
GlobalMed® Transportable
Exam Station™ Enterprise
User Manual
MAN-600087 Rev. D
2/14/18

Legal
While GlobalMedia Group, LLC. (GlobalMed) and its divisions make every
effort to ensure that the information contained on our website and product
literature is accurate, GlobalMed takes no responsibility for incorrect,
outdated, or otherwise inaccurate information. GlobalMed is not
responsible for typographical errors or omissions and shall not be liable for
any incidental or consequential damages caused directly or indirectly from
anything on any GlobalMed website or within product literature. Our
products are not meant to diagnose, cure or prevent any disease.
No license to any intellectual property rights is granted by this document,
whether express or implied, by estoppel or otherwise.
One or more GlobalMed products are covered under U.S. Patent(s) with
other patents pending. Any testimonials contained within any GlobalMed
website or document are individual cases and do not guarantee that you
will get the same results.
©2002-2018 GlobalMedia Group, LLC. (DBA GlobalMed). All Rights
Reserved. iREZ, LiteExam, TotalExam, ClearProbe, CapSure, CapSure
Cloud, eNcounter, EasyShare, ClearSteth, GlobalMed, TotalECG,
ClinicalAccess, WallDoc, and Transforming Healthcare Globally are
registered trademarks. All other trademarks are the property of their
respective holders.
2/14/18 Doc. No. MAN-600087 Rev. D | ii |
Confidential

Warranty and Return Policy
All GlobalMedia Group, LLC (GlobalMed) products are warranted under the
following terms:
Products that become defective during the first year after the order is
shipped will be repaired or replaced by GlobalMed free of charge. This
limited warranty is contingent upon proper use of the product and does not
cover products that have been damaged (scratches, bent metal, broken
components) misused, modified, or subject to unusual physical or electrical
stress. All returns for any other reason must be made within the first 30
days from time of shipment and will be subject to a 25% restocking charge.
Buyer must include all original components, literature, and packaging in the
same salable condition received to avoid any additional charges.
All returned materials must have a GlobalMed return materials
authorization (RMA) number. Authorized returns must be shipped freight
prepaid to GlobalMed. GlobalMed reserves the right to refuse any return
that is sent COD or without an RMA number visible on the exterior of the
package. Unauthorized returns, refused shipments, and authorized returns
of nondefective merchandise after the above stated return periods are
subject to additional charges.
Limited Liability Agreement
No claim made by the buyer shall be for an amount greater than the
purchase price of the goods in respect of which the claim was made,
regardless of whether the claim pertains to damage incurred in shipping,
failure to ship, or inherent defects. GlobalMedia Group will in no way be
liable for incidental or consequential charges. In all events, GlobalMed
reserves the option of repair or replacement at its discretion. GlobalMed
takes no responsibility for incorrect, outdated, or otherwise inaccurate
information, including pricing and product specifications. GlobalMed is not
responsible for typographical errors or omissions and shall not be liable for
any incidental or consequential damages caused directly or indirectly from
any GlobalMed product. In addition, GlobalMed reserves the right to
change prices, specifications or discontinue products at any time without
prior notice while reserving the right to refuse or conduct a cancellation on
its transaction activities due to price inconsistency from its suppliers.
©2002-2018 GlobalMedia Group, LLC All Rights Reserved
2/14/18 Doc. No. MAN-600087 Rev. D | iii |
Confidential

About GlobalMed
GlobalMed powers the largest telehealth programs in the world, facilitating
more than 15 million consults to date. We have helped 4,000+
organizations improve healthcare access in 55 countries and are honored
to be the telehealth provider for the White House. With a focus on security
and simplicity, GlobalMed designs, builds, manufactures and deploys fully
integrated, evidence-based hardware and software telehealth solutions that
enable medical groups, healthcare enterprises and government entities to
improve patient outcomes while lowering cost.
Founded in 2002 by a Marine Corps Reserve Veteran still serving as CEO,
GlobalMed is proud to be a Veteran Owned Small Business (VOSB).
Contact info:
®
Help Desk: 1.800.886.3692 or visit https://globalmed.desk.com
GlobalMed
15020 North 74th Street
Scottsdale, AZ 85260
+1.480.922.0044 phone
+1.480.922.1090 fax
telemed@globalmed.com
www.globalmed.com
2/14/18 Doc. No. MAN-600087 Rev. D | iv |
Confidential

