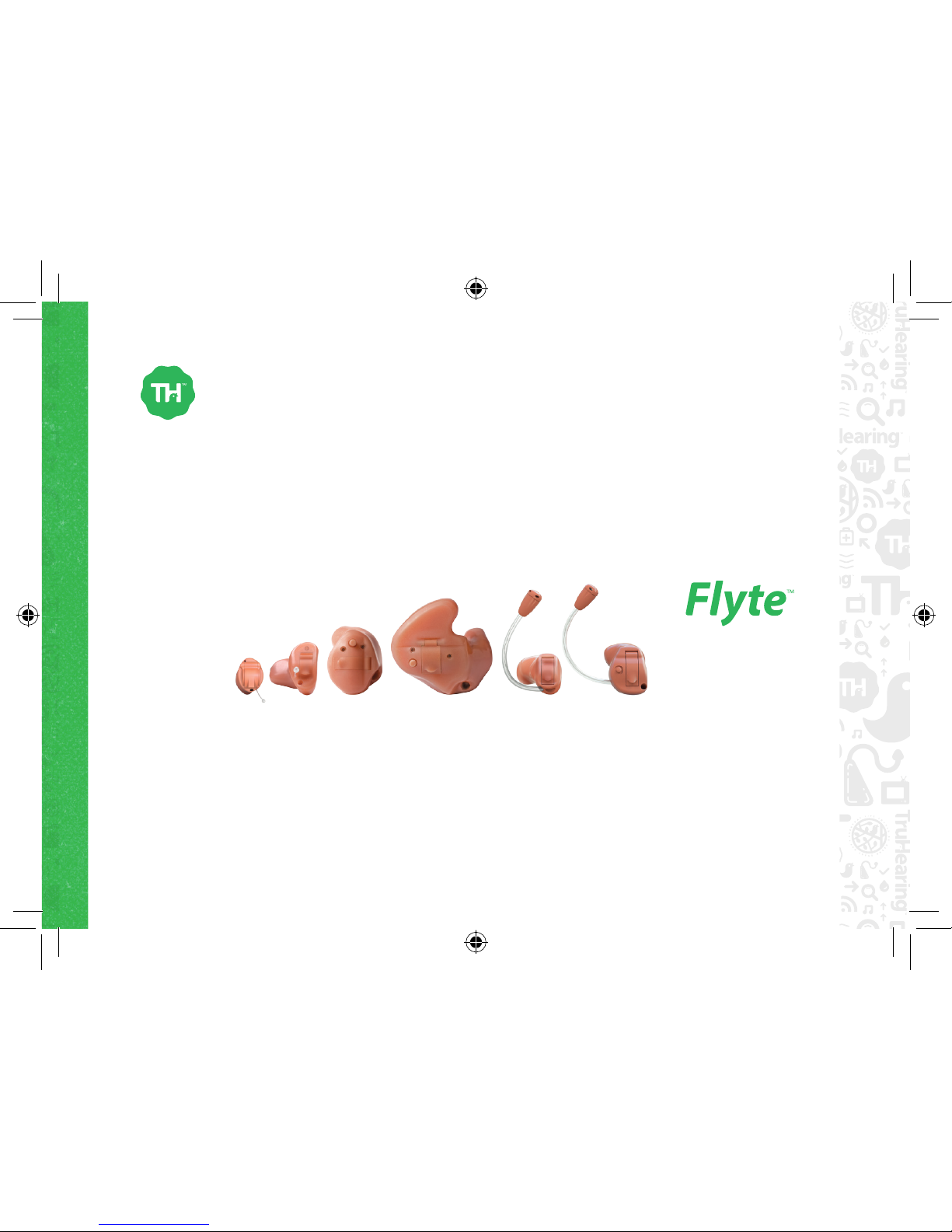
User Guide Flyte Custom
400847011US-17.10-Rev.A 1 02-10-2017 14:14:51
2
Hearing instrument type designations for models included in this user guide are: DA312r, FCC ID:
X26DA312r, IC: 6941C-DA312r; DA13r, FCC ID: X26DA13r, IC: 6941C-DA13r; DA312i, FCC ID: X26DA312i,
IC: 6941C-DA312i; and DA13 i, FCC ID: X26DA13i, IC: 6941C-DA13i. Please see page 10, 12 and 14 for lists
of models referring to these types.
Statement:
This device complies with part 15 of the FCC rules and ICES-003 of the ISED rules. Operation is subject to
the following two conditions:
(1) this device may not cause harmful inter ference, and
(2) this device must accept any interference received, including interference that may cause undesired
operation.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules and ICES-003 of the ISED rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses
and can radiate radio frequency energy and, if not installed and used in accordance with the instructions,
may cause harmful interference to radio communications. However, there is no guarantee that interference
will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try
to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from the one in which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
• Changes or modifications can void the user´s authority to operate the equipment.
400847011US-17.10-Rev.A 2 02-10-2017 14:14:51
3
The products are in compliance with the following regulatory requirements:
• In the EU: the device conforms to the Essential Requirements according to Annex I of Council Directive
93/42/EEC for medical devices (MDD) and essential requirements and other relevant provisions of Directive 2014/53/EU (RED).
• The declaration of conformity may be consulted at www.resound.com
• In US: FCC CFR 47 Part 15, subpart C.
• Other identified applicable international regulatory requirements in countries outside the EU and US.
Please refer to local country requirements for these areas.
• In Canada: these hearing instruments are certified under the rules of ISED.
• Japanese Radio Law and Japanese Telecommunications Business Law Compliance. This device is
granted pursuant to the Japanese Radio Law (電波法) and the Japanese telecommunications Business
Law (電気通信事業法) This device should not be modified (otherwise the granted designation number
will become invalid).
This device operates in the frequency range of 2.4 GHz - 2.48 GHz.
This device includes an RF transmitter that operates in the range of 2.4 GHz - 2.48 GHz.
Maximum RF output power transmitted is up to 0 dBm.
Intended use
Generic air-conduction hearing instruments are wearable sound-amplifying devices intended to compensate for impaired hearing. The fundamental operating principle of hearing instruments is to receive, amplify,
and transfer sound to the ear drum of a hearing impaired person.
400847011US-17.10-Rev.A 3 02-10-2017 14:14:51
4
Prescription use of a Tinnitus Sound Generator (TSG) hearing
instrument
The TSG module should be used as prescribed by your doctor, audiologist or hearing healthcare professional.
In order to avoid permanent hearing damages, the maximum daily usage depends on the level of the
generated sound. Should you develop any side effects from using the sound generator, such as dizziness,
nausea, headaches, perceived decrease in auditory function or increase in tinnitus perception, you should
discontinue use of the sound generator and seek medical evaluation.
The target population is primarily the adult population over 18 years of age. This product may also be used
with children 5 years of age or older. However, children and physically or mentally challenged users will require training by a doctor, audiologist, hearing healthcare professional or the guardian for the insertion and
removal of the hearing instrument containing the TSG module.
Important notice for prospective sound generator users
A tinnitus masker is an electronic device intended to generate noise of sufficient intensity and bandwidth to
mask internal noises. It is also used as an aid in hearing external noises and speech.
Good health practice requires that a person with a tinnitus condition have a medical evaluation by a licensed
physician (preferably a physician who specializes in diseases of the ear) before using a sound generator.
Licensed physicians who specialize in diseases of the ear are often referred to as otolaryngologists, otologists or otorhinolaryngologists.
400847011US-17.10-Rev.A 4 02-10-2017 14:14:51
5
The purpose of medical evaluation is to assure that all medically treatable conditions that may affect tinnitus
are identified and treated before the sound generator instrument is used.
