQWLQJWXEHORRSWKH
H[FHVVFDEOHUXQWKHWLH
DURXQGFDEOHDQGSXOOWLJKW
WRVHFXUH&XWRIIH[FHVV
FDEOHWLH
)HPDOHHQG
RI6WDII&RQWURO
&DEOH
(QGRI'RXEOH7&DEOH
DWIRRWHQG*UH\
7UXQVRQHLWKHU
VLGHRI6HDW3DQ
IRUDWWDFKLQJ\RXU
+DQG&RQWUROOHU
/RFNLQJ
(QG&DS
TROUBLESHOOTING/REPLACING YOUR APS STAFF CONTROL
REMOVING AND REPLACING THE
STAFF CONTROL ASSEMBLY
1. UNPLUG THE POWER CORD FROM
THE WALL OUTLET.
2. Unscrew the four Phillips Screws (#1) that attach the
Shroud (cable cover) to the footboard and set aside.
3. At the foot end of the bed, unscrew the retaining
cap on the T-Cable (#3) and unplug the two cables.
4. Unscrew the two remaining Phillips Screws (#2)
from the inside of the footboard that attach the
Staff Control Assembly bevel to the board. Set
aside screws for later re-assembly.
5. Remove the OLD Staff Control Assembly and fish
the old cable out through the large hole.
6. Feed the cable of the NEW Staff Control
Assembly from the outside through the
large hole and insert the bevel into the
cutout on the outside of the footboard.
7. Secure the Staff Control by
re-inserting the two Phillips
Screws (#2) you removed
in Step 4.
8. Re-position the Shroud on
the inside of the footboard
and secure with the four
Phillips Screws (#1) you
removed in Step 2.
9. Plug the T-Cable (#5) into the Staff Control
Cable (#4), making sure the phone jack plug
is seated correctly into the Staff Control cable plug.
Screw on the retaining cap (#3) to secure.
10. Run a Cable Tie through the large end hole on
the right-hand footboard mounting tube.
11. Loop the excess Staff Control Cable. Run the
cable tie around the cable and pull tight to secure.
Cut off any excess Cable Tie.
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
29
Page 30

Zenith aps seRies
RECOMMENDED MAINTENANCE AND INSPECTION
} Maintenance Inspection of All
Components (Receipt of shipment
• Make sure all parts/components are included
(Please see page 9 - “Unpacking Your Bed”).
• Check all bed components for obvious damage.
• Inspect the Power Supply Cord for any cuts and/or damage.
• Check to see all actuator cords are routed and connected
properly to the control box.
)
} Mechanical Inspection of Assemblies
(6 months)
• Inspect all welds on the sleeping surface, frame, and base
assemblies for stress fractures.
• Inspect all fasteners for wear and looseness.
• IMPORTANT: Lubricate all pivot points, actuator/motor
clevis pins, and control arm clevis pins as needed. White
Lithium Grease is recommended.
} Mechanical Inspection of Casters
& Pedal Locking Mechanism
(3 months)
• Check the pedal locking mechanism to make sure it engages
and disengages properly.
} Electrical Inspection of Control Box
(6 months)
• Check the external power cord that plugs into the control
box for any chafing cuts or wear.
• Make sure all attaching hardware is securely tightened.
• Check all electrical connections for any wear or fractures.
• Check your external or mounted battery (see page 24)
Replace if needed.
} Electrical Inspection of Pendant
and Staff Control (6 months)
• Check the pendant cord for any chafing, cuts, or wear.
• Check all pendant functions . . .
P Head raises and lowers properly
P Foot raises and lowers properly
P Entire bed raises and lowers properly
P Bed moves to Chair position properly
P Bed moves to Trendelenberg and
Reverse Trendelenberg positions properly
• Check to make sure each button and associated function
work properly (i.e. the head section raises when the Head
Up button is activated).
• Check the bottom foot pads on the foot end casters, making
sure they are clean of debris and undamaged. Replace if needed.
• Check all casters to ensure that they roll properly and
are unobstructed.
} Electrical Inspection of Actuators/Motors
(6 months)
• Check the actuator/motor cords for any chafing, cuts, or wear.
• Check the range of movement on all motors to ensure they
do not bind in the Full Up and Full Down positions.
WARRANTY
- 15 Years on Frame
- 5 Years on Motors and Control Box
(longest motor warranty in the industry)
- 3 Years on Other Electronics
The warranties contained herein contain all the representation and warranties with respect to the subject matter
of this document, and supersede all prior negotiations, agreements and understanding with respect thereto.
The recipient of this document hereby acknowledges and represents that it has not relied on any representation,
assertion, guarantee, warranty, collateral contract or other assurance, except those set out in this document.
30
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 31

