
PRESENTATION OF MANUAL
INSTRUCTIONS FOR USE
EQUIPMENT:
Technical Name: Dental Delivery Units and Accessories
Trade Name: Mini Delivery Unit
Brand: GNATUS
Manufacturer/ Distribuitor:
GNATUS - EQUIPAMENTOS MÉDICO-ODONTOLÓGICOS LTDA.
Rod. Abrão Assed , Km 53+450m - Cx. Postal 782 CEP 14097-500
Ribeirão Preto - S.P. - Brasil
Fone +55 (16) 2102-5000 - Fax +55 (16) 2102-5001
C.N.P.J. 48.015.119/0001-64 - Insc. Est. 582.329.957.115
www.gnatus.com.br - gnatus@gnatus.com.br
Technical Duties: Gilberto Henrique Canesin Nomelini
CREA-SP: 0600891412
Registration ANVISA #: 10229030047
ATTENTION
For greater safety:
Read and understand all the instructions contained in these
Instructions for Use before installing or operating this Equipment.
Note: These Instructions for Use must be read by all the operators
of this Equipment.
2

INDEX
PRESENTATION OF MANUAL .........................................................................02
IDENTIFICATION OF EQUIPMENT ................................................................04
- Dear Customer ....................................................................................... .....04
- Identification ................................................................................................04
- Principles and bases applied to the functioning of the product..............................05
- Description of Equipment ..............................................................................05
- Indication of Equipment ................................................................................06
MODULES, ACCESSORIES, OPTIONS AND MATERIALS OF CONSUMPTION .....07
TECHNICAL SPECIFICATIONS ......................................................................09
- Technical features of the Delivery Unit and its accessories ................................... 09
- Standards applied ........................................................................................11
- Dimension ...................................................................................................12
- Symbologies of packaging .............................................................................13
- Symbologies of product ..................................................................................13
- Contents of the accessible and inaccessible markings .........................................14
INSTALLATION OF EQUIPMENT ...................................................................14
OPERATION OF EQUIPMENT ........................................................................15
- Activation of Terminals ..................................................................................15
- Adjustment of Spray .....................................................................................15
- Activation of ejectors .....................................................................................15
- Use of 3-Way Syringe ...................................................................................16
- Curing Light Activation ...................................................................................16
- Laser Hand Activation ....................................................................................17
- How to supply the reservoirs ..........................................................................18
PRECAUTIONS, RESTRICTIONS AND WARNINGS .........................................19
- Conditions of transport and storage ................................................................19
- Environmental conditions of operation .............................................................19
- Sensitiveness to environmental conditions foreseeable in normal situations of use .19
- Precautions and warnings “during the installation” of equipment .........................19
- Recommendations for preserving the equipment ................................................20
- Precautions and warnings “during the use” of equipment ...................................20
- Precautions and warnings “after” the use of equipment .....................................20
- Precautions and warnings during the “cleaning and disinfection” of equipment ......21
- Precautions in case of alteration in the functioning of equipment .........................21
- recautions to be adopted against foreseeable or uncommon risks,
related to the deactivation and abandoning of equipment .....................................21
CORRECTIVE AND PREVENTIVE MAINTENANCE AND PRESERVATION ...........21
- Additional procedures for reuse ......................................................................21
- Cleaning .....................................................................................................22
- Preventive maintenance ................................................................................24
- Corrective maintenance..................................................................................24
UNFORESEEN EVENTS – SOLUTION OF PROBLEMS .......................................25
WARRANTY OF EQUIPMENT .........................................................................26
FINAL CONSIDERATIONS .............................................................................26
3

DESCRIPTION OF THE EQUIPMENT
Dear Customer
Congratulations. You have made a good choice when you decided to buy a GNATUS
QUALITY product comparable to the best products available in the World. This manual is a
general presentation of your product and it will give you important details to help you to
solve possible problems.
Please, read it and keep this with you.
Identication
Technical Name: Dental Delivery Units and Accessories
Trade Name: Mini Delivery Unit
Brand: GNATUS
4

