Gnatus Syncrus GL User manual


PRESENTATION OF MANUAL
INSTRUCTIONS FOR USE
Technical Name: Dental Chair
Brand: Gnatus
Trade Name: Syncrus GL Dental Chair
Manufacturer/ Distribuitor:
GNATUS - EQUIPAMENTOS MÉDICO-ODONTOLÓGICOS LTDA.
Rod. Abrão Assed , Km 53+450m - Cx. Postal 782 CEP 14097-500
Ribeirão Preto - S.P. - Brasil
Fone +55 (16) 2102-5000 - Fax +55 (16) 2102-5001
C.N.P.J. 48.015.119/0001-64 - Insc. Est. 582.329.957.115
www.gnatus.com.br - gnatus@gnatus.com.br
Technical Duties: Gilberto Henrique Canesin Nomelini
CREA-SP: 0600891412
Registration ANVISA nº: 10229030041
ATTENTION
For greater safety:
Read and understand all the instructions contained in these
Instructions for Use before installing or operating this Equipment.
Note: These Instructions for Use must be read by all the operators
of this Equipment.
2

INDEX
PRESENTATION OF MANUAL ........................................................................02
IDENTIFICATION OF EQUIPMENT .................................................................04
- Principles and bases applied to the functioning of the product..............................05
- Purpose of equipment ....................................................................................05
- Description of Equipment ...............................................................................05
- Indication of Equipment ................................................................................06
MODULES, ACCESSORIES, OPTIONS AND MATERIALS OF CONSUMPTION .....07
TECHNICAL SPECIFICATIONS .......................................................................09
- Technical Specications ..................................................................................09
- Standards applied .........................................................................................10
- List of pieces and circuit scheme .....................................................................10
- Electromagnetic emissions ..............................................................................11
- Contents of the accessible and inaccessible markings ........................................14
- Dimensions ..................................................................................................15
- Symbologies of packaging .............................................................................17
- Symbologies of product .................................................................................17
INSTALLATION OF EQUIPMENT ....................................................................18
OPERATION OF EQUIPMENT .........................................................................20
PRECAUTIONS, RESTRICTIONS AND WARNINGS ..........................................23
- Conditions of transport, storage and operation ..................................................23
- Sensitiveness to environmental conditions foreseeable in normal situations of use .23
- Precautions and warnings “during the installation” of equipment ..........................23
- Recommendations for preserving the equipment. ...............................................24
- Precautions and warnings “during the use” of equipment ....................................24
- Precautions and warnings “after” the use of equipment ......................................24
- Precautions and warnings during the “cleaning and disinfection” of equipment .......25
- Precautions in case of alteration in the functioning of equipment ..........................25
- Precautions to be adopted against foreseeable or uncommon risks,
related to the deactivation and abandoning of equipment ......................................25
CORRECTIVE AND PREVENTIVE MAINTENANCE AND PRESERVATION............26
- Additional procedures for reuse .......................................................................26
- Cleaning ......................................................................................................26
- Disinfection ..................................................................................................26
- Preventive maintenance .................................................................................27
- Corrective maintenance..................................................................................27
UNFORESEEN EVENTS – SOLUTION OF PROBLEMS ........................................27
WARRANTY OF EQUIPMENT ..........................................................................28
FINAL CONSIDERATIONS ..............................................................................28
33
DESCRIPTION OF THE EQUIPMENT
Dear Customer
Congratulations. You have made a good choice when you decided to buy a GNATUS
QUALITY product comparable to the best products available in the World. This manual is a
general presentation of your product and it will give you important details to help you to
solve possible problems.
Please, read it and keep this with you.
Identication
Technical Name Dental Chair
Trade Name: Syncrus GL Dental Chair
Brand: GNATUS
4

DESCRIPTION OF THE EQUIPMENT
Principles and fundamentals applied to the product functioning
Chair for patient accommodation during dental treatment, activated by foot control,
where commands are sent from the electronic board to two motors/engines responsible for
mechanism movement of seat and backrest of the chair.
Purpose of the equipment
Chair for patient accommodation during dental treatment.
Description of Equipment
Dental chair to accept patient during treatments odontological, with automatic
movements, used to work with – position left and right-handed professionals, it starting
by a motor DC.
Operated by foot control, which many function in the controls and it work with
gradual dental light intensity, seat and backrest movements, return to initial position, and
programmable work position which are set by the dentist.