Zenith aps seRies
SERVICE/REPLACEMENT PART RECORD (Page 1)
DECKING
Part Number Description QTY Order Date Price
999-0844-920SP Head/Back Deck & Hardware Pack 1 Deck
999-0844-930SP OR - Foot Deck & Hardware Pack 1 Deck
999-0844-940SP OR - Knee Deck & Hardware Pack 1 Deck
Deck Hardware Pack - INCLUDES . . . 1 Pkg
4 Clevis Pins
5 5/16” Retaining Rings (Deck Brackets)
2 12mm Retaining Ring (Cntrl Arms to Decks)
1 Installation Instruction Sheet
MOTORS (ACTUATORS)
Part Number Description QTY Order Date Price
999-0831-051SP Hi/Lo Motor (Cable not included) & Hardware Pack 1 Motor
999-0822-052SP OR - Head Motor (Cable not included) & Hardware Pack 1 Motor
999-0822-053SP OR - Foot Motor (Cable not included) & Hardware Pack 1 Motor
Motor Hardware Pack - INCLUDES . . . 1 Pkg
2 12mm Retaining Rings
2 Hair Pin Clips
2 Snap Rings
8 Standard Cable Ties
1 Installation Instruction Sheet
999-0806-200 Head and Hi/Lo Motor Cables 1 per Motor
999-0806-202 Foot Motor Cable 1 Cable
CONTROL BOX & POWER CABLES
Part Number Description QTY Order Date Price
999-0831-300
999-0775-208
999-0775-206
Standard 3 Prong Power Cable Ground Wire 1 Cable
Linak Control Box and Clip 1 Box
Optional 2 Prong Power Cable 1 Cable
PENDANT/HAND CONTROLLER
Part Number Description QTY Order Date Price
999-0806-301 Backlit Pendant with Underbed Light button 1 Pendant
999-0791-000
999-0806-305 Non-Backlit Pendant (no Underbed Light button) 1 Pendant
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Pendant Holster
1 Holster
31
Page 32

Zenith aps seRies
SERVICE/REPLACEMENT PART RECORD (Page 2)
DOUBLE T-CABLE
Part Number Description QTY Order Date Price
999-0806-200SP Double “T” Control Cable 1 Cable
Deck Hardware Pack - INCLUDES . . . 1 Pkg
2 Split Stem Cable Retainer
4 Push Cable Ties
8 Standard Cable Ties
1 Installation Instruction Sheet
UNDERBED LIGHT
Part Number Description QTY Order Date Price
999-0806-220SP Underbed Light Unit 1 Motor
Motor Hardware Pack - INCLUDES . . . 1 Pkg
12 Adaptor Cable - 999-0806-212
4 Standard Cable Ties
1 Attachment Material
1 Installation Instruction Sheet
STAFF CONTROL
Part Number Description QTY Order Date Price
999-0831-901
ZL831000
PLEASE SEE
PAGE 11
FOR
INSTALLATION
INSTRUCTIONS
Switch Pad with Bezel & Cables 1 Unit
Staff Control Service Pack - INCLUDES . . . 1 Pkg
1 Switch Pad with Bezel & Cables
1 Shroud (Cable Cover)
6 #6 Phillips Head Truss Screws
3 4” Nylon Cable Tie
1 Installation Instruction Sheet
CAPS & PLUGS
Part Number Description QTY Order Date Price
NOTE: CAPS & PLUGS CAN ONLY BE PURCHASED IN SETS OF 12
100-4200-004PK
100-4700-017PK 1.00” x 2.00” Rectangular Caps Pack 12/pkg
1.25” x 1.25” Square Caps Pack
12/pkg
100-4700-018PK 1.25” x 2.00” Rectangular Caps Pack 12/pkg
100-4715-011PK 1.50” Round End Caps Pack (cover arm bearings) 12/pkg
999-0775-001PK Half Moon End Caps Pack (caster bases) 12/pkg
100-4762-002PK 5/8” Round Plugs Pack (both sides of foot deck) 12/pkg
100-4738-005PK 3/8” Round Plugs (on main frame rails - foot end) 12/pkg
FOR GENERAL CAP/PLUG DIAGRAMS PLEASE SEE PAGES 36 & 37
32
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 33