DESCRIPTION OF THE EQUIPMENT
Principles and bases applied to the functioning of the product
Posee mangueras con aire comprimido y conectores para la alimentación de las piezas
de mano (alta y baja rotación) y jeringa con salida de agua y aire.
It has ejectors, with suction activated by a venturi system or vacuum pump with
compressed air.
Description of Equipment
MINI DELIVERY UNIT is a portable unit with great fastening versatility. (E.g. tables,
cabinets, benches, etc).
Body made of high impact polystyrene.
Activation and control of the syringe, rotary instruments etc., providing greater proximity
in the work field.
Saliva/blood ejector with intermediary filter easy to access.
Automatic selection of the handpieces through sensitive pneumatic valves.
Unique foot control of activation of the handpieces, which can be activated in any
position.
Smooth, rounded hoses, without grooves or riing.
It has translucent reservoirs easy to access with automatic pressurization of water for
syringe/spray of the handpieces and chlorinated water for the “optional” Bio-System. The
Bio-System is a disinfection system, which provides internal cleaning of the hoses and
terminals via bactericidal liquid, preventing risks of cross contamination.
To guarantee the safe functioning of your equipment, use only the assemble configurations
(Dental Chair, Dental and Water Units and Dental Light) supplied by Gnatus authorized
Dealers / Technical Assistance.
EN ISO 9001/2000 and EN ISO 13485/2003 Quality System, assuryng the products
are manufactured under standart procedures.
Products manufactured in agreement with RDC 59/00 - ANVISA - (Sanitary Surveillance
National Agency).
Laser Hand Kit (optional item) – Features of the product:
See the Owner’s Manual - Laser Hand
Curing light (optional item) – Features of the product:
The Curing Light belongs to the newest generation of LED photo-activation devices. This
abbreviation stands for Light Emitting Diode, a totally different type of light emission, if
compared to conventional halogen equipment.
Unlike traditional devices, which generate wide-spectrum light and heat, this technology
uses a cold light of the precise wave length needed to activate various dental products.
LED technology, which was recently introduced in Dentistry, brought about several useful
features to those light-curing devices used in composite resin restoration. Besides being
more durable, LED technology turned devices more compact, ergonomic and easier to install
and transport. The emission of cold light within a precise wave length range ensures the
safe cure of camphorquinone-activated composites, preventing dental heating, pulp damage
or discomfort for both patient and dentist. Although being relatively new, this technology
is nowadays in its second generation. LED safety and efficiency, now allied to high-energy
emission, are available to all clinic procedures which require light-curing power, including
bleaching treatments.
5

