GlucaGen HypoKit Instructions For Use Manual
Summary of
Instructions for Use
This is only a summary of the Instructions for Use. Please see the full Instructions for Use on the following pages.
–––––––––––––––
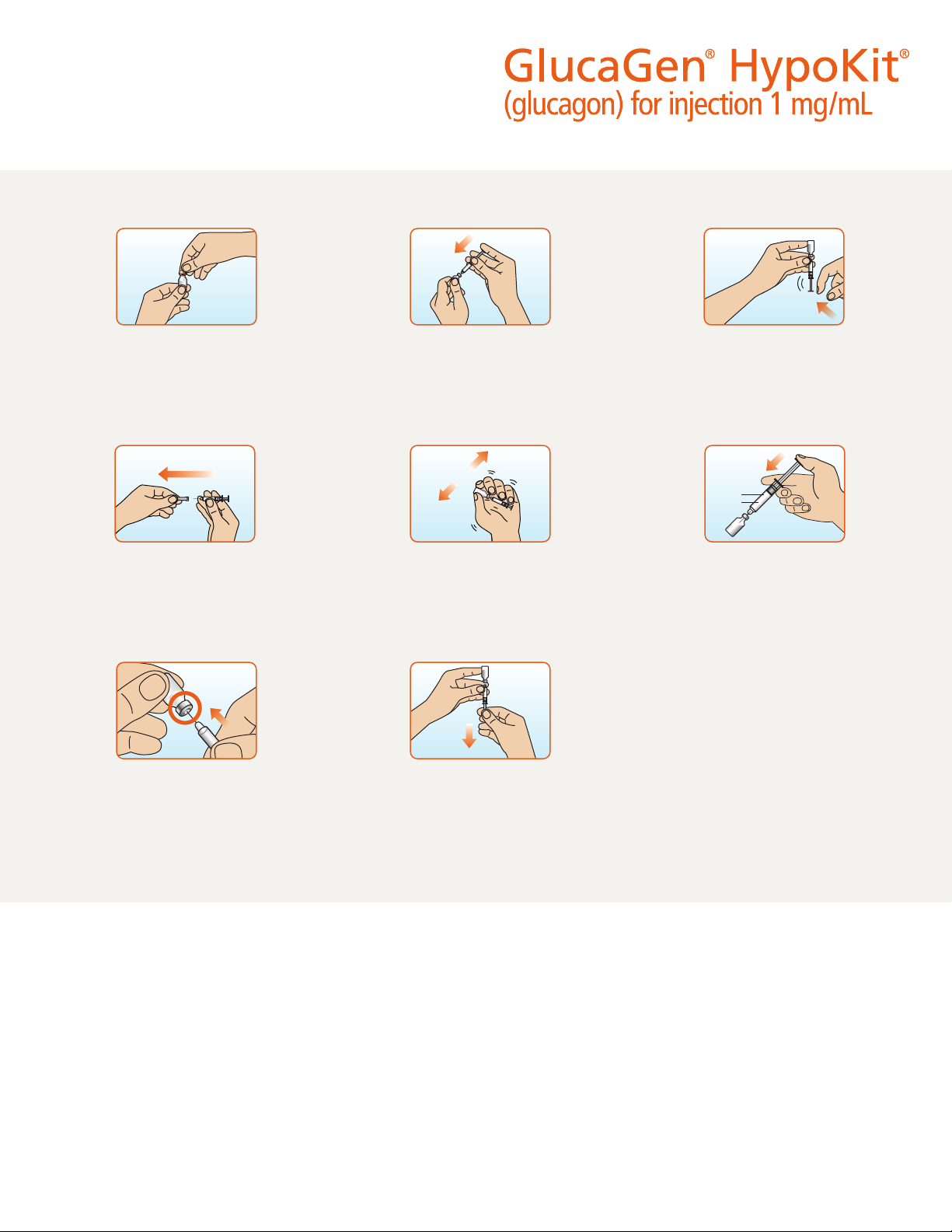
Step 1. Check that the orange plastic cap
on your vial of GlucaGen® is firmly attached.
Do not use if the cap is loose or missing.
With your thumb, flip the cap off the
GlucaGen® vial.