The sound generator instrument is a tool to generate sounds to be used with appropriate counselling and/
or in a tinnitus management programme to relieve patients suffering from tinnitus.
400847011US-17.10-Rev.A 5 02-10-2017 14:14:51
6
Introduction
Congratulations on the purchase of your new hearing instruments. The innovative sound technology and
design, combined with the customised device programming selected by your hearing care professional, will
make hearing a more enjoyable experience. Hearing instruments will enable you to hear sounds that you
may not have heard in years because of your hearing loss. Practice and a positive attitude are important
in learning to use hearing instruments. Your instruments have been adjusted according to your individual
hearing loss and needs. Some people adjust quickly to wearing hearing instruments in their ears and hearing new sounds; other people may need more time.
This product is a custom-made device.
Please read this manual carefully in order to wholly benefit from the use of your hearing instruments. With
proper care, maintenance, and usage, your hearing instruments will aid you in better communication for
many years. Ask your hearing care professional if you have any questions.
400847011US-17.10-Rev.A 6 02-10-2017 14:14:51
7
Hearing instrument model:
Model CIC: Battery size 10
Model IIC: Battery size 10
Model ITC: Battery size 13 or 312 (circle one)
Model ITE: Battery size 13 or 312 (circle one)
Model MIH: Battery size 13, 312, or 10 for MIH-S (circle one)
Left serial number:
Right serial number:
400847011US-17.10-Rev.A 7 02-10-2017 14:14:51
8
Contents
Statment ... ............................2
Intended use.... .........................3
Prescription use of a Tinnitus Sound
Generator (TSG) hearing instrument.. ........4
Important notice for prospective sound
generator users.........................4
Introduction............................6
Contents ..............................8
Getting started ........................16
On/Off function ........................16
SmartStart ...........................16
Inserting/Replacing the battery............17
Low battery indicator ...................18
Low battery indicator when paired with
wireless accessories only ................18
Inserting/removing hearing instruments .....19
Operation of the hearing instrument . . . . . . . . 22
Volume control (optional).................20
Program button (optional) ................21
Flight mode...........................24
Telephone use.........................25
Telecoil ..............................25
Listen to radio or TV ....................26
Using hearing instruments with smart
phone apps ..........................26
Using hearing instruments with iPhone®,
iPad®, and iPod touch® ....................26
Cellular phones .......................27
PhoneNow ...........................27
Tele-loop systems......................29
Care and maintenance ..................29
Daily maintenance......................30
Replacing wax filters....................30
General precautions ....................31
General warnings ......................32
Tinnitus Sound Generator (TSG) module ....34
Intended use for the TSG module ..........34
User instructions for the TSG module.......34
Using TSG with smart phone apps .........36
The scientific concepts that form the basis
for the device .........................36
Technical Specifications .................37
400847011US-17.10-Rev.A 8 02-10-2017 14:14:51
9
TSG warnings .........................38
TSG precautions.......................38
TSG warning to hearing healthcare
professionals..........................39
Battery warning information ..............40
Hearing instrument expectations. ..........41
Warning to hearing aid dispensers .........42
Important notice for prospective
hearing aid users ......................42
Children with hearing loss................43
Troubleshooting guide ..................44
Technical data.........................48
Warranty and repairs....................49
Temperature test, transport and
storage information .....................49
400847011US-17.10-Rev.A 9 02-10-2017 14:14:52
10
Mic in Helix (MIH-S) hearing instrument models
with size 10A battery are available in the following
variants:
FL9MIH-S-UP, FL9MIH-S-HP, FL9MIH-S-MP,
FL9MIH-S-LP
Mic in Helix (MIH) hearing instruments
(including type DA312r with FCC ID X26DA312r,
IC number 6941C-DA312r models designated by
a “W”) with size 312 battery and Custom Mic in
Helix hearing instruments (including type DA13 r
with FCC ID X26DA13r, IC number 6941C-DA13r
models designated by a “W”) with size 13 battery
are available in the following variants:
FL9MIH-W-UP, FL9MIH-W-HP, FL9MIH-W-MP,
FL9MIH-W-LP, FL9MIH-UP, FL9MIH-HP,