DESCRIPTION OF THE EQUIPMENT
The light of 440nm-460nm wave length, allied to the high energy emitted by Curing
Light, makes possible the multi-functionality of this device:
- Direct restoration procedures: composite resins, ionomers and adhesives.
- Indirect restorations: adhesive cementation of laminates, inlays, esthetic pins and
metal-free crowns.
- Dental Bleaching: activation of bleaching gel and polymerization of gingival barriers.
Compatible with 35% hydrogen peroxide-based bleaching gels.
- Attachment of braces and orthodontic accessories.
- Activation of light-cure materials, such as sealants, surgical cements and covering
bases.
Designed and built with cutting-edge technology, it meets the highest standards specified
by world’s dental authorities.
Operation control display in handpiece, sound alarm with beep every 10 seconds and 4
beeps at the end of the cycle.
Advantages offered by Curing Light:
- More spectrally-selective light than conventional lamps.*
- Cold light, it doesn’t heat up the resin nor the tooth**
• Light compact equipment that provides handling comfort.
• Low power consumption.
• Longer useful life of the light emitting diode (equivalent to 36.000.000 cycles of 10
seconds).
• It does not use optical filter.
• It does not require forced ventilation, thus avoiding noise emission.
We noted that the light emitted by the Curing Light is completely contained within the
absorption interval of the photo starter, therefore it’s 100% used, whereas the conventional
equipment running on halogen lamps has non-used wave-length regions.
The Curing Light doesn’t generate heat since it uses light emitting diodes.
The light conductor is removable, made out of high resistance polymer and of easy
maintenance.
Indication of Equipment
This equipment is for dental use use only. It must be operated and utilized by specialized
professional (certified professional, according to the legislation of the country) and following
the instructions of the manual. The operation of the equipment required, for the professional,
the utilization of correct instruments and it should to be in perfect conditions of the use, and
to protect the professional, the patients and others, in the eventual danger situation.
6
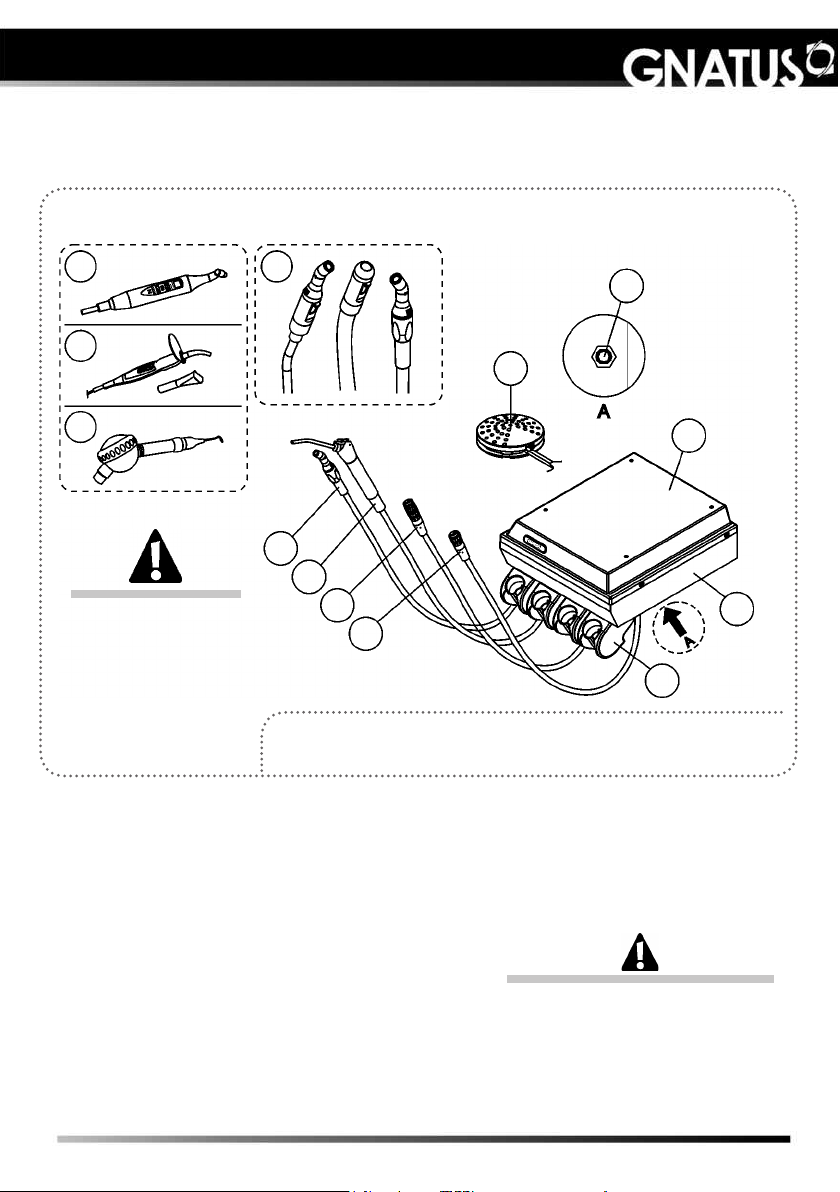
MODULES, ACCESSORIES, OPTIONS AND MATE
RIALS OF CONSUMPTION
Delivery Unit Mini
09
10
11
The Drawing illustrates
the equipment with all
the optional items. Your
delivery unit will only be
composed of the items
chosen during your
purchase option.
01 - “Venturi or vacuum pump” ejector (optional)
02 - Triple syringe
03 - High-speed-motor terminals (optional)
04 - Low-speed-motor terminal (optional)
05 - Support of the Points (optional Point four)
06 - Bod
07 - Delivery unit cover
08 - Foot control
09 - Kit Laser Hand (optional)
10 - Curing Light (optional)
11 - Bicarbonate jet “Hand”(optional)
12 - Bio-System activation (optional)
“available in the delivery units configured with
Bio-System”
01
01
02
03
04
Basic configuration of the product (composed of)
1-Triple syringe, 1-High-speed-motor terminal TB, 1-Low-speed-motor
terminal (without spray).
12
08
07
06
05
The contents of this page are of an
informative nature, the equipment
being able to differ from that illustrated.
So, upon acquiring the product check
the technical compatibilty between
equipment, coupling and accessories.
7
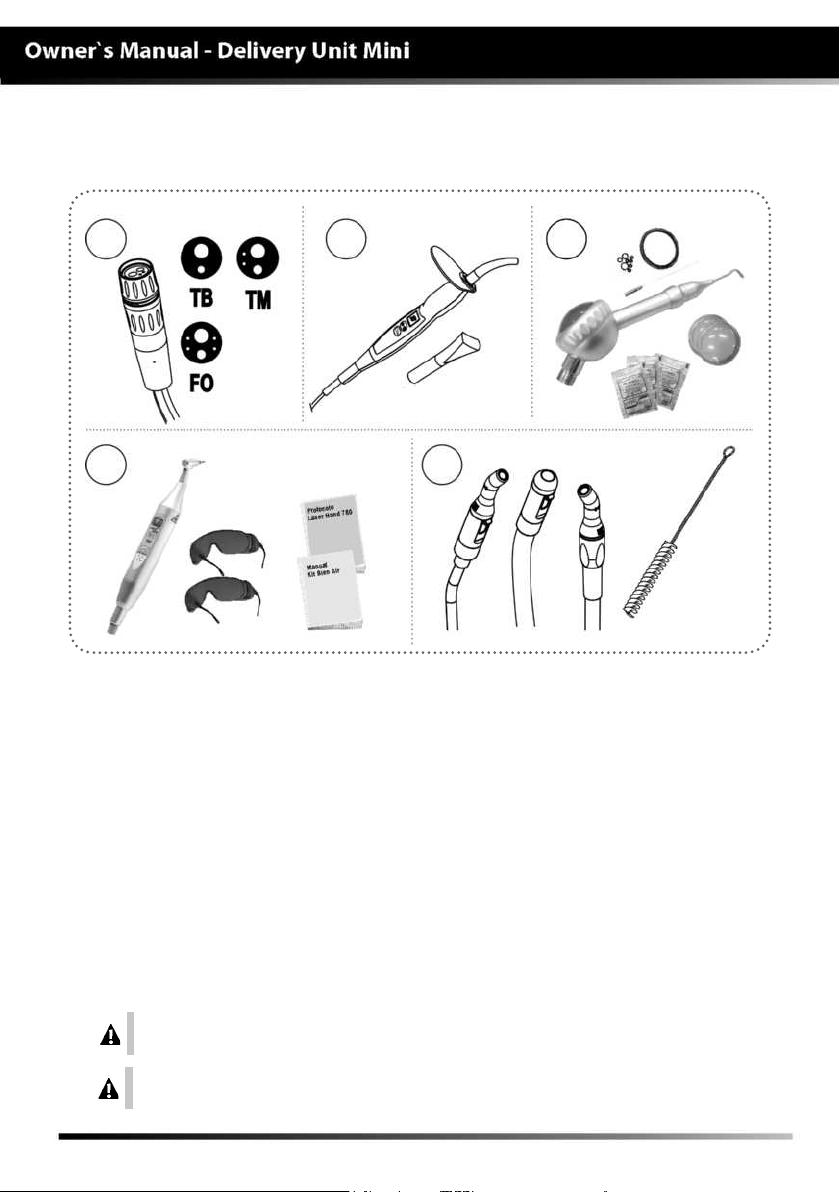
MODULES, ACCESSORIES, OPTIONS AND MATE
RIALS OF CONSUMPTION
01
04
01 - Terminals (optional)
- FO:Optical fiber terminal
- TM:Midwest terminal
- TB:Borden terminal
02 - Curing light + tip for 3 teeth
(optional)
03 - Bicarbonate jet kit “Jet
Hand”(optional)
- Bicarbonate jet
- Opener
- Covers for reservoir
- Rings for sealing
02
03
05
- Sachet of bicarbonate
- Manual
04 - Hand Laser kit (optional)
(Nº Registration Anvisa 80051420005)
- Laser Hand
- Safety goggles “patient and
professional”
- Manuals
05 - Ejectors (optional)
- Venturi Ejector
- Larger ejector for Vacuum Pump
- Smaller ejector for Vacuum Pump
- Brush for cleaning the ejector
The use of any part, accessory or material not specified or foreseen
in these instructions for use is entirely the user’s responsibility.
The accessories described above shall never be able to be sold separately
from the product.
8

