Goodlife Cloudia User Manual

User Guide
Blood Glucose
Monitoring System (Cloudia)

Contents
About GoodLife 4
About the System 5
Important Information 6
Important Safety Instruction 8
Blood Glucose Meter 10
Meter LCD Window 12
Blood Glucose Test Strip 14
Glucose Control Solution 17
Check Strip 20
Setting Meter Parameters 22
Performing Blood Test 26
Understanding Your Test Result 31
Data Transmission 33
Memory Recall 35
Replacing the battery 36
How to Clean and Disinfect 38
Storage and Handling 46
Display Messages and Problem-Solving Guide 47
Performance Evaluations
System Specication
54
FCC Regulations 66
Warranty 70
How to view transmitted reading
58
52
iglucose Diabets Management
5456
Please read this User Guide before using your GoodLife
Blood Glucose Meter (Cloudia). If you have any questions
or enquiries, please contact customer support at 1-855692-3511 for assistance.

4 5
About GoodLife™
About the System
Intended Use
Principle of the Test
GoodLife Blood Glucose Monitoring System(Cloudia)
is intended to be used for the quantitative measurement of glucose (sugar) in fresh capillary whole
blood sample drawn from the ngertips only. It is
intended to be used for single patient and should not
be shared. It is not indicated for the diagnosis or
screening of diabetes or for neonatal use.
The GoodLife System is an electrochemical biosensor
system that measures the amount of electric current
produced and displays the result as a blood glucose
level.
The GoodLife Blood Glucose Monitoring System
(Cloudia) (GoodLife System) is designed to provide
blood glucose measurements with easy and comfortable testing. The system requires 0.5μL of blood
sample and 5 seconds for the test to complete.
The GoodLife System consists of:
GoodLife Blood Glucose Meter (Cloudia), GoodLife
Blood Glucose Test Strips (Cloudia), Check Strip,
GoodLife Glucose Control Solution(Level I & II), Safety
Lancet (optional for
purchase).
These products are intended to be used together to
get accurate blood glucose test results. Do not use
other test strips or control solutions with your meter.
NOTE:
Not for use on patients who are dehydrated, hypotensive, in shock, or in a hyperosmolar state.ÿ
The GoodLife System is intended for in vitro diagnostic use with capillary whole blood. The system should
not be used for diagnosis and screening of diabetes
or for testing newborn infant (neonatal testing).
CAUTION
The test result does not suggest any medical
indication. The user should consult his or her
clinician for further diagnostic and treatment.
Call your doctor immediately if you experience
symptoms that are not consistent with your
blood glucose test results.
Severe dehydration or excessive water loss may
cause false, high results. Call your doctor right
away if you suspect you are suering from dehydration.
A sample with large amount of reducing
substances such as triglycerides(>1000mg/dL),
ascorbic acid (>2.25mg/dL), uric acid (>15mg/dL)
and xylose (> 50mg/dL).
1.
2.
3.
4.
A red blood cell count (hematocrit) that is either
very high (over 55%) or very low (under 30%) can
cause false result.
High altitudes above than 8,800 feet (2,750
meter) may aect the test results.
Temperatures outside the range of 50°F to104°F
(10°C to 40°C), 20%-80% RH may aect the test
results.
Do not use GoodLife system to test on critically ill
patient.
Not for use on patients who are dehydrated,
hypotensive,in shock, or in a hyperosmolar state.
5.
6.
7.
8.
9.
6
7
Important Information
Triglyserides
Ascorbic acid
Substance
Highest c oncentration
without interference
( mg/dL)
Toxic
Concertration
( mg/dL)
Theraprutic
Physiological
Levels
N/A
N/A
N/A
N/A
0.40 - 2.00
150 - 500
2.52 - 8.00
57
Uric acid
Xylose
2.25
1000
15
50
Theraprutic/