FL9MIH-MP, FL9MIH-LP
FP9MIH-W-UP, FP9MIH-W-HP, FP9MIH-W-MP,
FP9MIH-W-LP, FP9MIH-UP, FP9MIH-HP,
FP9MIH-MP, FP9MIH-LP
400847011US-17.10-Rev.A 10 02-10-2017 14:14:52
11
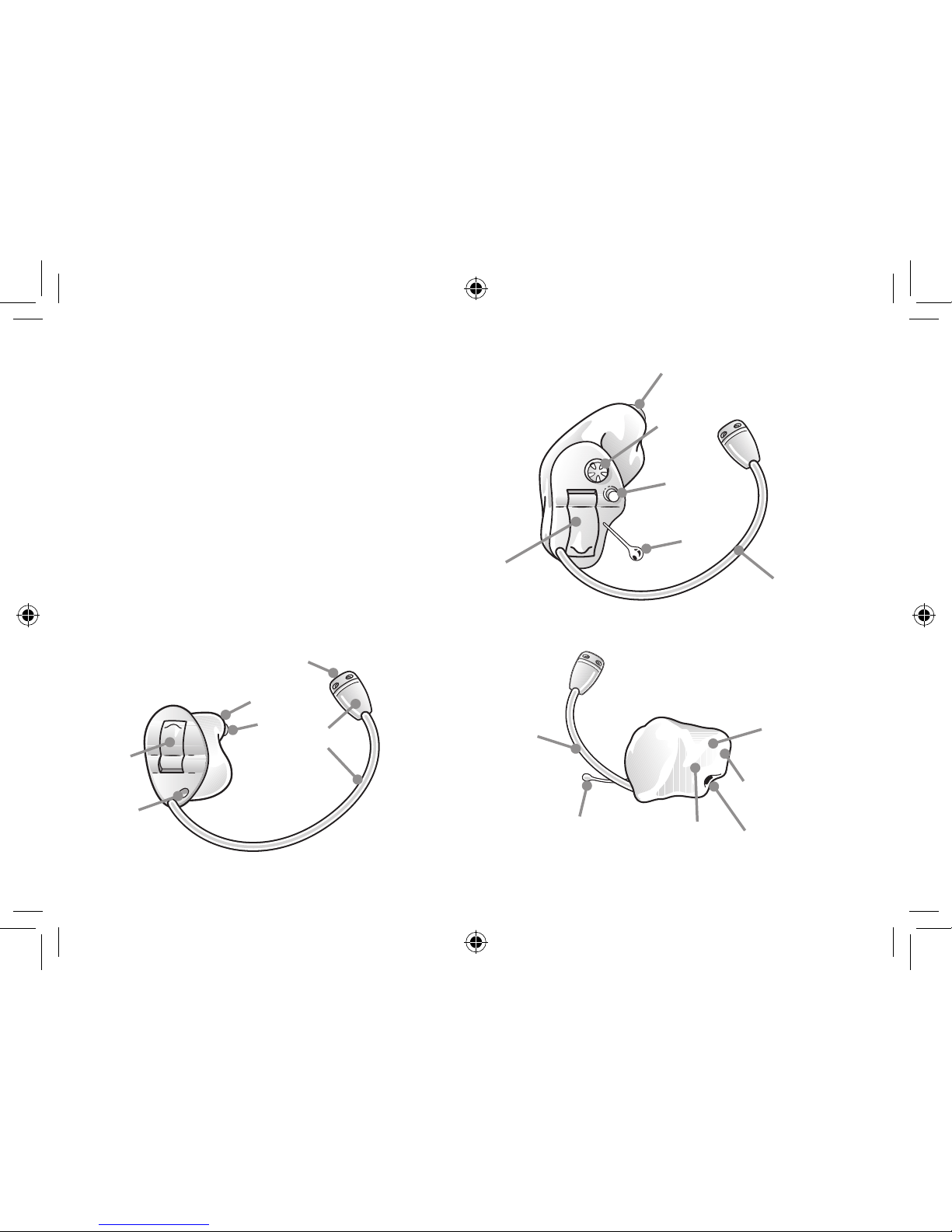
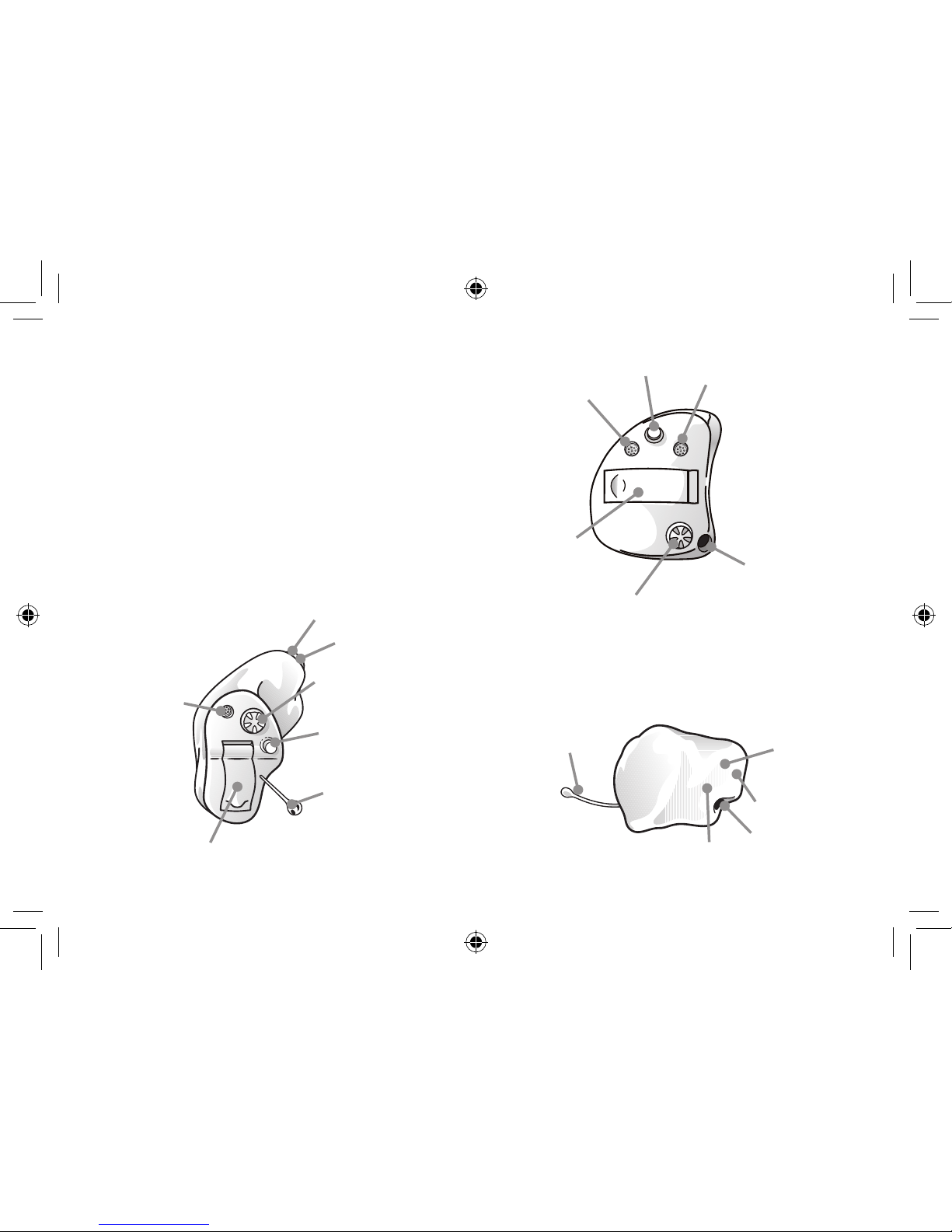
1. Program button (optional)
2. Battery compartment and On/Off switch
3. Removal cord (optional)
4. Sound outlet
5. Wax filter
6. Vent
7. Microphone sound inlet
8. Remote microphone and tubing
(for remote microphone devices)
9. Volume control (optional)
10. Model
11. Manufacturer
12. Serial number
8
6
7
4
5
2
2
4
9
1
3
8
11
12
10
3
8
6
XXXXXXX
XXXXXXX
XXXXXXX
400847011US-17.10-Rev.A 11 02-10-2017 14:14:56
12
Invisible-in-the-canal (IIC) and
completely-in-the-canal (CIC) hearing
instruments with size 10A battery are available in
the following variants:
FL9IIC, FL9CIC-UP, FL9CIC-HP, FL9CIC-MP,
FL 9 C I C- LP, F L 7 C IC -U P, FL7CI C - H P, F L 7 C IC - M P,
FL9CIC-LP
FP9IIC, FP9CIC-UP, FP9CIC-HP, FP9CIC-MP,
FP9CIC-LP, FP7CIC-UP, FP7CIC-HP, FP7CIC-MP,
FP9CIC-LP
In-the-canal (ITC) hearing instruments (including
type DA312i with FCC ID X26DA312i, IC number
6941C-DA312i models designated by a “W”)
with size 312 battery and In-the-canal (ITC)
hearing instruments (including type DA13i with
FCC ID X26DA13i, IC number 6941C-DA13i models
designated by a “W”) with size 13 battery are
available in the following variants:
FL9ITC-D-UP, FL9ITC-D-HP, FL9ITC-D-MP,
FL9ITC-D-LP, FL9ITC-UP, FL9ITC-HP,
FL9ITC-MP, FL9ITC-LP, FL7ITC-D-UP, FL7ITCD-HP, FL7ITC-D-MP, FL7ITC-D-LP, FL7ITC-UP,
FL7ITC-HP, FL7ITC-MP, FL7ITC-LP
FP9ITC-D-UP, FP9ITC-D-HP, FP9ITC-D-MP,
FP9ITC-D-LP
FP9ITC-DW-UP, FP9ITC-DW-HP, FP9ITC-DWMP, FP9ITC-DW-LP
FP9ITC-UP, FP9ITC-HP, FP9ITC-MP, FP9ITC-LP
FP9ITC-W-UP, FP9ITC-W-HP, FP9ITC-W-MP,
FP9ITC-W-LP
FP7ITC-D-UP, FP7ITC-D-HP, FP7ITC-D-MP,
FP7ITC-D-LP
FP7ITC-DW-UP, FP7ITC-DW-HP, FP7ITC-DWMP, FP7ITC-DW-LP
FP7ITC-UP, FP7ITC-HP, FP7ITC-MP, FP7ITC-LP
FP7ITC-W-UP, FP7ITC-W-HP, FP7ITC-W-MP,
FP7ITC-W-LP
400847011US-17.10-Rev.A 12 02-10-2017 14:14:56
XXXXXXX
XXXXXXX
XXXXXXX
13
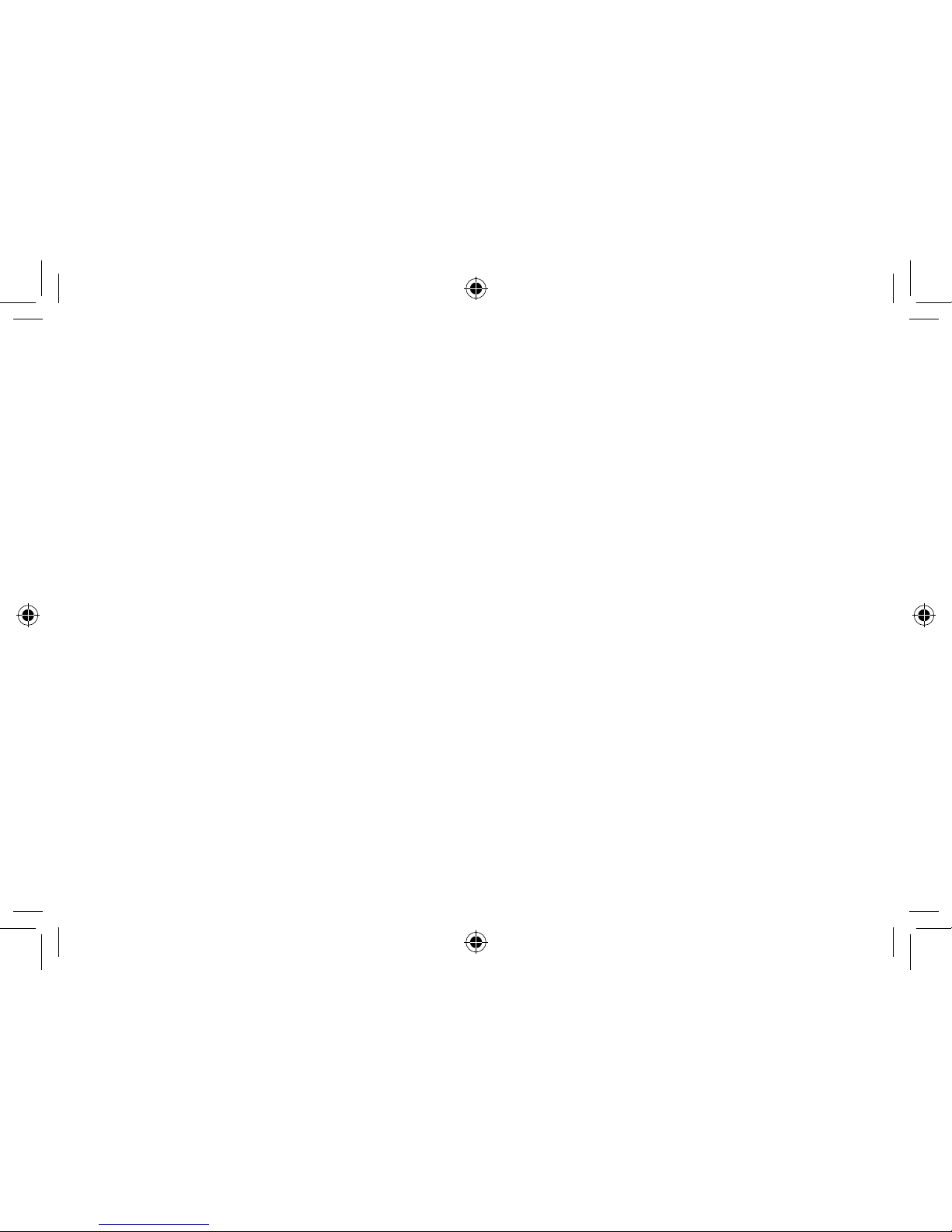
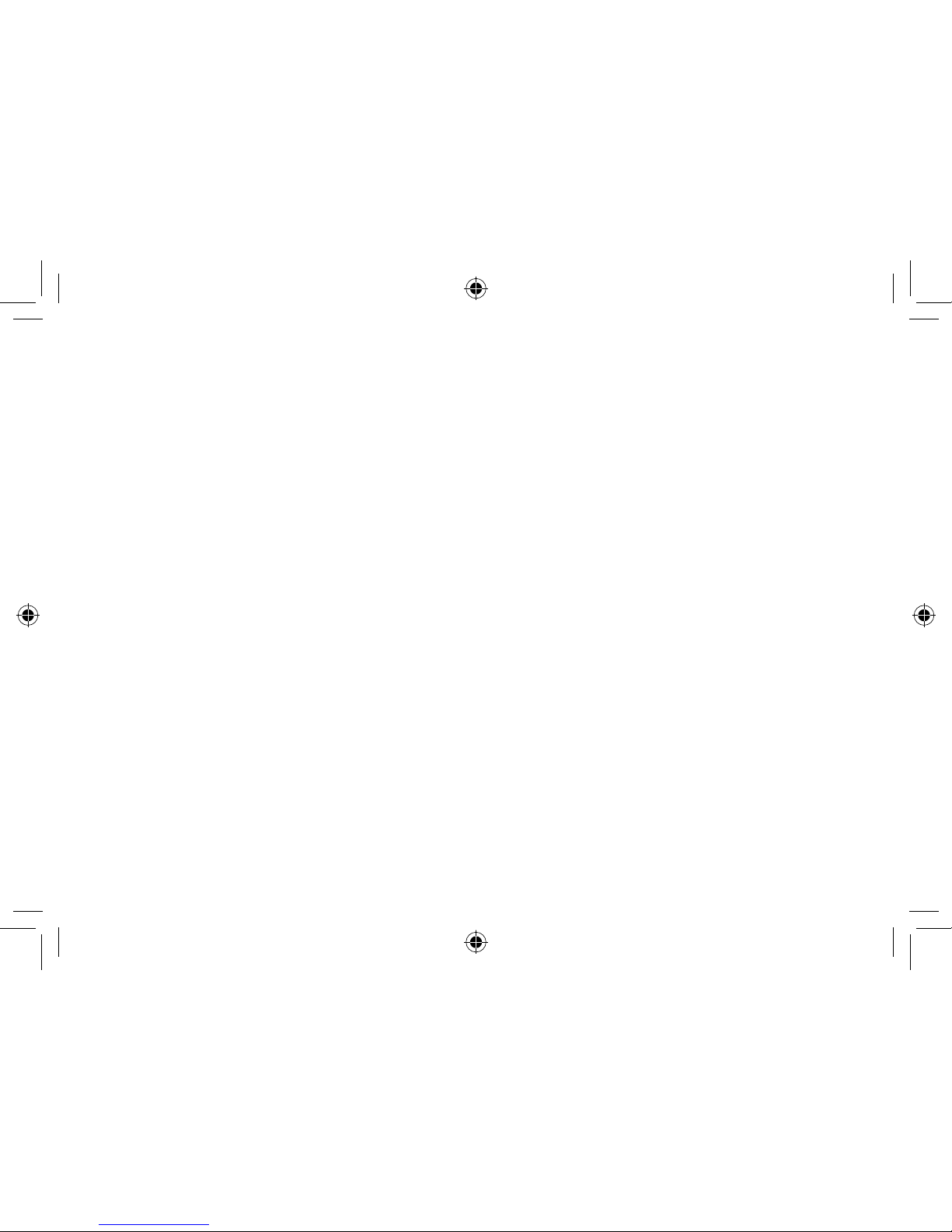
1. Program button (optional)
2. Battery compartment and On/Off switch
3. Removal cord (optional)
4. Sound outlet
5. Wax filter
6. Vent
7. Microphone sound inlet(s)
8. Volume control (optional)
9. Model
10. Manufacturer
11. Serial number
1
7
7
6
8
2
9
10
11
3
6
2
7
4
8
1
3
5
400847011US-17.10-Rev.A 13 02-10-2017 14:14:59
14
In-the-ear (ITE) hearing instruments (including
type DA13i with FCC ID X26DA13i, IC number
6941C-DA13i models designated by a “W”) with size
13 battery and In-the-ear (ITE) hearing instruments
(including type DA312i with FCC ID X26DA312i, IC
number 6941C-DA312i models designated by a “W”)
with size 312 battery are available in the following
variants:
FL9ITE-DW-UP, FL9ITE-DW-HP, FL9ITE-DW-MP,
FL9ITE-D-UP, FL9ITE-D-HP, FL9ITE-D-MP,
FL9ITE-W-UP, FL9ITE-W-HP, FL9ITE-W-MP,
FL9ITE-UP, FL9ITE-HP, FL9ITE-MP
FL7ITE-DW-UP, FL7ITE-DW-HP, FL7ITE-DW-MP,
FL7ITE-D-UP, FL7ITE-D-HP, FL7ITE-D-MP,
FL7ITE-W-UP, FL7ITE-W-HP, FL7ITE-W-MP
FL7ITE-UP, FL7ITE-HP, FL7ITE-MP
FP9ITE-DW-UP, FP9ITE-DW-MP, FP9ITE-DW-MP
FP9ITE-D-UP, FP9ITE-D-HP, FP9ITE-D-MP
FP9ITE-W-UP, FP9ITE-W-MP, FP9ITE-W-MP
FP9ITE-UP, FP9ITE-MP, FP9ITE-MP
FP7ITE-DW-UP, FP7ITE-DW-MP, FPITE-DW-MP
FP7ITE-D-UP, FP7ITE-D-HP, FP7ITE-D-MP
FP7ITE-W-UP, FP7ITE-W-MP, FP7ITE-W-MP
FP7ITE-UP, FP7ITE-MP, FP7ITE-MP
400847011US-17.10-Rev.A 14 02-10-2017 14:14:59
15
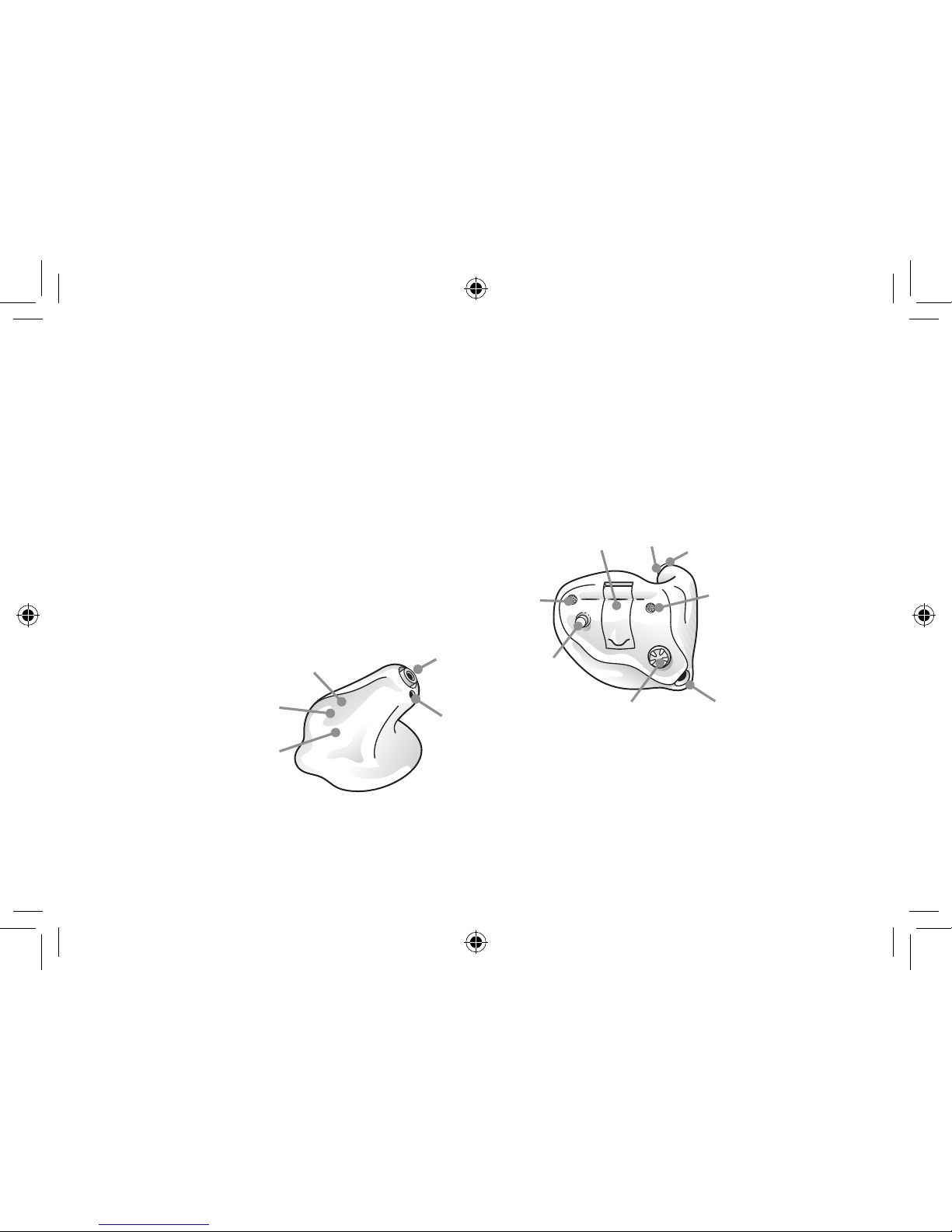
1. Program button (optional)
2. Battery compartment and On/Off switch
3. Sound outlet
4. Wax filter
5. Vent
6. Microphone sound inlet(s)
7. Volume control (optional)
8. Model
9. Manufacturer
10. Serial number
6
2
3
7
1
6
5
4
XXXXXXX
XXXXXXX
XXXXXXX
10
9
8
5
4
400847011US-17.10-Rev.A 15 02-10-2017 14:15:01
16
Getting started
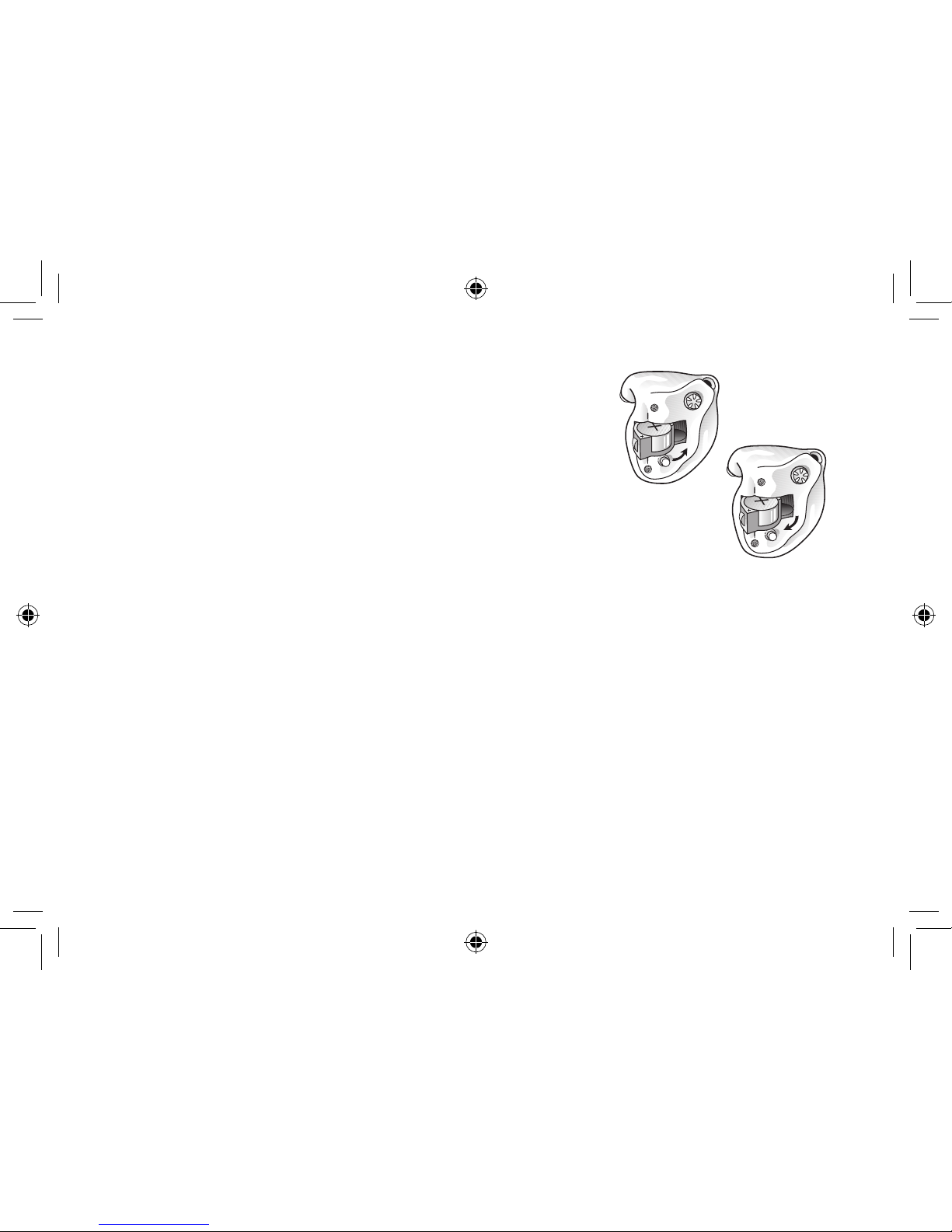
On/Off function
1. When the battery door is closed, the hearing instrument
will turn on, and the default program will be activated.
2. To turn the hearing instrument off, open the battery door.
Many individuals can use their fingernail to pull it open.
i
Tip: Whenever the hearing instruments are not in use, remember to open the
battery doors to avoid unnecessary battery consumption.
SmartStart
Hearing instruments can be turned on once you have placed them on your ears. If you
prefer to turn them on just prior to placing them in your ears, your hearing care professional can activate a function called SmartStart. This function will delay the time in which
the hearing instruments turn on by several seconds after the battery compartment is
closed. With SmartStart, a beep will be heard for each second of the delay period.
On
Off
400847011US-17.10-Rev.A 16 02-10-2017 14:15:03