TECHNICAL SPECIFICATIONS
Technical features of the Delivery Unit and its accessories
General
Model
Mini Delivery Unit
Classification of Equipment as per ANVISA:
Class II
Classification of Equipment as per standard IEC 60601-1:
Protection against Electric Shock - Type B and Class I Equipment (IEC 60601-1)
Degree of safety of application in presence:
Equipment not suited to an anesthetic mixture inammable with air, oxygen or nitrous
oxide.
Mode of Operation
Continuous operation with intermittent load
Power Supply
Inlet air pressure
80 PSI (5,52 BAR)
Other specications
Capacity of water reservoir
800ml
High rotation air consumption
9 l/min
High rotation water consumption
0,02 l/min
Inlet air pressure - Syringe
40 PSI (2,76 BAR)
Syringe air consumption
17 l/min
Syringe water consumption
0,1 l/min
Net weight (with all the options)
2.55 Kg
Gross weight (with all the options)
3.20 Kg
9

TECHNICAL SPECIFICATIONS
Venturi suction system – Maximum vacuum
220 mm/Hg
Venturi suction system – Volumetric displacement
30 l/min
“Bio Vac II Vacuum Pump” suction system – Maximum vacuum
400 mm/Hg
“Bio Vac II Vacuum Pump” suction system – Volumetric displacement
120 l/min
“Bio Vac IV Vacuum Pump” suction system – Maximum vacuum
550 mm/Hg
“Bio Vac IV Vacuum Pump” suction system – Volumetric displacement
350 l/min
Specications of Curring Light
Power
5,2VA
Light source
1 LED
Active medium
Semicondutor Led (InGaN)
Wavelength
440nm - 460nm
Timer
90 seconds
Timer alarm
sound alarm with beep every 10 seconds and 4 beeps at the end of the cycle
Activation
Through the hand-piece button
Light conductor
Made out of special polymer, rotational, removable and reuse sable.
Hand-piece body
ABS injected
10

TECHNICAL SPECIFICATIONS
Standards applied:
This product was tested and approved as per the standards:
EN 60601-1 (1990);
Amendment 1 EN 60601-1 (1992);
Amendment 2 EN 60601-1 (1995);
Amendment13 EN 60601-1 (1995);
EN 60601-1-3 (2001);
EN 60601-2-7 (2001);
EN 60601-2-28 (2001);
EN 60601-2-32 (2001);
Emenda 1 IEC 601-1;
IEC série 60601-1 Equipamento Eletromédico - Parte 1: Prescrições gerais para segurança;
EN ISO 980:2008 (Ed. 2) - Graphical symbols for use in the labelling of medical devices;
EN ISO 14971:2007 - Medical devices - application of risk management medical devices;
NBR ISO 9687: 2005 - Dental equipment - graphical symbols;
ISO 7494:2004 - Norma dental units;
EN ISO 13485-2003 - Quality systems - medical devices;
ISO 780:1997 - Packaging - pictorial marking for handling goods;
ISO 11144:1995 - Norma dental equipment - connections for suply and waste lines.
The Equipment maintains its condition of safety and efficacy, provided that
it is maintained (stored) as mentioned in this instruction for use. Thus, the
equipment will not lose or alter its physical and dimensional features.
11