Pull Straight Off
Step 2. Pick up the prefilled syringe
containing sterile water. Do not use any
other liquid to mix the medicine. Hold the
syringe with 1 hand. With your other hand,
pull the needle cover off the syringe. Do not
remove the plastic backstop from the syringe.
Step 3. Pick up the GlucaGen® vial of dry
powder. Hold the vial with 1 hand. With your
other hand, push the needle of the prefilled
syringe through the center of the rubber
st oppe r.
Preparing the GlucaGen® dose
Step 4. Hold the vial and syringe together,
with the needle still inserted into the vial.
Carefully turn the vial and syringe together
right side up. Slowly push the plunger down
until the syringe is empty. Do not take the
syringe out of the vial.
Step 5. Firmly hold both the vial and syringe
together in one hand, and gently shake until
the powder is completely dissolved. Do not
use if a gel has formed, or if you see particles
in the solution. Do not take the syringe out
of the vial.
Step 6. With the needle still inserted into
the vial, carefully turn the vial and syringe
together upside down. Gently pull down on
the plunger and slowly withdraw all of the
liquid into the syringe. Do not pull the plunger
out of the syringe.
––––––––––––––––
Step 7. Keep the needle inside the vial.
Check the syringe for air bubbles. If you see
bubbles, tap the syringe until the bubbles rise
to the top of the syringe. Gently push on the
plunger to move only the air bubbles back
into the vial.
1 mL
0.5 mL
Step 8. Hold the vial and syringe as shown.
• The usual dose for adults and children who
weigh more than 55 pounds (25 kg) is 1 mg
(1 mL). Use the content of the full syringe
(1 mL).
• The usual dose for children who weigh less
than 55 pounds (25 kg) is 0.5 mg (0.5 mL).
Gently push the plunger until it is at the
0.5 mL mark on the syringe to ensure there
is 0.5 mL liquid left in the syringe.
Take the syringe and needle out of the vial
when the correct dose of GlucaGen® is in the
syringe.
If you do not know how much the child
weighs:
• Give a child under 6 years of age 0.5 mg
(0.5 mL).
• Give a child 6 years of age and older 1 mg
(1 mL).
See instructions for giving the injection on the next page u
Indications and Usage
What is GlucaGen® (glucagon) for
injection 1mg/mL?
GlucaGen® is a prescription medicine
used to treat very low blood sugar (severe
hypoglycemia) in people with diabetes who
use insulin.
Important Safety Information
Who should not use GlucaGen®?
Do not use GlucaGen® if:
• you are allergic to glucagon or lactose or any
of the ingredients in GlucaGen
• you have a tumor in the gland on top
of your kidneys (adrenal gland) called a
pheochromocytoma
®
• you have a tumor in your pancreas called an
insulinoma
What should I tell my doctor before using
GlucaGen®?
Before using GlucaGen®, tell your doctor
about all of your medical conditions,
including if you:
• have kidney problems
• have pancreas problems. Tumors in your
pancreas called glucagonomas
• have not had food or water for a long time
(prolonged fasting or starvation)
• have low blood sugar that does not go away
(chronic hypoglycemia)
• have heart problems
• are pregnant or plan to become pregnant
• are breastfeeding or plan to breastfeed. It
is not known if GlucaGen® passes into your
breast milk
Tell your doctor about all the medicines
you take, including prescription and over
the counter medicines, vitamins and herbal
supplements. GlucaGen® may affect the way
other medicines work, and other medicines
may affect how GlucaGen® works.
Please see additional Important
Safety Information on next page, and
Prescribing Information on following
pages.