1 2 3
17
Inserting/Replacing the battery
1. Open the battery door completely by using your fingernail. Remove the used battery if present.
2. Prepare the new battery (please refer to page 5 for information on appropriate battery type/size for your
hearing instrument). Remove the protective foil and wait 2 minutes before inserting the battery into the
hearing instrument to allow activation of the battery.
3. Insert the new battery with the positive side in the correct position.
4. Gently close the battery door.
i
Tip:
1. Always use new Zinc-Air batteries that have a minimum remaining shelf life of 1 year.
2. To save battery, turn off your hearing aids when they are not in use.
3. At night, switch off the hearing aid and open the battery door
completely to allow moisture to evaporate. This prolongs the lifespan
of the hearing aid.
4. If the hearing aids frequently lose connection to resound wireless
accessories, contact your hearing care professional for a list of
appropriate batteries.
400847011US-17.10-Rev.A 17 02-10-2017 14:15:09

18
Low battery indicator
When the batteries are low on power, your hearing aids reduce the volume, and play a melody every 15
minutes until they are empty and turn off.
i
NOTE: Keep spare batteries on hand.
Low battery indicator when paired with wireless accessories only
The batteries drain faster when you use wireless functionalities like streaming from your smart phone or
from your TV.
As the battery power goes down, the wireless functions stop working. A short melody every five minutes
indicates that battery power is too low.
The table below shows how the functionality shifts with the power level of the battery.
Battery level Signal Hearing aid Remote Control Streaming
Fully charged
P P P
Low
P P O
Depleted
(change battery)
P O O
400847011US-17.10-Rev.A 18 02-10-2017 14:15:12