Revision History
Revision Changes Date
A Initial release. 09-26-2017
B Product name. 10-25-2017
C Added clarification to "WiFi Hotspot" on page 22 01-02-2018
D Added information to "Technical Specifications" on
page 5
02-13-2018
2/14/18 Doc. No. MAN-600087 Rev. D | v |
Confidential

Table of Contents
Chapter 1: About the TES Enterprise
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Chapter 2: Using the TES Enterprise
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
TES Enterprise Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Opening the TES Enterprise . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Charging the TES Enterprise for the First Time. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Power. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Surface Pro 4 Usage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Sound and Video . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Touch LED Light . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
WiFi Hotspot . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Device Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Thinklabs® One Stethoscope Usage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Chapter 3: Maintenance and Troubleshooting
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Required Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Basic Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Cleaning Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
2/14/18 Doc. No. MAN-600087 Rev. D | 1 |
Confidential

CHAPTER
About the TES Enterprise
1
1.1 Overview
Recognizing the need for mobility, GlobalMed® developed the
Transportable Exam Station™ (TES) Enterprise to take healthcare beyond
the static environment. With a tablet computer that integrates consultation
software and a variety of connected medical devices housed in an impact-,
dust- and weather-resistant rolling case, the TES Enterprise is an
interactive and fully mobile examination station.
The TES Enterprise is designed and manufactured in the USA by
GlobalMed.
2/14/18 Doc. No. MAN-600087 Rev. D | 1 |
Confidential
About the TES Enterprise
1.2 Precautions
Note: Refer to all component manuals for any additional precautions
before using this unit.
Table 1 Precautions
Precaution Type Warning Description