It is innovative and modern round-edged design. It curved of the backrest, which improves
for patient with high comfort but produce also approach of the doctor.
Base with ergonomic design, made of steel, protected by debrun antiskid. It has 2 holes
which allows the option of fastening the dental chair to the oor.
Steel-built structure with a resistant, smooth, high-shine, round-edged coating.
It High-shine smooth epoxy coating, polymerized in stove at 250ºC, with phosphate
process resistant to rust and cleaning products.
The headrest anatomic, removable with height adjustment. Optional cervical rest, which
improves high patient comfort.
Round edged xed armrest, designed to ease the patient access and improve the dentist
productivity, avoiding unnecessary movements and making cleaning and disinfections much
easier.
Ample seamless upholstery, with lumbar rest, mounted on a rigid structure covered
with a high-resistant polyurethane cover, this coverage use material plastic and seamless
upholstery.
ISO 9001/2000 and ISO 13485/2003 Quality System, assuryng the products are
manufactured under standart procedures.
Products manufactured in agreement with RDC-59 - ANVISA (Sanitary Surveillance
National Agency) with guarantees accomplishiment to the sanitary legislation according to
BPF (Good Manufacturing Procedures). In order to comply with directive 93/42/EEC for CE
Mark purpose, it is dened that the life span of this product is estimated in 05 years provided
that the enduser follows the requirements and instructions of this manual.
55

DESCRIPTION OF THE EQUIPMENT
IMPORTANT:
• This equipment is for dental use use only. It must be operated and utilized by specialized
professional (certied professional, according to the legislation of the country) and following
the instructions of the manual. The operation of the equipment required, for the professional,
the utilization of correct instruments and it should to be in perfect conditions of the use,
and to protect the professional, the patients and others, in the eventual danger situation.
• This equipment can’t be used in the presence of inammable anesthetics or products
that may cause explosion.
• After nish life-cycle of the equipment, this equipment is throw way, so it should to be
destroye in appropriate area (according to the legislation of the country).
•To guarantee the safe functioning of your equipment, use only the assemble
congurations (Dental Chair, Dental and Water Units and Dental Light) supplied by Gnatus