19
Inserting/Removing hearing instruments
Insertion (Mic in Helix)
1. Hold the hearing instrument between your thumb and index finger,
either above and below or on the sides.
2. Place the sound outlet portion into your ear canal. Turn the top part of
the hearing instrument gently backwards and forwards so that it tucks
behind the fold of skin above your ear canal.
3. Insert the hearing instrument into your ear canal. Opening and closing
your mouth may ease insertion.
4. Gently push the microphone into the creased area of the ear that is
located above the microphone entrance, and make sure the tubing is
in place.
400847011US-17.10-Rev.A 19 02-10-2017 14:15:13

20
Insertion (IIC, CIC, ITC, and ITE)
1. Hold the hearing instrument between your thumb and index finger, either above
and below or on the sides. For IIC, there is a dot on the top side of the shell to show
orientation for insertion.
2. Place the sound outlet portion into your ear canal. Turn the top part of the hearing
instrument gently backwards and forwards so that it tucks behind the fold of skin
above your ear canal.
3. Insert the hearing instrument into your ear canal. Opening and closing your mouth
may ease insertion.
By experimenting, an easier method may be discovered. With proper insertion, hearing
instruments should fit snugly but comfortably. If the hearing instruments cause irritation
of the ears, contact your hearing care professional.
i Never attempt to modify the shape of the hearing instrument yourself.
i
Tip: It may be helpful to pull your ear up and outward with your opposite hand during insertion.
IIC
400847011US-17.10-Rev.A 20 02-10-2017 14:15:16

21
Removal options (IIC, CIC and Mic in Helix)
1. Hold the removal cord with your thumb and index finger and pull outward.
2. Hold the edges of the hearing instrument with your thumb and forefinger
and pull outward while slightly rotating your hand forward.
3. If Mic in Helix hearing instruments do not have a removal cord, gently pull
outward with the microphone tubing.
Removal (ITC and ITE)
1. Hold the edges of the hearing instrument with your thumb and forefinger.
2. Pull outward while slightly rotating your hand forward.
i Note: Consult your hearing care professional if you have difficulty removing
the hearing instruments.
400847011US-17.10-Rev.A 21 02-10-2017 14:15:16

22
Operation of the hearing instrument
Volume control (Optional)
The volume control will allow the volume of hearing instruments to be increased or decreased.
1. To increase the volume, rotate the volume control forward (towards your face when you are wearing the
hearing instruments).
2. To decrease the volume, rotate the volume control backward (away from your face).
When volume is increased or decreased,
a beep signal will be heard for each incremental change. When the upper or lower
limits of the volume range are reached, a
beep signal with a longer duration will be
heard.
400847011US-17.10-Rev.A 22 02-10-2017 14:15:19

23
Programbutton (Optional)
Depending on your experience level with hearing instruments, individual hearing needs, and the type of listening environments you experience, your hearing care professional may activate additional programs in the
hearing instrument. If additional programs have been activated, the following list explains how they work.
1. You can switch between programs by pushing the push button once.
2. You will then hear one or more beeps. The number of beeps indicates which program you have selected
(one beep=program one, two beeps=program two, etc.).
3. When the hearing instruments are turned off and then back on, the hearing instrument always returns
to the default setting (program one).
4. If you have two hearing instruments with the synchronization function enabled, program changes to one
instrument will automatically repeat in the second instrument. When a program change is made in one
instrument, you will hear the same amount of confirmation beeps in the second instrument.
Your hearing care professional can fill out the following table for you.
Program Description of when to use
1
2
3
4
400847011US-17.10-Rev.A 23 02-10-2017 14:15:19

24
i Flight mode*
When boarding a flight or entering an area where RF transmitters are prohibited, wireless functionality must be deactivated, as it is not allowed to radiate radio signals during flights or in otherwise restricted
areas.
For wireless hearing instruments follow the following steps to enter and leave flight mode:
It is possible to disable wireless operation by opening and closing the battery compartment three times
within a ten second period (open-close, open-close, open-close). Your instruments will now be in flight
mode.
If the hearing instrument is in flight mode, the hearing instrument must have been operating in flight mode
for at least 10 seconds before attempting to enable wireless again. It is possible to re-enable wireless
operation by opening and closing the battery door once. 10 seconds after this operation is completed,
wireless operation will begin again.
i
Note: It is important to wait an additional 15 seconds after wireless function resumes before opening and closing the battery compartment again for any reason. If the battery compartment is opened and
closed during this 15 second window, flight mode will resume.
* For wireless models only
400847011US-17.10-Rev.A 24 02-10-2017 14:15:19