TECHNICAL SPECIFICATIONS
Dimensions (mm)
12

TECHNICAL SPECIFICATIONS
Packing symbols
Maximum stacking:
It determines the maximum
quantity of boxes which can be
stacked during transportation
and storage “as per packaging”.
Packing to be transported and / or
stored with the harrows up.
Packing to be transported and /
or stored with care (should not
suffer drop and neither receive
impact).
Product symbols
Careful: It indicates an important
instruction for the operation of the
product. Not following it can cause
dangerous malfunctioning.
Note: It indicates useful
information for operation of the
product.
Packing to be transported and
/ or stored avoiding humidity,
rains and wet oor.
The packing must be stored and
transported away from direct
sun light exposure.
Temperature limit for the
packing to be stored or
transported.
Bio-System operation
High-speed with FO
Curring Light
Important: It indicates an
instruction of safety for operation
of the product. Not following it,
can lead to serious danger to the
patient.
Landing (in many parts of the
equipment) indicates the condition
of being landed.
B type equipment
Triple syringe
BV ejector
Ejector type Venturi
13

TECHNICAL SPECIFICATIONS
Content of accessible and non-accessible demarcations
INSTALLATION OF EQUIPMENT
The installation of this equipment requires specialized technical
assistance (Gnatus).
OBS: These information also make part of the Manual of Installation
and Maintenance of the equipment that can be found with the
authorized Gnatus technician.
- This equipment shall only be able to be unpacked and installed by a Gnatus authorized
technician, under penalty of losing the warranty, as only (s)he has the information, suitable
tools and training required to execute this task.
- Gnatus bears no responsibility for damages or accidents caused by poor installation
executed by a technician not authorized by Gnatus.
- Only after the equipment has been installed and duly tested by the authorized technician
representing Gnatus, will it be ready to start work operations.
14

OPERATION OF EQUIPMENT
Activation of the Terminals
- For the functioning of the rotary
instruments, remove the instrument to be
used from the support, activate the foot
control pressing it with your feet.
The power (air power supply) can be
controlled by the operator with greater or
less pressure on the foot control.
Adjustment of Spray of “TB/
TM/FO high and low rotation
terminals”
- The adjustment is made via a valve
positioned in the terminal. Turn it in a
clockwise direction to reduce the spray
and in a counter- clockwise direction to
increase it.
Note: As the “TB” double terminal does
not have a spray this adjustment is not
required.
Ejectors operation
The ejectors (both BV and Venturi) start
working automatically when retired from the
tips supportThe BV ejectors feature suction
ow adjustment , and its regulated moving
the lever located at the ejector up or down.
Substituição do padrão de
acoplamento da cânula
Caso haja necessidade de utilização da
cânula de 6,5 mm no suctor BV, faça a substituição do acoplamento da cânula conforme
procedimento abaixo:
• Retire o acoplamento de 11 mm (A)
desenroscando-o do conjunto suctor BV.
• Enrosque o acoplamento de 6,5 mm (B)
no conjunto suctor BV e encaixe o engate
para cânula.
B
A
15

OPERATION OF EQUIPMENT
Engate da cânula de 6,5mm
A curva do engate da cânula foi projetada para uma
melhor a manipulação, mas também pode ser cortada no local
indicado com auxílio de um estilete.
Use of 3-Way Syringe
- Press button (A) for water to come
out, (B) for air to come out or both
simultaneously to obtain a spray.
Curing Light Activation
- Select application time, press time
selection button (01), which values are: 10s
(standard mode), 20s, 60s, 80s and 90s.
- To initiate a polymerization cycle, press
the timer trigger (02), which generates a
short beep every 10 seconds and a 4 beeps
at the end of cycle.
- To interrupt a polymerization cycle
just activate the timer trigger again (02).
02
01
04
03
IMPORTANT:
- Keep the light conductor tip (03) at least 2mm away from the restoration.
- Keep the light conductor (03) always protected by an expendable PVC film, which must
be changed for every patient. This procedure protects the light conductor from scratches
and other residues.
- Use the polymerization time recommended by the compound resin manufacturer and
always perform restorations in incremental layers with a maximum thickness of 2mm.
WARNING
- Never aim the blue light beam towards the eyes
- Use the eyesight protection (04)
- In order to protect the eyes, the eyesight protection (04) filters only the blue light used
for the resins polimerization, and it allows ambient light to pass through.
16