General • Always open the TES Enterprise fully on a steady tabletop or flat
surface.
• Only use manufacturer-recommended or approved accessories to
ensure compatibility.
• Retain the original packaging material.
Liquid and Moisture • Never spray or use liquid directly on components or connectors.
• If any liquid penetrates any component or connector, switch off
and unplug the power cord. Contact your service provider. Continuous
use may lead to further damage and/or result in electrical shock or
may create a fire hazard.
• Never operate or touch any electrical component with wet hands.
Power • Unplug power supply from any component before cleaning.
• Unplug main power supply during lightning storm to prevent possible damage from power surge.
• Do not pull any power supply by the cord; only pull from the plug.
Failure to comply may result in damage to the power supply, electrical
shock or may create a fire hazard.
• If any abnormalities occur, such as smoke, odor or other, turn off
the TES Enterprise, unplug main power supply, and contact your service provider immediately.
• This system should be stored in a controlled environment (see
"Technical Specifications" on page 5 for acceptable condi-
tions). Powering down the TES Enterprise is recommended for longterm storage.
Note: This device is only intended for use by licensed medical
professional
1.2.1 Warnings
• Do not sit or stand on the TES Enterprise. Excess weight on the unit
may result in injury to the user or damage to the unit.
• Do not use the TES Enterprise in an oxygen-rich environment. The
TES Enterprise has not been certified for this use.
2/14/18 Doc. No. MAN-600087 Rev. D | 2 |
Confidential
• Do not use devices that deliver or support microwave radiation for
diagnostic or therapeutic uses. The TES Enterprise is not capable of
using these devices.
• Do not use around flammable agents to avoid risk of injury or damage to the unit.
• Do not use the TES Enterprise in conjunction with a pacemaker or
microwave as the TES Enterprise may interfere with their function.
1.2.2 Cautions
• The TES Enterprise is delivered in non-sterile condition and is not
intended to be sterile for use.
• Modification to equipment may void your warranty or may cause
damage to the unit. Consult with your service provider prior to making changes.