25
Telephone use
Finding the optimal position for holding a tele phone may require practice for
some individuals, and one or more of the following suggestions may be helpful.
• Hold the telephone as you would normally.
• Hold the telephone towards the top of the ear (closer to where the microphones are).
• If whistling occurs, holding the tele phone in the same position may help the
hearing instrument to eliminate the feedback.
• Any whistling may also be decreased by holding the telephone slightly away
from the ear.
• Depending on your individual needs, your hearing care professional may
activate a program specifically for telephone use.
Telecoil (optional on some ITC, ITE, and Mic in Helix models)
If equipped, a telecoil can be activated by your hearing care professional and accessed through one of the
additional programs. A telecoil picks up a telephone’s magnetic signal and converts it to sound. An optional
telephone program may help to improve speech understanding on the telephone. When using a telecoil
program, the receiver of the telephone may need to be held closer to the hearing instrument. The handset
of the telephone may need to be moved to slightly different positions in order to find the best reception.
400847011US-17.10-Rev.A 25 02-10-2017 14:15:21

Listen to radio or TV
When listening to the TV or the radio, start out by listening to news commentators since they usually speak
clearly, then try other programs.
If you find it difficult to listen to TV or radio, your hearing care professional will be able to give you advice on
available wireless accessories to enhance your listening capabilities for TV and radio.
Using GN Hearing hearing instruments with smart phone apps
i
Intended use of smart phone apps:
GN Hearing smart phone apps are intended to be used with GN Hearing wireless hearing aids. GN Hearing
smart phone apps send and receive signals from the GN Hearing wireless hearing aids via smart phones for
which the apps have been developed.
Use with smart phone apps:
• Notifications of app updates should not be disabled, and it is recommended that the user installs all updates to ensure that the app will function correctly and will be kept up to date.
• The app must only be used with GN Hearing devices for which it is intended, and GN Hearing takes no
responsibility if the app is used with other devices.
• If you would like a printed version of the user guide for a smart phone app please consult customer support.
Using GN Hearing hearing instruments with iPhone®, iPad®, and iPod touch
®
(TruHearing Flyte)
TruHearing Flyte is a Made for iPhone instrument and allows for direct communication and control with an
iPhone, iPad, or iPod touch. For assistance in pairing and using these products with your TruHearing Flyte
wireless device, please contact your hearing care professional.
400847011US-17.10-Rev.A 26 02-10-2017 14:15:21

27
Cellular phones
Your hearing instrument is designed to comply with the most stringent Standards of International
Electromagnetic Compatibility. However, not all cell phones are hearing instrument compatible. The varying degree of disturbance can be due to the nature of your particular cellular phone or of your wireless
telephony service provider.
If you find it difficult to obtain a good result while using your cellular phone, your hearing care professional
will be able to give you advice on available wireless accessories to enhance listening capabilities.
PhoneNow (not available in IIC instruments)
The PhoneNow function allows your hearing instrument to automatically switch to your tele phone program
when a telephone receiver is raised to the ear. When the telephone receiver is removed from the ear, the
hearing instrument automatically returns to the previous listening program.
Placement of PhoneNow magnets
Place PhoneNow magnet on your telephone receiver to allow operation of
the PhoneNow function. In order to place PhoneNow magnet properly:
1. Clean the telephone receiver thoroughly.
2. Hold the telephone vertically, in a position similar to when making a
telephone call.
3. Place the magnets just below the telephone receiver. Make sure not to cover the microphone openings.
If necessary, move the magnet to another position to improve ease of use and comfort while speaking.
4. If you are not satisfied with the strength of PhoneNow, you can reposition the PhoneNow magnet or
add additional PhoneNow magnets.
400847011US-17.10-Rev.A 27 02-10-2017 14:15:23

28
i
Only use recommended cleaning agent to clean the telephone prior to placing the magnet on the
phone in order to obtain best possible adherence.
PhoneNow usage
Telephones can be used in a normal manner. A short melody will indicate that the PhoneNow feature has
automatically switched the hearing instrument to your telephone program. Initially, you may need to move
the telephone receiver slightly to find the best position for reliable PhoneNow activation and good hearing
on the telephone.
i PhoneNow warnings
1. Keep magnets out of reach of pets, children and individuals who are mentally challenged. If a magnet
is swallowed, please seek advice from a medical practitioner.
2. The magnet may affect some medical devices or electronic systems. The manufacturer of any
magnetically sensitive devices (e.g. pacemakers) should advise you regarding appropriate safety
precautions when using your hearing instrument and magnet in close proximity to the medical device
or electronic system in question.If the manufacturer cannot issue a statement, we recommend keeping
the magnet or a telephone equipped with the magnet 30 cm (12”) away from magnetically sensitive
devices (e.g. pacemakers).
i PhoneNow precautions
1. High distortion during dialing or phoning may mean that the magnet is not in the optimal position relative
to the telephone receiver. To avoid the issue, please move the magnet to another place on the telephone
receiver.
2. Only use magnets supplied by GN Hearing.
400847011US-17.10-Rev.A 28 02-10-2017 14:15:23

29
Tele-loop systems
Many places, such as theaters, houses of worship, and schools are equipped with tele-loop systems.
When using a telecoil program with tele-loop systems, sound is picked up directly and may improve speech
understanding. If there is no sound from the hearing instruments in a tele-loop system and with a telecoil
program activated, the tele-loop system may not be turned on or is not operating correctly. If a facility is not
equipped with a tele-loop system, sitting as close as possible to the front may be helpful.
i
Care and maintenance
Please follow the advices below to have the best user experience and to prolong the life of your hearing
aids.:
1. Keep your HAs dry and clean.
2. Open the battery door to dry out your HAs when you are not wearing them.
3. Wipe the HAs with a soft cloth after use to remove grease or moisture.
4. Do not wear your HAs when putting on cosmetics, perfume, after-shave, hair spray, suntan lotion etc.
These might discolour the HA or get into the HA causing damage.
5. Do not immerse your HA in any liquid.
6. Keep your HAs away from excessive heat and direct sunlight. The heat may deform the shell, damage
the electronics and deteriorate the surfaces.
7. Do not swim, shower or steam bathe while wearing your HAs.
400847011US-17.10-Rev.A 29 02-10-2017 14:15:23

30
i
Daily maintenance
It is important to keep your hearing instrument clean and dry. On a daily basis, clean the hearing instruments using a soft cloth or tissue. Remove any wax or debris from hearing instruments using a brush and/
or a wire loop. In order to avoid damage due to humidity or excessive perspiration, the use of a drying kit
is recommended.
Replacing wax filters
Custom hearing instruments may have wax filters that protect against wax and moisture. It is recommended that these are changed as needed.
For changing HF3 wax filters, the following steps are needed:
1. Brush the sound outlet area with the sound outlet pointed down.
2. Insert the threaded end of the wax filter tool into the used wax filter, and gently rotate clockwise.
3. Gently pull until the used filter is removed.
4. Discard the used filter in the slot located in the wax filter kit by pressing it into the center, sliding it to
one end of the slot, and pull until the filter is discarded.
5. Flip the wax filter tool around, locate a new filter in the dial, and press the tip of the tool into the center
of the dial.
6. Gently pull the new filter out of the dial.
7. Align the new filter to the sound outlet.
8. Press the new filter into the opening, and simultaneously pull and rock back and forth until the new
wax filter is in place.
400847011US-17.10-Rev.A 30 02-10-2017 14:15:23

31
For changing Cerustop (white) wax filters, the following steps are needed:
1. To remove the old wax guard, insert the removal side of the wax guard tool into the used wax guard so
that the shaft of the tool is touching the rim of the wax guard. Slowly pull the wax guard straight out.
2. To insert the new wax guard, gently press the replacement side of the wax guard tool straight into the
hole of the sound outlet until the outer ring lies flush with the outside of the receiver. Pull the tool straight
out -the new wax guard will remain in place.
i
Tip: Pressing on the new filter with the flat side of the wax filter tool can ensure that the filter is correctly
in place.
i
Note: If a different type of wax filter is used for your hearing instruments, or if your hearing instruments
do not use wax filters, consult your hearing care professional for guidance.
i Use only original GN Hearing consumables e.g. wax filters.
i General precautions
1. When wireless function is activated, the device uses low-powered digitally coded transmissions in
order to communicate with other wireless devices. Although unlikely, nearby electronic devices may be
affected. In that case, move the hearing instrument away from the affected electronic device.
2. When using wireless functionality and the devices are affected by electromagnetic interference, move
away from the source.
3. Only connect GM Hearing hearing instruments to GN Hearing accessories intended and qualified to be
used with GN Hearing hearing instruments.
4. Never attempt to modify the shape of the hearing instrument, earmolds, or tubing yourself.
5. For use of wireless functionality only use GN Hearing accessories. For further guidance please refer to
the user guide of the relevant GN Hearing accessory.
400847011US-17.10-Rev.A 31 02-10-2017 14:15:23