8 9
Important Safety Instruction
The meter and lancing device are for single
patient use. Do not share them with anyone
including other family members. Do not use on
multiple patients.
Because all parts of the kit may come in contact
with your blood, all parts of the kit are considered
biohazardous and can potentially transmit infectious diseases. Please refer to "How to Clean and
Disinfect" section to help mitigate biohazard.
Users should wash hands thoroughly with soap
and water and dry thoroughly after handling the
meter, test strips and any lancing device.
For customer support contact at 1-855-692-3511.
(Mon-Fri 9:00 am~4:30 pm Pacic Time)
NOTE:Please make sure that all products listed on the
"contents" of the box are contained in the package before
using this system. If you nd any imperfection in our products,
please contact customer support at 1-855-692-3511.
(Mon~Fri 9:00am~4:30pm Pacic Time)
1.
2.
3.
“FDA Public Health Notication: Use of Fingerstick
Devices on More than one Person Poses Risk for
Transmitting Bloodborne Pathogens: Initial Communication” (2010)
http://www.fda.gov/MedicalDevice/
Safety/AlertsandNotices/ucm224025.htm
“CDC Clinical Reminder: Use of Fingerstick
Devices on More than One Person Poses Risk for
Transmitting Bloodborne Pathogens” (2010)
http://www.cdc.gov/injectionsafety/FingerstickDeviceBGM.html
References
1.
2.