––––––––––––––––
Giving the GlucaGen® injection
Front
Back
––––––––––––––––
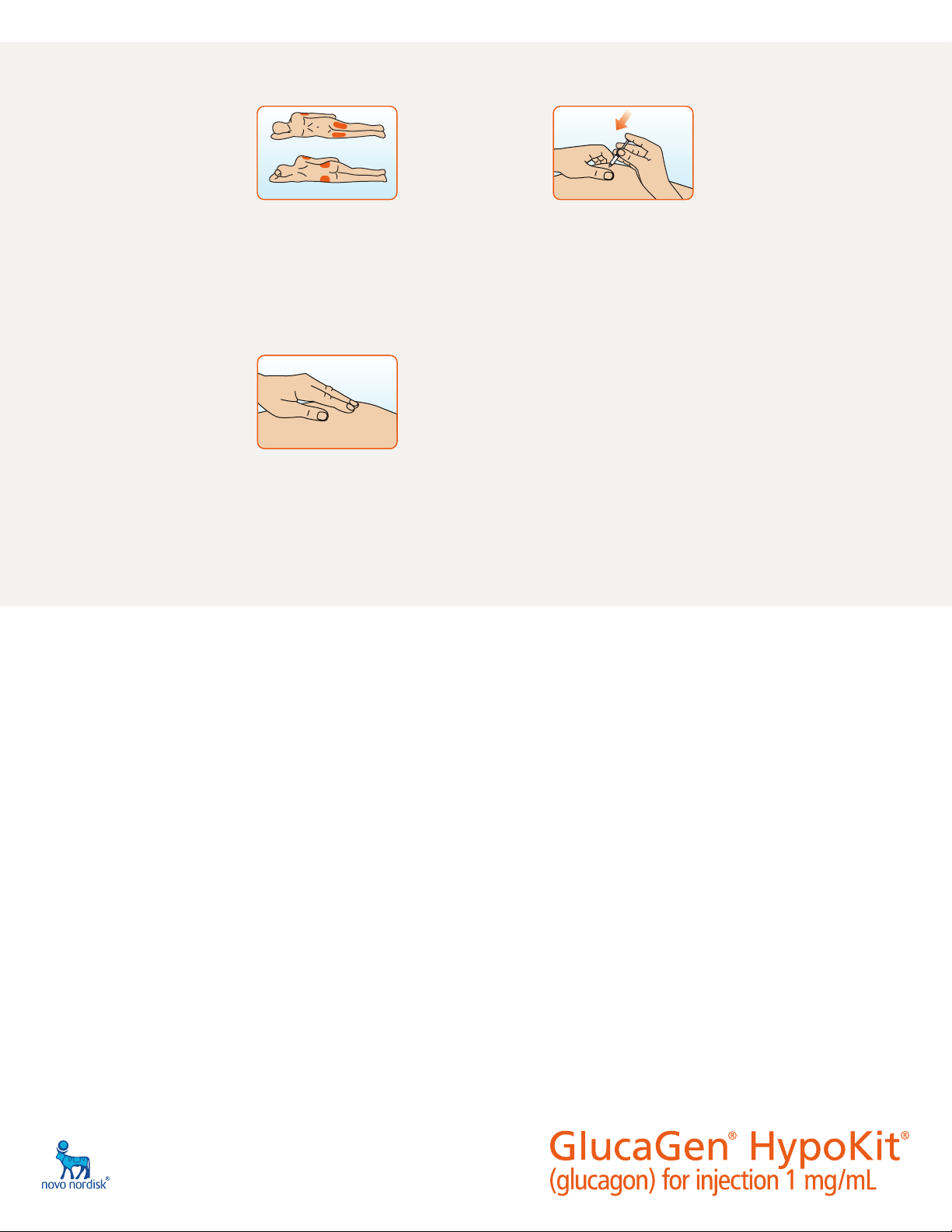
Step 9. Choose the injection site. Common
injection sites for GlucaGen® are upper arms,
thighs, or buttocks.
––––––––––––––
Step 11. Pull the needle out of the skin
and press on the injection site. Throw away
your used syringe with the needle attached
and any GlucaGen® you did not use.
See “How should I dispose of (throw
away) used GlucaGen® prefilled syringes”
in the full Instructions for Use on the
following pages.
Early symptoms of hypoglycemia may include: sweating, drowsiness, dizziness, sleep disturbances, irregular heartbeat (palpitation), anxiety,
tremor, blurred vision, hunger, slurred speech, depressed mood, tingling in the hands, feet, lips, or tongue, irritability, abnormal behavior,
lightheadedness, unsteady movement, inability to concentrate, personality changes, headache, and restlessness.
If not treated early, hypoglycemia may worsen and the person may have severe hypoglycemia.
Signs of severe hypoglycemia include: confusion, unconsciousness, seizures, and death.