32
i General warnings
1. If a hearing aid is broken, do not use it
2. Consult a hearing care professional if you think there may be a foreign object in your ear canal, if you
experience skin irritation, or if excessive earwax accumulates with the use of the hearing aid
3. Different types of radiation, e.g. from NMR, MRI or CT scanners, may damage hearing aids. It is
recommended not to wear hearing aids during these or other similar procedures. Other types of
radiation, such as burglar alarms, room surveillance systems, radio equipment, mobile telephones,
contain less energy and will not damage hearing aids. However, they have the potential to momentarily
affect the sound quality or temporarily create undesired sounds from hearing aids
4. Do not wear hearing aids in mines, oil fields, or other explosive areas unless those areas are certified
for hearing aid use
5. Do not allow others to use your hearing aids. This may cause damage to the hearing of the other
individual or to the hearing aids
6. Hearing aid usage by children or mentally disabled persons should be supervised at all times to ensure
their safety. The hearing aid contains small parts that could be swallowed by children. Please be mindful
not to leave children unsupervised with this hearing aid
7. Hearing aids should be used only as prescribed by your hearing care professional. Incorrect use may
result in sudden and permanent hearing loss
8. Warning to hearing care professionals: Special care should be exercised in selecting and fitting hearing
aids with maximum sound pressure level that exceeds 132dB SPL with an IEC 60711:1981 occluded
ear simulator. The remaining hearing may risk further impairment.
400847011US-17.10-Rev.A 32 02-10-2017 14:15:23

33
9. When boarding a flight or entering an area where RF transmitters are prohibited, deactivate wireless
functionality. Turn off your wireless functionality by using the flight mode in areas where radio frequency
emission is prohibited
10. External devices connected to the electrical input must be safe according to the requirements of IEC
60601-1-1, IEC 600 65, or IEC 60950-1, as appropriate ( wired connection, for example HIPRO, SpeedLink)
i
Note:
• GN Hearing wireless devices operate in the frequency range of 2.4 GHz - 2.48 GHz.
• GN Hearing wireless devices include a RF transmitter that operates in the range of 2.4 GHz - 2.48 GHz.
• For use of wireless functionality only use GN Hearing accessories. For further guidance regarding e.g.
pairing, please refer to the user guide of the relevant GN Hearing accessory.
400847011US-17.10-Rev.A 33 02-10-2017 14:15:23

34
Tinnitus Sound Generator (TSG) module
Intended use for the TSG module
Your ReSound hearing instrument includes the Tinnitus Sound Generator function, a tool for generating
sounds to be used in tinnitus management programmes to relieve suffering from tinnitus.
The Tinnitus Sound Generator can generate sounds adjusted to the specific therapeutic needs and your
personal preference as determined by your doctor, audiologist, or hearing healthcare professional. Depending on the selected hearing instrument programme and the environment you are in, you will sometimes
hear the therapeutic sound resembling a continuous or fluctuating humming.
User instructions for the TSG module
Description of the device
The Tinnitus Sound Generator (TSG) Module is a software tool that generates sounds to be used in tinnitus
management programmes to relieve suffering from tinnitus.
Explanation of how the device functions
The TSG module is a frequency and amplitude shaped white-noise generator. Noise signal level and frequency characteristics can be adjusted to the specific therapeutic needs as determined by your doctor,
audiologist or hearing healthcare professional.
Your doctor, audiologist or hearing healthcare professional can modulate the generated noise with the
purpose of making it more pleasant. The noise can then resemble, for example, crashing waves on a shore.
400847011US-17.10-Rev.A 34 02-10-2017 14:15:23

35
Modulation level and speed can also be configured to your likes and needs. An additional feature can be
enabled by your hearing healthcare professional that allows you to select predefined sounds that simulate
sounds from nature, such as breaking waves or running water.
If you have two wireless hearing aids that support ear-to-ear synchronisation this functionality can be enabled by your hearing healthcare professional. This will cause the Tinnitus Sound Generator to synchronise
the sound in both hearing aids.
If your tinnitus troubles you only in quiet environments, your doctor, audiologist or hearing healthcare professional can set the TSG Module so that it becomes audible exclusively in such surroundings. The overall
sound level can be adjusted via an optional volume control. Your doctor, audiologist or hearing healthcare
professional will review with you the need for having such a control.
For hearing aids where ear to ear synchronisation is enabled your hearing healthcare professional can also
enable environmental monitoring synchronisation so that the TSG noise level is automatically adjusted simultaneously in both hearing aids dependent on the background sound level. Additionally if the hearing aid has
a volume control then the background noise level monitored by the hearing aid and the volume control can
be used simultaneously to adjust the generated noise level in both hearing aids.
TSG volume control
The sound generator is set to a specific loudness level by the hearing healthcare professional. When switching the sound generator on, the volume will have this optimal setting. Therefore, it might not be necessary
to control the volume (loudness) manually. However, the volume control provides the ability to adjust the
volume, or amount of stimulus, to the liking of the user.
400847011US-17.10-Rev.A 35 02-10-2017 14:15:23

36
Using TSG with smart phone apps
The tinnitus sound generator control via hearing aid push buttons can be enhanced with wireless control
from a TSG control app on a smart phone or mobile device. This functionality is available in supported
hearing aids when a hearing healthcare professional has enabled the TSG functionality during fitting of the
hearing aid.
To use smart phone apps the hearing aid must be connected with the smart phone or mobile device.
The scientific concepts that form the basis for the device
The TSG module provides sound enrichment with the aim of surrounding the tinnitus sound with a neutral
sound which is easily ignored. Sound enrichment is an important component of most approaches to tinnitus management, such as Tinnitus Retraining Therapy (TRT). To assist habituation to tinnitus, this needs
to be audible. The ideal level of the TSG module, therefore, should be set so that it starts to blend with the
tinnitus, and so that you can hear both your tinnitus as well as the sound used.
In a majority of instances, the TSG module can also be set to mask the tinnitus sound, so to provide temporary relief by introducing a more pleasant and controllable sound source.
400847011US-17.10-Rev.A 36 02-10-2017 14:15:23

37
Technical Specifications
Audio signal technology
Digital
Available sounds
White noise signal which can be shaped with the following configurations:
The white noise signal can be modulated in amplitude with an attenuation depth of up to 14dB.
High-pass filter Low-pass filter
500 Hz 2000 Hz
750 Hz 3000 Hz
1000 Hz 4000 Hz
1500 Hz 5000 Hz
2000 Hz 6000 Hz
400847011US-17.10-Rev.A 37 02-10-2017 14:15:23

38
i
TSG warnings
• Sound generators can be dangerous if improperly used.
• Sound generators should be used only as advised by your doctor, audiologist, or hearing healthcare
professional.
• Sound generators are not toys and should be kept out of reach of anyone who might cause themselves
injury (especially children and pets).
i
TSG precautions
• Should the user develop any side effects from using the sound generator, such as dizziness, nausea,
headaches, perceived decrease in auditory function or increase in tinnitus perception, the user should
discontinue use of the sound generator and seek medical evaluation.
• Children and physically or mentally challenged users will require guardian supervision while wearing the
TSG hearing instrument.
• The volume control is an optional feature in the TSG module used for adjusting the sound generator
output level. To prevent unintended usage by pediatric or physically or mentally challenged users, the
volume control must, if enabled, be configured to only provide a decrease of the sound generator output
level.
400847011US-17.10-Rev.A 38 02-10-2017 14:15:23

39
i
TSG warning to hearing healthcare professionals
A hearing healthcare professional should advise a prospective sound generator user to consult promptly
with a licensed physician (preferably an ear specialist) before getting a sound generator if the hearing
healthcare professional determines through inquiry, actual observation, or review of any other available
information concerning the prospective user that the prospective user has any of the following conditions:
(i) Visible congenital or traumatic deformity of the ear.
(ii) History of active drainage from the ear within the previous 90 days.
(iii) History of sudden or rapidly progressive hearing loss within the previous 90 days.
(iv) Acute or chronic dizziness.
(v) Unilateral hearing loss of sudden or recent onset within the previous 90 days.
(vi) Audiometric air-bone gap equal to or greater than 15dB at 500 hertz (Hz), 1000 Hz, and 2000 Hz.
(vii) Visible evidence of significant cerumen accumulation or a foreign body in the ear canal.
(viii) Pain or discomfort in the ear.
i
CAUTION: The maximum output of the sound generator falls into the range that can cause hearing
loss according to OSHA regulations. In accordance with NIOSH recommendations the user should not use
the sound generator for more than eight (8) hours a day when this is set to a level of 85db SPL or above.
When the sound generator is set to levels of 90db SPL or above the user should not use the sound generator for more than two (2) hours per day. In no case should the sound generator be worn at uncomfortable
levels.
400847011US-17.10-Rev.A 39 02-10-2017 14:15:23

40
i Battery warning information
Batteries, although very small, contain dangerous substances, and should be disposed of carefully. This
is for the safety of you and the environment. Please note:
1. DO NOT RECHARGE ZINK AIR BATTERIES – THEY MAY LEAK OR EXPLODE.
1. DO NOT attempt to dispose of batteries by burning them. Used batteries are harmful to the environment.
1. Please dispose of them according to local regulations or return them to your hearing care practitioner.
1. DO NOT place batteries in your mouth. Consult a physician immediately if a battery has been
swallowed, as they can be harmful to your health.
1. Keep batteries away from pets, children and individuals who are mentally challenged.
2. BATTERIES MAY LEAK. REMOVE THE BATTERY IF YOU LEAVE THE HEARING AIDS UNUSED FOR
LONGER PERIODS.
3. If the batteries are not inserted correctly, the device will not work and the batteries may build up heat.
If this happens, please remove the batteries
400847011US-17.10-Rev.A 40 02-10-2017 14:15:23

41
i
Hearing instrument expectations
A hearing instrument will not restore normal hearing and will not prevent or improve a hearing impairment resulting from organic conditions. Consistent use of the hearing instrument is recommended. In most cases,
infrequent use does not permit you to attain full benefit from it.
The use of a hearing instrument is only part of hearing rehabilitation and may need to be supplemented by
auditory training and instructions in lip-reading.
400847011US-17.10-Rev.A 41 02-10-2017 14:15:23