authorized Dealers / Technical Assistance.
Indication of Equipment
This equipment is for dental use use only. It must be operated and utilized by specialized
professional (certied professional, according to the legislation of the country) and following
the instructions of the manual. The operation of the equipment required, for the professional,
the utilization of correct instruments and it should to be in perfect conditions of the use,
and to protect the professional, the patients and others, in the eventual danger situation.
6
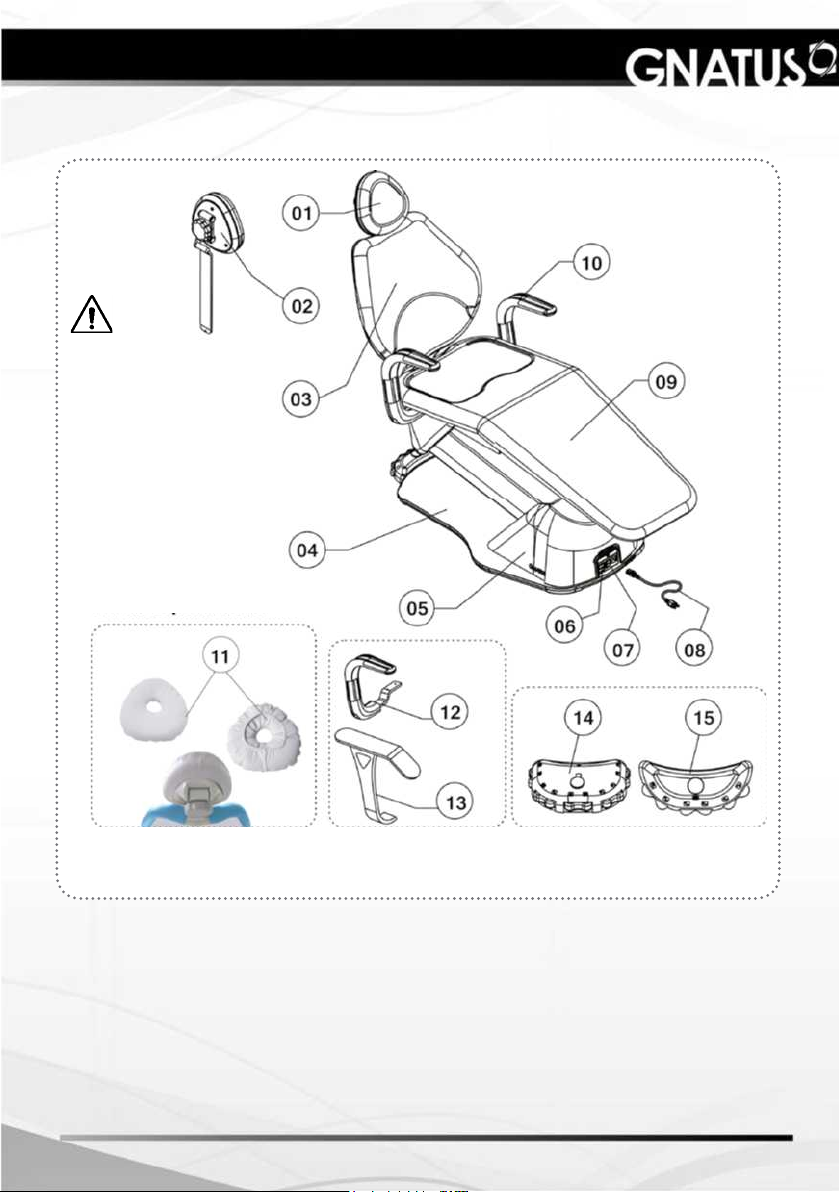
MODULES, ACCESSORIES, OPTIONS AND MATERIALS
OF CONSUMPTION
Syncrus GL Dental Chair
(without any built-in junction box)
The Drawing illustrates the
equipment with all the optional
items. Your delivery unit will only
be composed of the items chosen
during your purchase option.
The contents of this page are
of an informative nature, the
equipment being able to differ
from that illustrated. So, upon
acquiring the product check
the technical compatibilty
between equipment, coupling
and accessories.
Items Optional:
WARNING: This dental chair meets the requirements of stability dened by international
safety standards which dispenses with fastening it to the oor. If the customer opts to fasten the
chair to the oor, it has 2 holes in the base for this purpose.
01 - Headrest
02 - Headrest Bi-articulated (optional)
03 - Backrest
04 - Base
05 - Motor lid
06 - Fuse
07 - On/off switch
08 - Inlet cord
09 - Seat
10 - Armrest (optional)
11 - Cervical rest (optional)
12 - Swivel armrest (optional)
13 - Fixed armrest (optional)
14 - Foot control with 11 functions (optional)
15 - Foot control with 07 functions (optional)
77
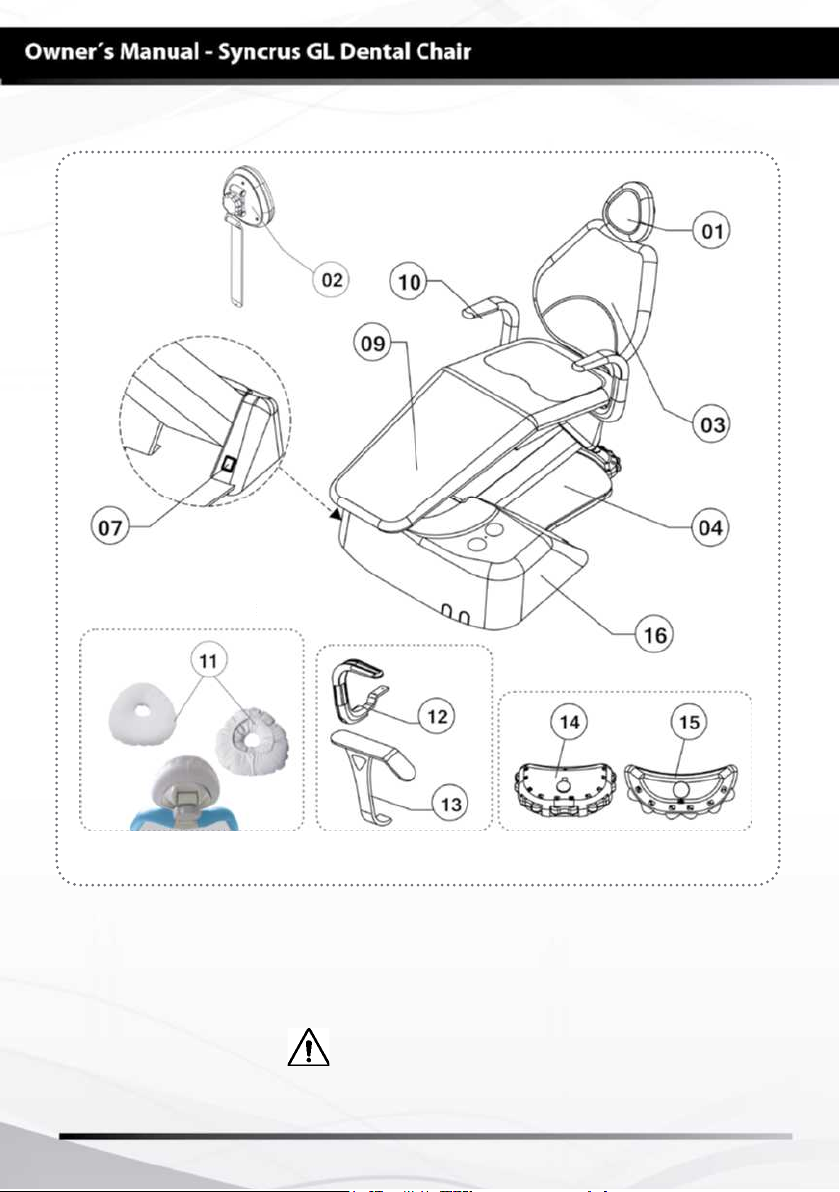
MODULES, ACCESSORIES, OPTIONS AND MATERIALS
OF CONSUMPTION
Syncrus GL Dental Chair
(with any built-in junction box)
Items Optional:
WARNING: For the chair with integrated connection box, it is recommended to x the base of the chair to
the oor, according to guidelines in the “Setup” section of this manual.
01 - Headrest
02 - Headrest Bi-articulated (optional)
03 - Backrest
04 - Base
07 - On/off switch
09 - Seat
10 - Armrest (optional)
11 - Cervical rest (optional)
informative nature, the equipment being able to differ from that
illustrated. So, upon acquiring the product check the technical
compatibilty between equipment, coupling and accessories.
The Drawing illustrates the equipment with all the optional items.