About the TES Enterprise
• When using the TES Enterprise, position the unit in a manner that
does not cause a trip hazard between the unit and the electrical wall
outlet.
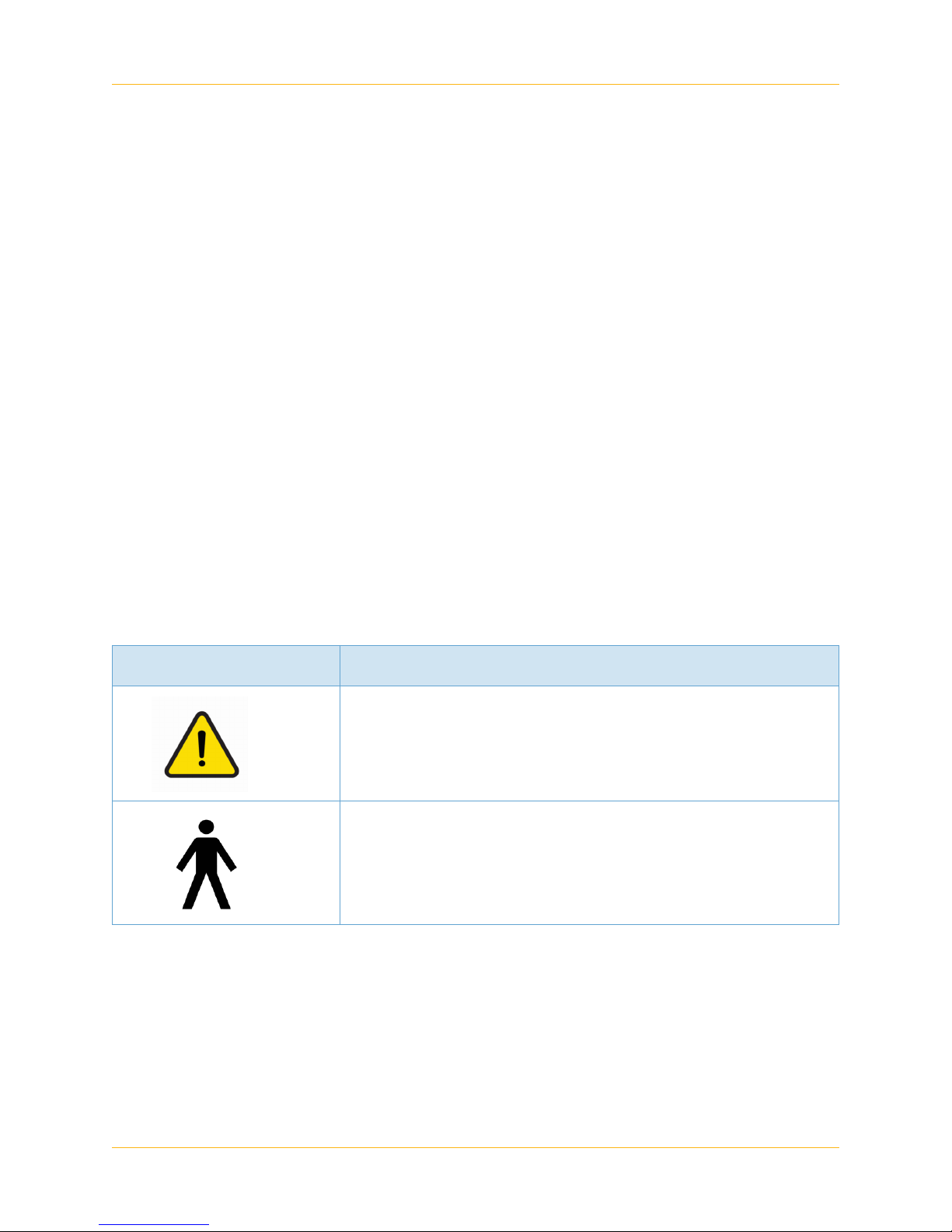
Table 2 Warning Symbols
Symbol Description
General Warning Sign (ISO 7010 – W001)
Type B Applied Part (IEC 60601 – 1)
2/14/18 Doc. No. MAN-600087 Rev. D | 3 |
Confidential
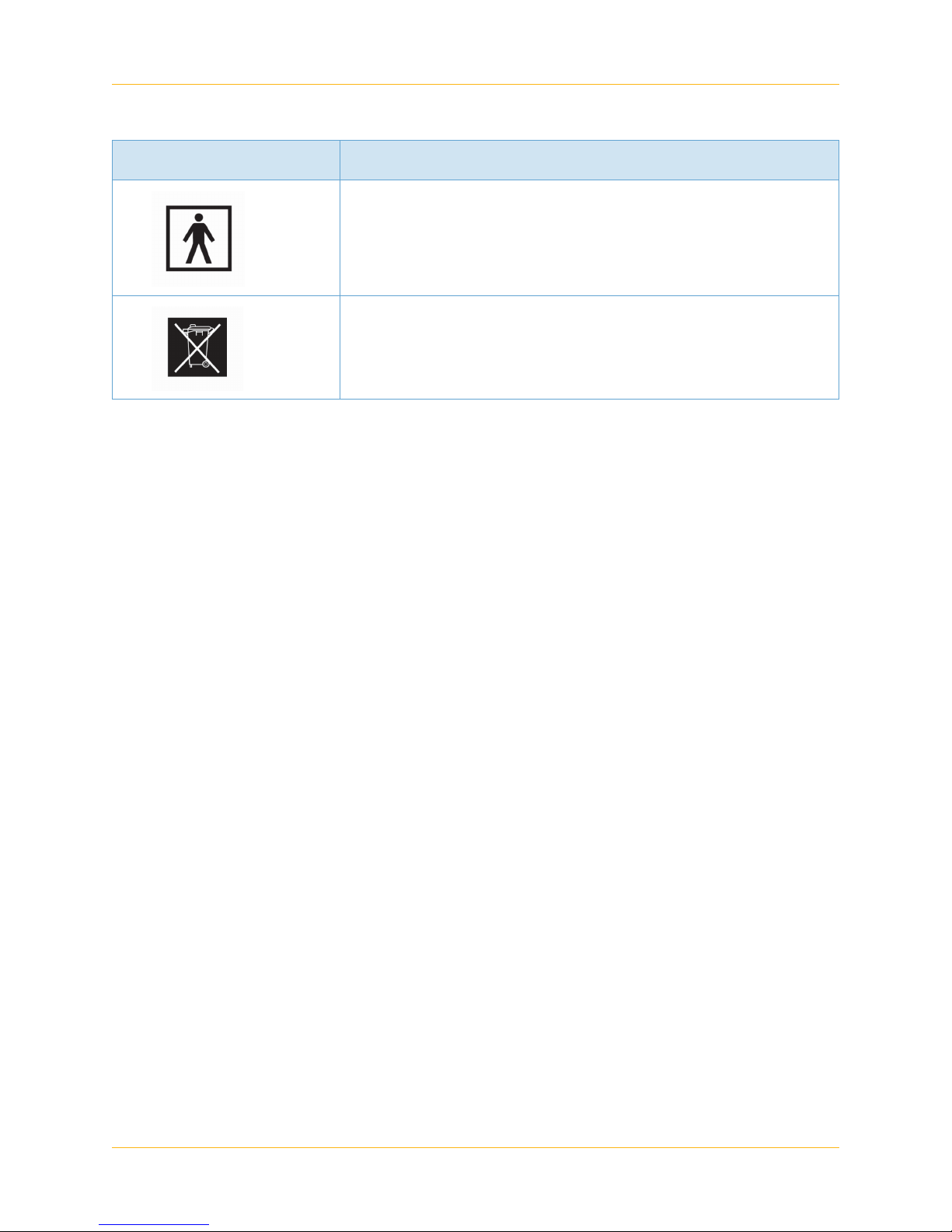
Table 2 Warning Symbols (Continued)
Symbol Description
Follow Operating Instructions (IEC 60878)
Take all used batteries to a battery collection site according to
your national legislation and the Batteries Directive 2006/66/EU.
Do not discard in standard trash or at a trash site.
About the TES Enterprise
2/14/18 Doc. No. MAN-600087 Rev. D | 4 |
Confidential
About the TES Enterprise
1.3 Technical Specifications
Table 3 TES Enterprise Specifications
Component Specification Description
Dimensions Exterior: 22”L x 14”W x 9”H
Interior: 20.5”L x 11.3W x 7.5”H
Weight 33 pounds
Power Tablet has Surface Pro (180 WHr) primary battery
Battery Life TES Enterprise battery at max configuration runs for a minimum of
8 hours.
Computer Surface
Operating System Windows
Display 3:2 aspect ratio, 2736 x 1824 resolution
Keyboard Attached keyboard. Touchpad with scroll zone and gestures sup-
port is integrated into tablet.
®
Pro 4
®
7 or 10
Connectivity WiFi, Ethernet, 3G/4G, (depending on your network’s capability)
Ports 4 USB 3.0 ports, 1 HDMI port, 1 Ethernet port
Cables PC cables- Integrated
Device cables- Not integrated
Audio Integrated speaker and microphone
Portability 2 wheels with retractable handle
Main Camera Integrated 1080p camera
Devices Compatible devices include but are not limited to: TotalExam® line
of cameras; ClearProbe Ultrasound devices; Stethoscope solutions:
ClearSteth, TotalECG 12 Lead Wireless System.
Security • Powerclaw Latching system
• Reinforced metal padlock holes for padlocks
Power Consumption Lithium ion battery:
12V - 20V DC at 4.5A MAX
185 Wh
Note: This battery is nonremovable.
Designated Battery Charger Input 90-264VAC
47-63 Hz
Output 16.8VDC 2.5A
Storage Transport Conditions Water-resistant IP67 certified
2/14/18 Doc. No. MAN-600087 Rev. D | 5 |
-29° to 60°C (-20 to 140°F)
5% to 95%, 38.7°C (101.6°F) Max Wet Bulb Temperature
Confidential