OPERATION OF EQUIPMENT
Laser Hand
The “Laser Hand Kit” is low intensity (780nm) and provides relief of acute and chronic pain,
and speeds repair of damaged tissue by means of biostimulation effects of radiation.
Eminently analgesic, anti-inammatory and biomodulation effect.
Applications:
• Inammations;
• Oral mucous lesions;
• Dental hypersensitivity;
• Analgesia;
• Paresthesia;
• Alveolitis and pericoronitis;
• Acceleration of post surgical and
injury cicatrisation;
Activation of the “Laser Hand”
Turn on the main unit power switch, which will
automatically turn on the laser.
To select application time, press the time selection
button (01) with variations of: 01s to 90s. Maintain
pressure on the key until desired time selection,
which can be at 1-second intervals (1s, 2s, 3s, 4s,
5s, 6s, 7s...) or 10-second intervals (10s, 20s, 30s,
40s, 50s..).
To start, press timer activation button (01). A
single beep will be heard, followed by 5 beeps at each
conclusion.
The laser will remain active with a 10-minute
program. After 10 minutes, a beep will inform that the
laser is in standby mode.
To restart the cycle, press the key (02) which will
sound 2 beeps and the last programmed selection will
appear on the screen. To interrupt the cycle, press
button (03).
• Decrease of edemas, bruising and
scabbing;
• Distension, muscular spraining and
articular pain;
• Acupuncture (optional).
03
02
01
Note: For a new program, in case desired time is less than the previous program,
press (01) until the start of time “00”.
WARNING: Never direct the red light towards eyes;
17

OPERATION OF EQUIPMENT
How to provision the reservoirs
Water - Syringe / Handpieces
Remove the reservoir uncoiling it on clockwise and make the replacement of water. After
the replacement put it back coiling on anticlockwise. Always use filtered water or aseptic
products.
Bio-System
Remove the reservoir uncoiling it on clockwise and make the replacement. Use a
chlorinated water solution 1:500
Preparing the solution:
From a solution of hypochlorite of sodium at 1% a solution of chlorine at 500 p.p.m. is
prepared.
How to prepare the solution: Take 25ml of hypochlorite of sodium at 1% and dilute
it in 500 ml of water (1 to 20). Such solution should be prepared daily.
IMPORTANT:
Follow this proportion strictly to avoid damages in the equipment and to have an efficient
result in the disinfection.
Supports fastened at the side of or under tables, cabinets,
benches, etc...
Bearing in mind that:
The equipment should only be installed by Gnatus authorized technical
assistance or technicians.
18

PRECAUTIONS, RESTRICTIONS AND WARNINGS
Transportation, storage and operation
This equipment must be transported and stored observing the following directions:
- Avoid falls and impacts;
- Keep it dry, do not expose it to rain, water drops or wet oor;
- Keep it away from water and direct sunlight, and in it original wrapping;
- Don’t move it over irregular surfaces, protect it from rain and observe the maximum
stack quantity specified in the packaging;
- Transportation and storage temperature range: -12°C to 50°C.
- Ambient temperature range recommended by Gnatus +10 ° C to +35 ° C.
Sensitivity to environmental conditions in normal situations
of use
- The equipment has been planned not to be sensitive to interference such as magnetic
fields, external electrical factors, electrostatic discharge, pressure or variance of pressure,
provided that the equipment is installed, maintained, clean, preserved, transported and
operated as per this instruction for use.
Precautions and warnings “during the installation” of
quipment
- The equipment should only be installed by Gnatus authorized technical assistance or
technicians.
- Check that the socket in which the device will be connected has a ground connection.
According to the ABNT standard, this is essential for the safe operation of the system;
- Position the unit in a place where it will not get wet.
- Install the unit in a place where it will not be damaged by the pressure, temperature,
humidity, direct sunlight, dust, salts, or sulfur compounds.
- The unit should not be submitted to inclination, excessive vibrations, or blows (including
during transportation and handling).
- This equipment was not planned for use in an environment where vapors, anesthetic
mixtures inammable with air, or oxygen and nitrous oxide can be detected.
- Check the voltage of the equipment at the moment of executing the electrical
installation.
- The equipment must be grounded correctly.
- Before the first use and/or after long interruptions from work such as vacations, clean
and disinfect the equipment; eliminate air and water deposited in the internal hoses.
These information also make part of the Manual of Installation and
Maintenance of the equipment that can be found with the authorized
Gnatus technician.
19

PRECAUTIONS, RESTRICTIONS AND WARNINGS
Recommendations for the dental equipment maintenance.
Your Gnatus equipment has been designed and developed according to the standards
of modern techology. Similarly to other kinds of equipment, it requires special care, which
is many times neglected due to several reasons and circunstances.
Therefore, here are some important reminders for your daily routine. Try to follow these
simple rules, which will save you a lot of time and will avoid unnecessary expenses once
they start making part of your working procedure.
Precautions and warnings “during the use” of equipment
- The equipment should only be operated by duly enabled and trained technicians (Dental
Surgeons, Capacitated Professionals)
- If any maintenance should be required, only use services of the Gnatus Authorized
Technical Assistance.
- The equipment has been manufactured to handle both continuous and intermittent
operation; so follow the cycles described in these Instructions for Use.
- Although this equipment has been planned in accordance with the standards of
electromagnetic compatibility, it can, in very extreme conditions, cause interference with
other equipment. Do not use this equipment together with other devices very sensitive to
interference or with devices which create high electromagnetic disturbance.
- Do not expose the plastic parts to contact with chemical substances, use in the routines
of dental treatment, such as: acids, mercury, acrylic liquids, amalgams, etc.
Gnatus shall not be responsible for:
- Use of the equipment differing from that for which it is intended.
- Damages caused to the equipment, the professional and/or the patient by the incorrect
installation and erroneous procedures of maintenance, differing from those described in these
Instructions for use which come with the equipment or by the incorrect operation of it.
Precautions and warnings “after” the use of equipment
- Turn off the main switch of the dental set when it is not in use for an extended period
of time.
- Always maintain the equipment clean for the next operation.
- Do not modify any part of the equipment. Do not disconnect the cable or other
connections without need.
- After using the equipment, clean and disinfect all the parts which may be in contact
with the patient.
- Upon noticing irremovable stains, splits or cracks in the light conductor or in the eye
protector, replace the damaged components.
20