After Giving the GlucaGen® injection
Hypoglycemia may happen again after receiving GlucaGen® treatment.
Step 10. With one hand gently pinch the skin
at the injection site. With your other hand
insert the needle into the skin and push the
plunger down until the syringe is empty.
–––––––––––––––
Step 12. Turn the person on their side. When an
unconscious person awakens, they may vomit. Turning the
person on their side will lessen the chance of choking.
Step 13. Call for emergency medical help right away.
Step 14. Feed the person as soon as they are awake
and able to swallow.
Give the person a fast-acting source of sugar (such as a
regular soft drink or fruit juice) and a long-acting source of
sugar (such as crackers and cheese or a meat sandwich).
Step 15. Even if the GlucaGen® treatment wakes
the person, tell their doctor right away. The doctor
should be told whenever a severe drop in blood sugar
(hypoglycemia reaction) happens. The person’s dose of
diabetes medicine may need to be changed.
Important Safety Information
(cont ’d)
How should I use GlucaGen®?
• Use GlucaGen® exactly as your doctor tells
you to
• Make sure that you and your family know
how to use GlucaGen® the right way before
you need it
• Act quickly. Having very low blood sugar for
a period of time may be harmful
• Call for emergency medical help right
after you use GlucaGen
• Eat sugar or a sugar-sweetened product such
as a regular soft drink or fruit juice as soon
as you are able to swallow
• Tell your doctor each time you use
GlucaGen®. Your doctor may need to change
the dose of your diabetes medicines
GlucaGen® and HypoKit® are registered trademarks of Novo Nordisk A/S.
Novo Nordisk is a registered trademark of Novo Nordisk A/ S.
© 2018 Novo Nordisk A ll rights res erved. US18GLGN0 0007 Au gust 2018
®
What should I avoid while using
GlucaGen®?
While using GlucaGen® do not:
• drive or operate machinery until you have
eaten sugar or a sugar-sweetened product
such as a regular soft drink or fruit juice
What are the possible side effects of
GlucaGen®?
GlucaGen® may cause serious side effects,
including:
• High blood pressure. High blood pressure
is common after taking GlucaGen® and can
be severe
• Low blood sugar. GlucaGen® can cause
low blood sugar in patients with tumors
in their pancreas called insulinomas and
glucagonomas by making too much insulin in
their bodies
• Allergic reactions. Symptoms of a serious
allergic reaction to GlucaGen® may include
rash, difficulty breathing, or low blood
pressure (hypotension)
The most common side effects of GlucaGen®
include:
• nausea
• vomiting
• temporary fast heartbeat or pounding in
your chest (tachycardia)
Please see additional Important Safety
Information on previous page, and
Prescribing Information on following
pages.
GlucaGen® HypoKit® is available by prescription
only.
You are encouraged to report negative side effects
of prescription drugs to the FDA.
Visit www.fda.gov/medwatch, or call
1-800-FDA-1088.
If you need assistance with prescription costs,
help may be available. Visit pparx.org or call
1-888-4PPA-NOW.
(glucagon) for injection 1 mg/mL
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GlucaGen
and effectively. See full prescribing information for GlucaGen®.
GlucaGen® (glucagon) for injection, for subcutaneous, intramuscular or intravenous use
Initial U.S. Approval: 1998
——— RECENT MAJOR CHANGES ———
• Warnings and Precautions (5.5) ..........................................................................................7/2018
——— INDICATIONS AND USAGE ———
GlucaGen® is an antihypoglycemic agent and a gastrointestinal motility inhibitor indicated for:
• Treatment of severe hypoglycemia (1.1)
• Use as a diagnostic aid (1.2)
——— DOSAGE AND ADMINISTRATION ———
Treatment of severe hypoglycemia (GlucaGen® HypoKit®)
• Reconstitute before administration. (2.1)
• Inject 1 mL (adults and children, weighing more than 55 lbs (25 kg)) or 0.5 mL (children weighing
less than 55 lbs (25 kg)) subcutaneously, intramuscularly, or intravenously. (2.1)
• If the weight is not known: Children younger than 6 years should be given 0.5 mL and children
6 years and older should be given 1 mL. (2.1)
• Seek emergency assistance immediately after subcutaneous or intramuscular injection of glucagon.