Note: Before flying with TES Enterprise, contact the airline regarding
baggage restrictions. Refer to"Technical Specifications" on page 5 for
internal component details.
1.4 Devices
Table 4 TES Enterprise peripherals
About the TES Enterprise
Device
Tot alExam® 3 with AutoFocus Head
Tot alExam® 3 with Otoscope Head
Fundus Camera
ClearProbe™ Ultrasound
ClearSteth™
SpiroPerfect Spirometer
TotalVitals
Tot alECG™ & Wireless 12-lead ECG system
Cameras
Other Peripherals
Note: Not all devices are shown in the figures below. Devices and
storage locations can vary based on your TES Enterprise
configuration.
2/14/18 Doc. No. MAN-600087 Rev. D | 6 |
Confidential

About the TES Enterprise
Figure 1 Device storage locations in the TES Enterprise, top tray
2/14/18 Doc. No. MAN-600087 Rev. D | 7 |
Confidential

About the TES Enterprise
Figure 2 Device storage locations in the TES Enterprise, bottom tray
2/14/18 Doc. No. MAN-600087 Rev. D | 8 |
Confidential

CHAPTER
Using the TES Enterprise
2
2.1 Overview
This chapter describes the basic functions when using the TES Enterprise.
The following topics are discussed:
• "TES Enterprise Features" on page 9
• "Battery" on page 15
• "Power" on page 17
• "Sound and Video" on page 19
• "Device Connections" on page 23
2.2 TES Enterprise Features
The following figures identify the TES Enterprise features.
2/14/18 Doc. No. MAN-600087 Rev. D | 9 |
Confidential