PRECAUTIONS, RESTRICTIONS AND WARNINGS
Precautions and warnings during the “cleaning and disinfection”
of equipment
Delivery Unit:
- Avoid spilling water, even accidentally, or other liquids inside the equipment, which
could cause short circuits.
- Do not use microabrasive material or steel wool when cleaning, or employ organic
solvents or detergents which contain solvents such as ether, stain remover, gasoline etc.
Curring Light:
- The equipment and the light conductor cannot be placed in the oven or autoclaves.
- The conductor can’t be immersed in solvents or substances that contain acetone in its
composition.
- Avoid the light conductor to terminal to touch the resin to be polymerized.
- When using the Curring Light check if the light conductor output doesn’t have residues
that might obstruct the light beam.
Hand Laser:
For further information, please see the Hand Laser manual which comes with the
product.
Precautions in case of alteration in the functioning of equipment
- If the equipment has any abnormality, check if the problem is related to any item
listed in the topic of unforeseen events (failures, causes and solutions). If it is not possible
to resolve the problem, turn off the equipment, remove the power supply cable from the
socket and contact your representative (Gnatus).
Precautions to be adopted against foreseeable or uncommon
risks, related to the deactivation and abandoning of equipment
In order to avoid environmental contamination or undue use of the Equipment after it
has become useless, it should be discarded in the suitable place (as per the local legislation
of the country).
- Pay attention to the local legislation of the country for the conditions of installation
and disposal of residue.
CORRECTIVE AND PREVENTIVE MAINTENANCE
AND PRESERVATION
Additional procedures for reuse
The equipment can be reused in undetermined, i.e. unlimited, quantities, only needing
to be cleaned and disinfected.
Disinfection
Use clean and soft cloth dampened in alcohol 70% to disinfection of the equipment.
Never use corrosive disinfectants or solvents.
21

CORRECTIVE AND PREVENTIVE MAINTENANCE
AND PRESERVATION
Cleaning
The cleaning procedure below should be executed at the start of the
working day and after each patient.
Always turn off the main switch before executing the procedures of
daily maintenance.
To clean the equipment, we recommend the use of “BactSpray
(Reg nº MS: 3.2079.0041.001-5) or any other similar product:
Active component: Benzalkonium chloride (tri-quaternary
ammonium)
Solution 50%................................................. 0.329%
Chemical composition: Butyl Glycol, Decyl polyglucose, Sodium
Benzoate, Sodium Nitrate, Essence, Deodorized Propane / Butane,
demineralized Water.
For more information concerning cleaning procedures, see
manufacturer’s instructions.
WARNING:
- In order to prevent risks and damages to equipment, make sure
that the liquid does not enter into the unit.
- The application of other solvent-based cleaning products or
sodium hypochloride isn’t recommended, because they may damage
the equipment.
NOTE: The registration at the Ministry of Health of the
“BactSpray” is executed separately from the product described in
this manual, as the “BactSpray” is not manufactured by Gnatus.
Note: Use gloves
and other systems of
protection, during the
disinfection.
Attention: Do not use any
disinfectant spray, as the
vapor may be inflammable, or
it may cause injury.
Cleaning
Curring Light
The light conductor cleaning and the optical protector must be done using only neutral
soap and cotton. To the exterior of the pen use neutral soap or alcohol 70% vol.
Never use any other chemical based product than previous mentioned, because along
the time these products attack the surface of the instrument.
Never immerse the instrument in disinfection baths.
22

CORRECTIVE AND PREVENTIVE MAINTENANCE
AND PRESERVATION
Bio-System
Remove hanpieces from terminals. Take terminals to
bowl or water unit’s sink.
Open the terminal’s spray valves completelly.
Press the Bio-system key (12) see pag 07, for some
seconds, to disinfect the equipment’s components internally
with disinfectant.
Then, press the command pedal for some seconds to
rinse, in order to eliminate the disinfectant residues that
could have remained.
IMPORTANT:
Repeat this procedure before working day and after
each patient.
Reservoirs
It’s highly recommended the cleaning of the water reservoirs,
using chlorinated water solution 1:500 (as described previously).
Cleaning
Gnatus suggests performing a daily suction of the clearance
and disinfectant solution, avoiding the risk of cross contamination
and increasing equipment service life. To perform the disinfection
of your equipment we recommend the use of the “Sugclean” (MS
Reg. No.: 31.080.003-2) product.
• Indication: It is indicated for clearance of sucker and hose
suction system. It is important to perform the suction solution in
all suction terminals, which it is also important to be open. Then,
remove suckers from hose for asepsis.
• Preparing the Solution: Add “Sugclean” 30mL in 1 liter of
water. Aspirate the solution with maximum power of the suckers,
and also put the liquid in the water unit bowl.
In the first use of “Sugclean” product, we suggest adding 60mL
of concentrated product in 1 liter of water during the first 5 days
in order to remove accumulated residues.
• Composition:
• Active Drug: Phosphoric Acid 13.6%
• Excipients: Isopropyl Alcohol, Acidulant, Dye and
Thickener.
Warning: do not use foaming product.
NOTE: The registration at the Ministry of Health of the
“Sugclean” is executed separately from the product described
in this manual, as the “Sugclean” is not manufactured by
Gnatus.
Fig.A
23