10 11
Blood Glucose Meter
DISPLAY: The large, easy
to read display shows
the test results, messages, blood glucose results stored in memory,
time and date.
Mem Button: Press Mem button to enter memory mode to
recall the information stored in
meter’s memory.
Set Button: Press Set button to
enter date and time setting.
STRIP SLOT: Holds a Blood Glucose Test Strip or Check
Strip in place when you perform blood glucose test
or perform check test.
: Holds
-
Button: For data transmission
3L-02-XXXXXX V1.0
Blood Glucose
Monitoring System
Cloudia
For Self-testing
Battery:AAA,6V
HMD BioMedical Inc.
Hsinchu , Taiwan
serial no.:
XXXXX-0000001
SIM:
8901204043780370392
For In Vitro Diagnostic Use Only FCC ID:
AYY0000001
3L-02-XXXXXX V1
SIM
AAA
AAA
AAA
AAA
SMM
AAAAAA
AA
3L-02-810035 V1.0
Blood Glucose
Monitoring System
Cloudia
For Self-testing
Battery:AAA,6V
serial no.: 65534-XXXXXXX
For In Vitro Diagnostic Use Only
FCC ID: AYY0000002
Distrubutor:
Kannact Inc. / KRemedy
10260 SW. Greenburg Road Suite 525,
Portland OR 97223
Battery Compartment
four AAA batteries. Before us
ing the meter, please install
Meter SN Label: The label shows the
meter serial number.
STRIP EJECTOR:
For easy ejection
of the test strip.
12 13
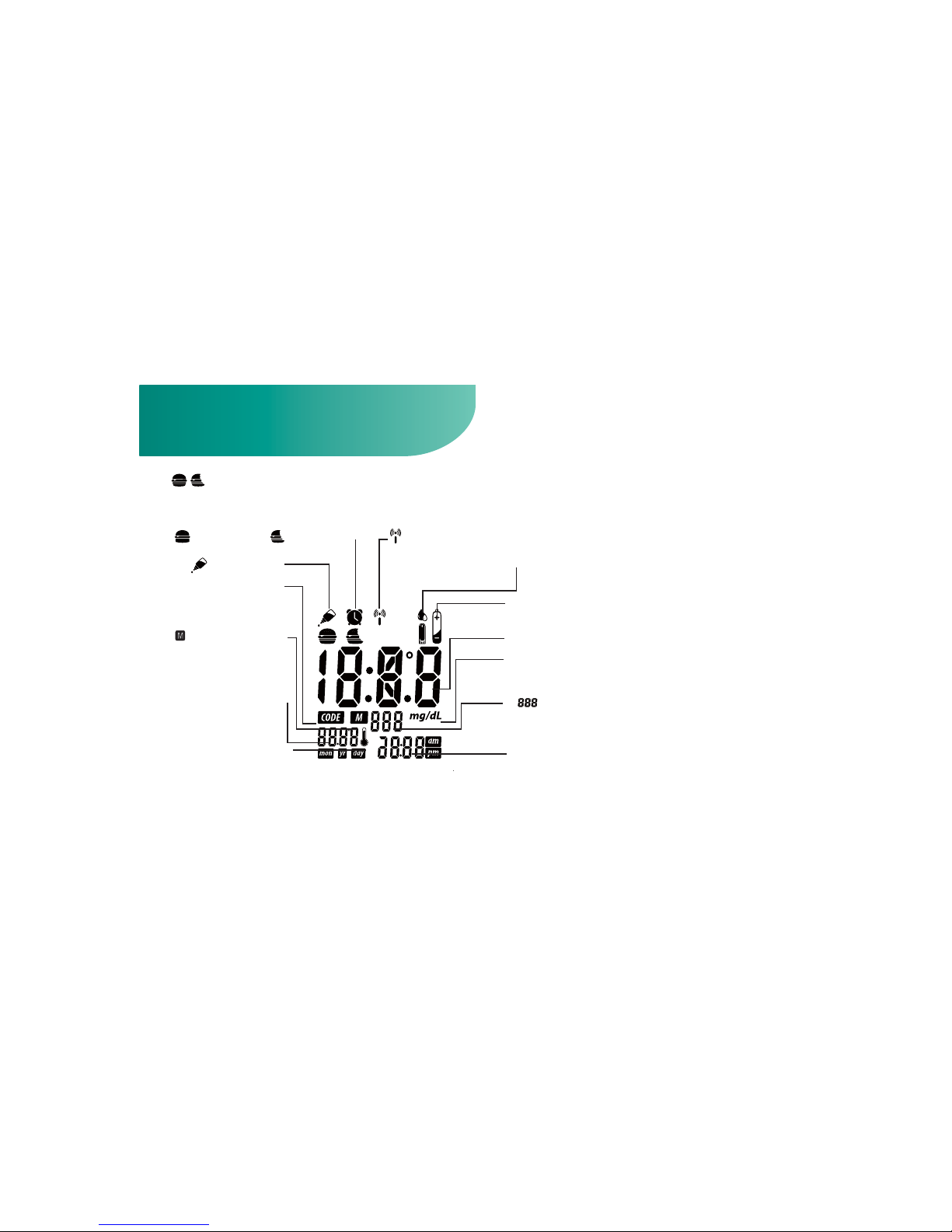
Meter LCD Window
DATE: Month/Year/Day
and the temperature at
the moment of the test.
THERMOMETER SYMBOL:
Appears when ambient
temperature is above or below the acceptable range
needed for testing.
CODE: A code number
should appear before meter is used to test sample.
Ř
Meter is at a Mem-
ory mode. Prior analysis
results can be read.
Symbol:The test
can be adjusted and
stored into 3 types of test
modes - Before Meal Test
(
), After Meal Test(
) and Control Solution
Test(
).
Alarm Symbol:
Appears when
alarm is set.
Symbol: Appears
when data transmission.
Test Result
Time: 12 and 24 hour period format.
mg/dL: The units of measurement in the U.S. are mg/dL.
Ř
Memory capacity is 999 entries. The display shows to
ensure that all digits are working properly.
Strip Symbol: Appears when the strip is inserted and meter is
ready for blood testing. Replace vial cap after removing test strips.
Battery Symbol: Appears when battery is low.