Figure 3 TES Enterprise: Front view closed
Using the TES Enterprise
Figure 4 TES Enterprise: Back view closed
2/14/18 Doc. No. MAN-600087 Rev. D | 10 |
Confidential

Figure 5 TES Enterprise: Front view open
Using the TES Enterprise
2/14/18 Doc. No. MAN-600087 Rev. D | 11 |
Confidential

Figure 6 TES Enterprise: Front view, tray displayed
Using the TES Enterprise
2/14/18 Doc. No. MAN-600087 Rev. D | 12 |
Confidential

Figure 7 TES Enterprise: Top view with top tray
Using the TES Enterprise
2/14/18 Doc. No. MAN-600087 Rev. D | 13 |
Confidential

Figure 8 TES Enterprise: Top view without top tray
Using the TES Enterprise
2.3 Opening the TES Enterprise
1. Place case horizontally on flat, sturdy surface.
2. If you have a padlock on the case, unlock the padlock.
3. Lift up the Powerclaw latches from the bottom and pull out to open.
4. Lift up the upper lid until completely upright.
5. Carefully unpack any devices and cables needed from the individual
storage compartments.
2/14/18 Doc. No. MAN-600087 Rev. D | 14 |
Confidential

Using the TES Enterprise
2.4 Charging the TES Enterprise for the First Time
The TES Enterprise must be plugged into a wall outlet overnight before first
use.
Tip: When charging the TES Enterprise make sure to fully insert the
charging adapter into the AC power input. The battery level gauge
flashes when the TES Enterprise is charging.
1. Use the included charging adapter found in the bottom tray.
2. Plug the adapter into the AC power input and into a wall outlet.
2.5 Battery
It will take at least 6-8 hours to fully charge the TES Enterprise. The
Surface Pro 4 also charges while the TES Enterprise is charging. The TES
Enterprise operates on its internal batteries for up to 8 hours.
Tip: The TES Enterprise may be used while charging the battery,
AFTER the initial overnight charge has been completed, as long as
one light is lit on the battery level gauge.
Note: Battery life will vary depending on the devices in use.
The TES Enterprise has a battery level gauge on the front of the TES
Enterprise (see Figure 9). The blue LED lights indicate the battery charge
levels:
2/14/18 Doc. No. MAN-600087 Rev. D | 15 |
Confidential

Figure 9 Battery charge indicator
Using the TES Enterprise
• One LED light indicates a 0-25% charge.
• Two LED lights indicate a 25-50% charge.
• Three LED lights indicate a 50-75% charge.
• Four LED lights indicate a 75-100% charge.
• When one LED light is flashing, the TES Enterprise is about to shut
down, which also causes all devices to power down.
2/14/18 Doc. No. MAN-600087 Rev. D | 16 |
Confidential

2.6 Power
1. To power the TES Enterprise on, press the power button located at
Figure 10 Power button location
Using the TES Enterprise
the front of the unit (see Figure 10).
2. Press the power button located at the top of the Surface Pro 4 to
turn on the tablet.
3. To power down the TES Enterprise:
a. Turn off the Surface Pro 4 first.
b. Press the TES Enterprise power button for three seconds.
Note: The Surface Pro 4 must be powered on for the speaker, LED
light, and USB hub to operate.
2.7 Surface Pro 4 Usage
The Surface Pro 4 is on a Z-tilt bracket and may be positioned for better
viewing during a consultation.
2/14/18 Doc. No. MAN-600087 Rev. D | 17 |
Confidential

Using the TES Enterprise
1. Gently pull the Surface Pro 4 out and down.
2. For a better typing surface, you may use the top tray to support the
attached keyboard (see Figure 11).
Figure 11 TES Enterprise with pulled out keyboard
2/14/18 Doc. No. MAN-600087 Rev. D | 18 |
Confidential