Your delivery unit will only be composed of the items chosen during
your purchase option.
12 - Swivel armrest (optional)
13 - Fixed armrest (optional)
14 - Foot control with 11 functions (optional)
15 - Foot control with 07 functions (optional)
16 - Built-in junction box (optional)
The contents of this page are of an
8

TECHNICAL SPECIFICATIONS
General
Classification of Equipment:
According to norm NBR IEC 60601-1
Supply voltage
127/220 V~ Selecionable
Frequency
50/60 Hz
Electric shock protection type
Class one equipment
Degree of protection against electric shock
B type
Mode of Operation
Continuous with intermittent load: T-on 1min. - T-off 4min.
Water leak protection
IPX0 – All the chair, excepting foot control
IPX1 – foot control
Power
200VA
Protection fuses
F1 e F2 5A - Time-lag
Rising capacity
200 Kg
Net Weight
104 Kg
Gross Weight
137,3 Kg
Delivery unit tray’s maximum load capacity
Syncrus line HS - 1 Kg / Syncrus lines LS and S - 2 Kg
99

TECHNICAL SPECIFICATIONS
Chair for left and right-handed dentists use
Chair was designed for easy installation for left and right handed users, without losing
its features.
When the Gnatus authorized technician installs the equipment, tell him whether position
you want, and he will make the necessary adjustments.
Emergency stop
An “emergency stop” key is provided. When pressed, all functions and movements
are automatically interrupted. When pressed again, unit returns to operation, keeping the
programming and positions previously preset.
Current surge protection
The equipment is endowed with high current, safety device that uses 2 fuses and internal
overpower protection for the equipment that is attached to the chair.
List of pieces and circuit scheme
Gnatus Company declares that the supply of the circuit scheme, list of pieces or any
other information that propitiate technical attendance for the user, can be request if there
is an agreement between the user and Gnatus Company.
Standards applied:
NBR 60601-1:1997 - Equipamento Eletromédico- Parte 1: Prescrições gerais para segurança;
NBR ISO 6875:1998 - Equipamento odontológico – Cadeira odontológica de paciente;
NBR ISO 14971:2004 - Medical devices - application of risk management medical devices;
NBR ISO 9687: 2005 - Dental equipment - graphical symbols;
EN ISO 13485-2003 - Quality systems - medical devices;
IEC 60601-1-2:2007 - Compatibilidade Eletromagnética.
The materials used to produce the equipment are Biocompatible.
10
 Loading...
Loading...