14 15
(correct) (incorrect )
Blood Glucose Test Strip
Aperture: apply
the blood to the
front of the strip.
Black Electrode End:
Insert this end of test
strip into meter.
Blood Reaction
Zone: It must be
lled with blood
entirely.
GoodLife System measures the amount of
glucose in capillary whole blood. Blood can be
applied to the front of the test strip and is automatically drawn to the test strip through capillary
action.
GoodLife Blood Glucose Test Strips (Cloudia) are
intend ed for in vitro diagnostic use with capillary
whole blood or GoodLife Glucose Control
1.
2.
Solution.
Results will not be accurate if used with plasma or
serum samples.
Do not use test strips beyond the expiration date
indicated on the strip vial label.
The discard date for test strips is 90 days after rst
opening the vial. Record the discard date on the
vial when you open a new vial of test strips.
Blood Glucose Test Strip can be damaged by heat
and light. Keep them sealed in the original vial.
Store the vial in a cool, dry place between
104°F(40°C) and 50°F (10°C). Do not refrigerate.
Do not use damaged test strips in any way. Use
test strip immediately after taking it out from the
vial or foil packet; replace the vial cap and close it
tightly.
3.
4.
5.
6.
7.

16 17
Glucose Control Solution
IMPORTANT INFORMATION
Do not transfer test strips to a new vial. Always
carry test strips in their original vial.
Do not place in direct heat or sunlight.
Do not carry loose test strips in your carrying
case.
Test strips are for single use only.
8.
9.
10.
11.
GoodLife Glucose Control Solution is
used to check if the GoodLife Blood
Glucose Monitoring System(Cloudia)
and Test Strip are working correctly as
a system.
Users should perform a control
solution test when:
a new vial of test strip is opened.
a meter is dropped
whenever results are not consistent with patient’s symptoms.
1.
2.
3.
Do not use control solution beyond the expiration date indicated on the bottle label.
The discard date for control solution is 90 days
after rst opening. Record the discard date on the
bottle when you open a new bottle of control
solution.
1.
2.

18 19
Test result will show up in 5 seconds. The result
should correspond to the range printed on the
label of strip vial used.
NOTE:
Repeat test if the result falls outside the control
range. If you continue to get the result falling outside the
control range, the potential causes are
1. the test strip is damaged or get moist
2. the test strip is expired.
3. the meter and strip may not be working properly.
Do not use the system to test your blood until you get a
test result falls within the control range.
Contact customer support at 1-855-692-3511 or your
healthcare provider for help.
5.Store the control solution closed at temperatures
between 50ºF (10ºC) and 86ºF (30ºC).
Please carefully read the label before use.
For purchase of control solution, please contact
your local distributor or customer support at
1-855-692-3511 for more information.
3.
4.
5.
Perform Control Test
Insert a new test strip into the strip slot, the
meter will activate.
The code number will appear on the screen.
Compare the code number shown on the screen
against the code number on the test strip vial. If
the two numbers match, you may begin test,
otherwise contact customer support at 1-855692-3511 or your healthcare provider for help.
When shows up, press Set button and choose
control solution test.
Gently shake the control solution and apply a
drop to the aperture of strip. Make sure that the
control solution has saturated the blood reaction
zone.
1.
2.
3.
4.

20 21
Check Strip
CHECK
How to check meter by check strip
How to delete memory
The Check Strip can be used in 2
ways:
1.2.To test only the function of
the meter and not complete
BGM test system. Please
check the complete test
system with Control Solution
To delete all test memories.
Insert the check strip into strip slot with label side
up as above.
You should obtain an “OK“ reading within 10
seconds, which means your meter is working
properly.
Remove the check strip to exit. Meter will automatically turn o.
NOTE
:
If you do not get "OK" reading but appear other
error message, turn o the meter by remove check strip
from the meter. Then check the battery and repeat the
test. If the second result persists, contact customer
support at 1-855-692-3511 or your healthcare provider.
1.
2.
Insert check strip into strip slot with label side up.
After “OK” displayed, press and hold the Set
button until ashing “dEL” shows up with a beep
sound.
Press and hold the Mem button until you hear a
beep sound, Meter will display “OK” before
turning o and all the memory has been deleted
successfully.
Remove the check strip from the meter.
1.
2.
3.
4.
 Loading...
Loading...