Glucagon injection may be repeated while waiting for emergency assistance. (2.1)
• Intravenous glucose MUST be administered if the patient fails to respond to glucagon. (2.1)
• When the patient responds to treatment, give oral carbohydrates to restore the liver glycogen and
prevent recurrence of hypoglycemia. (2.1)
Use as a diagnostic aid (GlucaGen
• Reconstitute before administration. (2.2)
• The dose ranges from 0.2 mg to 2 mg depending on the diagnostic technique and the route of
administration. (2.2)
• After the end of the diagnostic procedure, give oral carbohydrates to patients who have been fasting,
if this is compatible with the diagnostic procedure applied. (2.2)
——— DOSAGE FORMS AND STRENGTHS ———
• For injection: 1 mg of glucagon as powder for reconstitution in a single dose vial, alone or
co-packaged with Sterile Water for Reconstitution. (3)
——— CONTRAINDICATIONS ———
• Do not use in patients with known hypersensitivity to glucagon or lactose (4)
• Do not use in patients with pheochromocytoma (4)
• Do not use in patients with insulinoma (4)
®
Diagnostic Kit and GlucaGen® 10-Pack)
®
safely
——— WARNINGS AND PRECAUTIONS ———
• Administer cautiously to patients suspected of having glucagonoma due to risk of secondary
hypoglycemia. Glucagon may release catecholamines from pheochromocytomas and is
contraindicated in patients with this condition. (5.1, 5.2)
• Allergic reactions may occur and include generalized rash, anaphylactic shock with breathing
difficulties, and hypotension. (5.3)
• In order for GlucaGen® treatment to reverse hypoglycemia, there must be adequate amounts of
glycogen stored in the liver. GlucaGen® should be used with caution in patients with conditions
resulting in low levels of releasable glucose in the liver. (5.4)
• Use caution when GlucaGen® is used as a diagnostic aid in diabetic patients because it may cause
hyperglycemia. (5.4)
• Necrolytic Migratory Erythema (NME), a skin rash, has been reported postmarketing following
continuous glucagon infusion and resolved with discontinuation of the glucagon. Should NME
occur, consider whether the benefits of continuous glucagon infusion outweigh the risks. (5.5)
• Use with caution in patients with known cardiac disease, as glucagon increases myocardial oxygen
demand. (5.6)
——— ADVERSE REACTIONS ———
Adverse reactions seen with GlucaGen
• Nausea and vomiting (6)
• Temporary increase in blood pressure and pulse may occur after administration. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at
1-800-727-6500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
——— DRUG INTERACTIONS ———
• Beta-blockers may cause a greater increase in both pulse and blood pressure after administration.
(7.1)
• Glucagon may lose its ability to raise blood glucose or may produce hypoglycemia when given with
indomethacin. (7.2)
• Coadministration with an anticholinergic drug is not recommended due to increased
gastrointestinal side effects. (7.3)
• Glucagon may increase the anticoagulant effect of warfarin. (7.4)
• Insulin reacts antagonistically towards glucagon. (7.5)
——— USE IN SPECIFIC POPULATIONS ———
• Nursing mothers: unknown whether drug is excreted in human milk, therefore caution should be
exercised. (8.3)
• Pediatrics: reported safe and effective for treatment of severe hypoglycemia. Safety and effectiveness
for use as a diagnostic aid have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
®
are:
Revised: 07/2018
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of severe hypoglycemia
1.2 Use as a diagnostic aid
2 DOSAGE AND ADMINISTRATION
2.1 Treatment of severe hypoglycemia
2.2 Use as a diagnostic aid
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pheochromocytoma
5.2 Insulinoma and Glucagonoma
5.3 Hypersensitivity and Allergic Reactions
5.4 Glycogen Stores and Hypoglycemia
5.5 Necrolytic Migratory Erythema
5.6 Cardiac Disease
5.7 Laboratory Tests
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Beta-blockers
7.2 Indomethacin
7.3 Anticholinergic Drugs
7.4 Warfarin
7.5 Insulin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Recommended Storage
17 PATIENT COUNSELING INFORMATION
17.1 Physician Instructions
*Sections or subsections omitted from the full prescribing
information are not listed.
1
 Loading...
Loading...