CORRECTIVE AND PREVENTIVE MAINTENANCE
AND PRESERVATION
Cleaning
01
Sucker lters
After the suction of the solution through the suctor, take
the lid (01) and the filter (02) and wash them in running
water.
Triple syringe
Only the syringe tip is autoclavable (03). The other
pieces must be cleaned using a piece of cotton wool and
alcohol 70% vol. Never use a hot air sterilizer.
02
03
Preventive Maintenance
The equipment should be calibrated routinely, as per the legislation in force in the
country.
But never with a period exceeding 3 years.
In order to protect your equipment, seek Gnatus technical assistance for periodic revisions
of preventive maintenance.
Corrective Maintenance
If the equipment has any abnormality, check if the problem is related to any of the items
listed in the item Unforeseen Events (situation, cause and solution).
If it is not possible to solve the problem, turn off the equipment, and request Gnatus
technical assistance.
24

UNFORESEEN EVENTS SOLUTION OF PROBLEMS
Upon coming across any problem in operation, follow the instructions below to
check and repair the problem, and/or get in touch with your representative.
Problem Probable cause Solution
Delivery Unit
-Handpiece is not working. - Compressor disconnected.
-Plug the compressor in.
-Handpiece with low speed. -Inlet pressure below speci-
-No water from syringe. -Reservoir run out of water.
-When Bio-system is operated no disinfectant come
from handpiece terminals.
- Ejector without suction. - Insufficient air pressure
Curring Light
-Equipment’s not working.
-Equipment is not polymerizing resins.
fied (80 PSI).
-Compressor disconnected.
-Bio-system reservoir run
out of water.
from compressor.
-Filter clogged with particles.
-Filter lid misplaced.
-Main or vacuum pump switch is off.
-Power cut. -Check power supply.
-Resin is not appropriate
for LED’s photopolymerizer
wave length range.
-Adjust inlet pressure (80
PSI).
-Put filtered water in reservoir.
-Plug compressor in.
-Put disinfectant in the reservoir.
-Adjust air ow.
-Remove and clean filter.
-Remove lid and place it
correctly.
-Switch main/ vacuum pump
switch on.
-Get the indicated resin for
the photopolymerizer’s wave
length range, one with contains photoinitiators based
on camphorquinone.
25

WARRANTY OF EQUIPMENT
This equipment is covered by the warranty terms counting from the date of installation,
as specified below; provided that the defect has occurred in normal conditions of use and
that the equipment has not remained stored for more than 06 months counting from the
issue date of the sales document until the date of the actual installation.
- WARRANTY TERMS: 24 months;
- LOSS OF THE WARRANTY:
A) Attempt to repair using an inadequate tool or by unauthorized technicians;
B) Installation of the equipment by an unauthorized technician;
C) Damage arising from inappropriate storage or signs of infringement;
D) Incorrect use of the equipment;
E) Use of a cleaning product not indicated by the factory;
F) Falls or blows which the equipment may undergo or lack of observation of an compliance
with the guidelines of the Owner’s Manual, which was delivered with the present document,
together with the equipment. Repair or replacement of parts during the warranty period
shall not extend the validity term of their warranty.
- This warranty doe snot exempt the customer from paying the service charge for the visit
and the travel expenses of the technician, except when the customer sends the equipment
to execute the maintenance inside the establishment of the technical assistance.
“Consumer Defense Code - art. 50, unique paragraph”.
- The Warranty Certificate comes with the product and must be filled in upon the date
of installation by the Gnatus Authorized Technician.
- Queries and information: GNATUS Help Desk (+55) 16 2102-5000.
- Check the warranty term attached to this manual.
FINAL CONSIDERATIONS
The most important aspect related to equipment care is that concerning spare parts.
To guarantee the life span of your equipment, use only original Gnatus spare parts.
They are sure to follow the technical specifications and standards required by Gnatus.
We must also point out to you our chain of authorized dealers. Only dealers that make
part of this chain will be able to keep your equipment constantly new for they count on
technical assistants who have been trained and on spedific tools for the correct maintenance
of your equipment.
Doubts and information: GNATUS Call center (55-16) 2102-5000.
26

27

 Loading...
Loading...