42
i Warning to hearing aid dispensers (US Only)
A hearing aid dispenser should advise a prospective hearing aid user to consult promptly with a licensed
physician (preferably an ear specialist) before dispensing a hearing aid if the hearing aid dispenser determines through inquiry, actual observation, or review of any other available information concerning the
prospective user, that the prospective user has any of the following conditions:
(i) Visible congenital or traumatic deformity of the ear.
(ii) History of active drainage from the ear within the previous 90 days.
(iii) History of sudden or rapidly progressive hearing loss within the previous 90 days.
(iv) Acute or chronic dizziness.
(v) Unilateral hearing loss of sudden or recent onset within the previous 90 days.
(vi) Audiometric air-bone gap equal to or greater than 15 decibels at 500 hertz (Hz), 1,000 Hz, and
2,000 Hz.
(vii) Visible evidence of significant cerumen accumulation or a foreign body in the ear canal.
(viii) Pain or discomfort in the ear.
Important notice for prospective hearing aid users (US Only)
Good health practice requires that a person with a hearing loss have a medical evaluation by a licensed
physician (preferably a physician who specializes in diseases of the ear) before purchasing a hearing aid.
Licensed physicians who specialize in diseases of the ear are often referred to as otolaryngologists, otologists or otorhinolaryngologists. The purpose of medical evaluation is to assure that all medically treatable
conditions that may affect hearing are identified and treated before the hearing aid is purchased.
400847011US-17.10-Rev.A 42 02-10-2017 14:15:23

43
Following the medical evaluation, the physician will give you a written statement that states that your
hearing loss has been medically evaluated and that you may be considered a candidate for a hearing aid.
The physician will refer you to an audiologist or a hearing aid dispenser, as appropriate, for a hearing aid
evaluation.
The audiologist or hearing aid dispenser will conduct a hearing aid evaluation to assess your ability to hear
with and without a hearing aid. The hearing aid evaluation will enable the audiologist or dispenser to select
and fit a hearing aid to your individual needs. If you have reservations about your ability to adapt to amplification, you should inquire about the availability of a trial-rental or purchase-option program. Many hearing
aid dispensers now offer programs that permit you to wear a hearing aid for a period of time for a nominal
fee after which you may decide if you want to purchase the hearing aid.
Federal law restricts the sale of hearing aids to those individuals who have obtained a medical evaluation
from a licensed physician. Federal law permits a fully informed adult to sign a waiver statement declining
the medical evaluation for religious or personal beliefs that preclude consultation with a physician. The
exercise of such a waiver is not in your best health interest and its use is strongly discouraged.
Children with hearing loss (US Only)
In addition to seeing a physician for a medical evaluation, a child with a hearing loss should be directed
to an audiologist for evaluation and rehabilitation since hearing loss may cause problems in language
development and the educational and social growth of a child. An audiologist is qualified by training and
experience to assist in the evaluation and rehabilitation of a child with a hearing loss.
400847011US-17.10-Rev.A 43 02-10-2017 14:15:23

44
Troubleshooting Guide
SYMPTOM CAUSE
POSSIBLE REMEDY
No sound
Not turned on
Turn on by closing the battery door
Dead battery
Replace battery
Battery door will not close
Insert battery properly
Blocked wax filter
Replace wax filter or consult your hearing care professional
Not loud enough
Incorrect earmold placement
Reinsert hearing instrument carefully
Blocked sound outlet filter
Change filter or consult your hearing care professional
Change in hearing sensitivity
Consult your hearing care professional
Excessive ear wax
Consult your physician
Volume set too low
Increase the volume control if available or consult your hearing care professional
400847011US-17.10-Rev.A 44 02-10-2017 14:15:23

45
POSSIBLE REMEDY
Turn on by closing the battery door
Replace battery
Insert battery properly
Replace wax filter or consult your hearing care professional
Reinsert hearing instrument carefully
Change filter or consult your hearing care professional
Consult your hearing care professional
Consult your physician
Increase the volume control if available or consult your hearing care professional
400847011US-17.10-Rev.A 45 02-10-2017 14:15:23

46
* If there are any other problems not mentioned in this guide, please contact your hearing care professional.
Troubleshooting Guide
SYMPTOM CAUSE
POSSIBLE REMEDY
Excessive
whistling / feedback
Incorrect custom placement in ear
Re-insert custom product carefully
Excessive ear wax
Consult your hearing care professional
Feedback control may need adjustment
Consult your hearing care professional
Hearing instrument settings not optimal
Consult your hearing care professional
Sound distorted / not clear
Weak battery
Replace battery
Improper fit
Consult your hearing care professional
Hearing instrument damaged
Consult your hearing care professional
Hearing instrument settings not optimal
Consult your hearing care professional
Wireless does not work
Possible Root Cause - Device is in flight mode
Open and close the battery compartment once. Wireless will reactivate 10 seconds later.
(If Root Cause is device in flight mode)
400847011US-17.10-Rev.A 46 02-10-2017 14:15:23

47
POSSIBLE REMEDY
Re-insert custom product carefully
Consult your hearing care professional
Consult your hearing care professional
Consult your hearing care professional
Replace battery
Consult your hearing care professional
Consult your hearing care professional
Consult your hearing care professional
Open and close the battery compartment once. Wireless will reactivate 10 seconds later.
(If Root Cause is device in flight mode)
400847011US-17.10-Rev.A 47 02-10-2017 14:15:24

48
Technical Data
HEARING INSTRUMENT MODEL
MAXIMUM OUTPUT
(2ccCoupler / IEC 60118-7
and ANSI S3.22-2009)
All Low Power (LP) models including IIC 115 dB SPL (typical)
All Medium Power (MP) models 119 dB SPL (typical)
All High Power (HP) models 121 dB SPL (typical)
All Ultra Power (UP) models 130 dB SPL (typical)
400847011US-17.10-Rev.A 48 02-10-2017 14:15:24

49
Warranty and repairs
GN Hearing provides a warranty on hearing instruments in the event of defects in workmanship or materials, as described in applicable warranty documentation. In its service policy, GN Hearing pledges to secure
functionality at least equivalent to the original hearing instrument. As a signatory to the United Nations
Global Compact initiative, GN Hearing is committed to doing this in line with environment-friendly best
practices. Hearing instruments therefore, at GN Hearing’s discretion, may be replaced by new products
or products manufactured from new or serviceable used parts, or repaired using new or refurbished replacement parts. The warranty period of hearing instruments is designated on your warranty card, which
is provided by your hearing care professional.
For hearing instruments that require service, please contact your hearing care professional for assistance.
GN Hearing hearing instruments that malfunction must be repaired by a qualified technician. Do not attempt to open the case of hearing instruments, as this will invalidate the warranty
Temperature test, transport and storage information
GN Hearing Aids are subjected to various tests in temperature and damp heating cycling between -25°C
(-13°F) and +70°C (+158°F) according to internal and industry standards.
During transport or storage, the temperature should not exceed the limit values of -20°C (-4°F) to +60°C
(+140°F) and relative humidity of 90% RH, non-condensing (for limited time). The air pressure between 500
hPa and 1,100 hPa is appropriate.
400847011US-17.10-Rev.A 49 02-10-2017 14:15:24

50
Be aware of information marked with the warning symbol
WARNING points out a situation that could lead to serious injuries,
i CAUTION indicates a situation that could lead to minor and moderate injuries.
i
Advice and tips on how to handle your hearing instrument better.
Equipment includes RF transmitter.
i
“Made for iPhone” means that an electronic accessory has been designed to connect specifically
to iPhone and has been certified by the developer to meet Apple performance standards. Apple is
not responsible for the operation of this device or its compliance with safety and regulatory standards. Please note that the use of this accessory with iPhone may affect wireless performance.
TruHearing Flyte is compatible with iPhone 7 Plus, iPhone 7, iPhone 6s Plus, iPhone 6s, iPhone 6 Plus, iPhone 6, iPhone SE, iPhone
5s, iPhone 5c, iPhone 5, iPad Pro (12.9-inch), iPad Pro (9.7-inch), iPad Air 2, iPad Air, iPad mini 4, iPad mini 3, iPad mini 2, iPad
mini, iPad (4th generation), iPod touch (6th generation) and iPod touch (5th generation) using iOS 8.X or later. Apple, the Apple logo,
iPhone, iPad Pro, iPad Air, iPad mini, iPad and iPod touch are trademarks of Apple Inc., registered in the U.S. and other countries.
Android is a trademark of Google Inc.
©2016 TruHearing, Inc. All Rights Reserved. TruHearing® is a registered trademark of TruHearing, Inc. All other trademarks, product
names, and company names are the property of their respective owners.
400847011US-17.10-Rev.A 50 02-10-2017 14:15:25

51
Please ask your local hearing ca re profe ssional
concerning disposal of your hearing instrument
400847011US-17.10-Rev.A 51 02-10-2017 14:15:26

User Guide Chime RIE
Distributed by:
TruHearing Inc.
12936 S. Frontrunner Blvd
Draper, UT 84020
USA
(844) 278-8672
Manufacturer
GN Hearing A/S
8001 E. Bloomington Freeway
Bloomington, MN 55420-1036
40 08 47 0 11U S -17.10-R ev
400847011US-17.10-Rev.A 52 02-10-2017 14:15:42
 Loading...
Loading...