Zenith aps seRies
SERVICE/REPLACEMENT PART DIAGRAM - ELECTRONICS
FOOT-END
HI/LO MOTOR
CONTROL BOX ATTACHED TO MOTOR
END OF
BATTERY CABLE
(BLACK)
LONGER SIDE OF T-CABLE
WITH PUSH CABLE TIE IN
SMALL HOLE
FOOT/KNEE
MOTOR
HEAD
END
POWER CABLE
PLUG AT HEAD END
HEAD-END
HI/LO MOTOR
FOOT-END
OF T-CABLE
(GREY)
FOOT
END
LONG “T” OF T-CABLE
AND BATTERY CABLE
SECURED TO ROD
AND MAIN FRAME
BRACKET WITH
CABLE TIES
FOOT MOTOR
SHORTER SIDE OF T-CABLE WITH
PUSH CABLE TIE IN SMALL HOLE
CABLE PROTECTION GROMMET
IN SEAT PAN KEY HOLE
POWER CABLE SECURED TO ROD
WITH CABLE TIES
1. TO ORDER A REPLACEMENT
HI/LO MOTOR USE SERVICE PACK
999-0831-051SP (Includes motor,
mounting hardware & installation
instruction sheet).
4. TO ORDER A REPLACEMENT
CONTROL BOX PLEASE USE
999-0831-300 (Includes Control
Box and mounting clip. Power
Cord must be ordered separately
2. TO ORDER A REPLACEMENT
HEAD MOTOR USE SERVICE PACK
999-0822-052SP (Includes motor,
mounting hardware & installation
instruction sheet).
5. TO ORDER A REPLACEMENT
DOUBLE T-CABLE USE SERVICE
PACK 999-0806-200SP (Includes
T-Cable, cable ties, grommets &
installation instruction sheet).
3. TO ORDER A REPLACEMENT
FOOT MOTOR USE SERVICE PACK
999-0822-053SP (Includes motor,
mounting hardware & installation
instruction sheet).
6. TO ORDER A REPLACEMENT
BATTERY MOTOR USE SERVICE
PACK 999-0711-001SP (Includes
battery cable, long cable ties, and
installation instruction sheet).
- see page 31 for order numbers)
WHEN ORDERING REPLACEMENT PARTS WITH CUSTOMER SERVICE,
PLEASE HAVE YOUR BED’S SERIAL NUMBER AVAILABLE
TO CONFIRM WHETHER THE PART IS COVERED UNDER WARRANTY.
(SEE PAGE 5 FOR LOCATION OF SERIAL NUMBER ID LABEL.)
33
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 34

Zenith aps seRies
SERVICE/REPLACEMENT PART DIAGRAM - DECKING
HEAD DECK
TO MAIN FRAME
SEAT PAN BRACKETS
DETAIL OF DECK
ATTACHMENT
(Similar for all decks)
DETAIL OF EDEMA
RATCHET RE-ATTACHMENT
(Use existing ratchets
and hardware)
KNEE DECK
TO MAIN FRAME
SEAT PAN BRACKETS
FOOT DECK
TO KNEE DECK
MOUNTING BRACKETS
1. TO ORDER A REPLACEMENT
HEAD DECK USE SERVICE PACK
999-0844-920SP (includes deck,
mounting hardware & installation
instruction sheet).
2. TO ORDER A REPLACEMENT
KNEE DECK USE SERVICE PACK
999-0844-940SP (includes deck,
mounting hardware & installation
instruction sheet).
3. TO ORDER A REPLACEMENT
FOOT DECK USE SERVICE PACK
999-0844-930SP (includes deck,
mounting hardware & installation
instruction sheet). Re-use existing
edema ratchets & ratchet hardware.
WHEN ORDERING REPLACEMENT PARTS WITH CUSTOMER SERVICE,
PLEASE HAVE YOUR BED’S SERIAL NUMBER AVAILABLE
TO CONFIRM WHETHER THE PART IS COVERED UNDER WARRANTY.
(SEE PAGE 5 FOR LOCATION OF SERIAL NUMBER ID LABEL.)
34
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 35

HEAD
END
Zenith aps seRies
SERVICE/REPLACEMENT PART DIAGRAM -
MAIN FRAME CAPPING
FOOT
END
CAPS OR PLUGS ARE NOT COVERED UNDER WARRANTY
(PACKS OF 12 CAN BE ORDERED IN THE EVENT YOU DAMAGE OR LOSE
ANY CAPS/PLUGS ON YOUR BED - SEE BOTTOM OF PAGE 32 FOR ORDER NUMBERS)
35
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 36

Zenith aps seRies
SERVICE/REPLACEMENT PART DIAGRAM -
CAPPING ON HEAD, KNEE, AND FOOT DECKS
CAPPING ON ARMS & LEGS
2X 100-4700-018
End Caps
2X 100-4700-018
Rectangular End Caps
4X 100-4715-011
Round End Caps
2X 100-4700-021
Rectangular End Caps
CAPS OR PLUGS ARE NOT COVERED UNDER WARRANTY
(PACKS OF 12 CAN BE ORDERED IN THE EVENT YOU DAMAGE OR LOSE
ANY CAPS/PLUGS ON YOUR BED - SEE BOTTOM OF PAGE 32 FOR ORDER NUMBERS)
36
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 37

Zenith aps seRies
NOTES:
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
37
Basic American Medical Products, a division of GF Health Products, Inc. www.grahamfield.com 999-0831-190A Service Manual
Page 38

336 Trowbridge Dr. • Fond du Lac, WI 54937
Customer Service: 1.800.365.2338 • Fax: 920.929.8213
www.grahamfield.com
© 2012 GF Health Products, Inc.
All Rights Reserved
Basic American Medical Products and Zenith
are registered trademarks of GF Health Products, Inc.
GF121699RevA12
 Loading...
Loading...