2.8 Sound and Video
The headphone jack and volume controls are located at the top and left
side of the Surface Pro 4.. The Surface Pro 4 integrated camera and headphone jack and built-in speakers are used for video conferencing. .
Figure 12 Surface Pro 4 button locations
Using the TES Enterprise
Figure 13 Surface Pro 4 camera
2/14/18 Doc. No. MAN-600087 Rev. D | 19 |
Confidential

Using the TES Enterprise
3. Located on the top left of the TES Enterprise is the integrated
speaker/microphone with the following functions shown in
Figure 14:
Figure 14 Speaker functions
Note: The pick up and hang up call buttons on the speaker are not
currently active.
2/14/18 Doc. No. MAN-600087 Rev. D | 20 |
Confidential

Using the TES Enterprise
4. Lights around the speaker light up to indicate the volume level, with
all lights showing for the highest volume setting (see Figure 15).
Only one light is lit for the lowest volume setting (see Figure 14).
Figure 15 Volume lights on speaker
5. The lights around the speaker show in red when Mute is selected
(see Figure 16).
Figure 16 Speaker on mute mode
2/14/18 Doc. No. MAN-600087 Rev. D | 21 |
Confidential

2.9 Touch LED Light
Figure 17 LED light
Using the TES Enterprise
1. To turn the LED light located at the top of the TES Enterprise on/off,
touch once.
2. To dim the light, hold your finger on the left upper corner of the light
until it dims to the desired level.
2.10 WiFi Hotspot
The TES Enterprise is equipped with a Jetpack™ MiFI 6620L that can be
used to set up a WiFi hotspot. For more information on how to do this
please refer to the Jetpack User Guide.
Note: WiFi is not enabled as a default; you must register your own SIM
card and set up your WiFi capability.
2/14/18 Doc. No. MAN-600087 Rev. D | 22 |
Confidential

2.11 Device Connections
The TES Enterprise has four USB 3.0 ports to connect devices (see Figure
18).
Figure 18 USB 3.0 hub
Using the TES Enterprise
For more information on using GlobalMed software for device data
collection with your TES Enterprise unit, refer to the relevant software
manuals. These include:
• eNcounter® Web
• eNcounter® Station
• eNcounterView
®
• CapSure® and its iterative versions
• ClearSteth
2/14/18 Doc. No. MAN-600087 Rev. D | 23 |
®
Confidential

Using the TES Enterprise
2.12 Thinklabs® One Stethoscope Usage
If your TES Enterprise includes a Thinklabs One Stethoscope use the
following information to connect it with TES Enterprise:
1. Plug the Thinklabs One Stethoscope into the monitor end of the
Thinklabs One Thinklink (see Figure 19).
2. Plug the USB audio device into the source end of the Thinklabs One
Thinklink (see Figure 19).
Note: The Source switch must be to the right and the Monitor switch
must to the left on the Thinklabs One Thinklink.
Figure 19 Thinklabs One Thinklink
3. Plug the USB audio device into the USB 3.0 hub (see Figure 20).
4. Plug your headphones into the Surface Pro 4 headphone jack (see
Figure 20).
2/14/18 Doc. No. MAN-600087 Rev. D | 24 |
Confidential

Using the TES Enterprise
Figure 20 Thinklabs usage with TES Enterprise
For more information on using the Thinklabs One Stethoscope refer to the
Thinklabs One User Manual.
2/14/18 Doc. No. MAN-600087 Rev. D | 25 |
Confidential

CHAPTER
Maintenance and
3
Troubleshooting
3.1 Overview
This chapter describes the basic maintenance and troubleshooting required
for the TES Enterprise. The following topics are discussed:
• "Required Maintenance" on page 26
• "Basic Troubleshooting" on page 28
• "Cleaning Procedures" on page 28
3.2 Required Maintenance
Due to the vibration from movement and use of the mobile unit, perform a
weekly maintenance check. In addition, any time a component or device
seems loose or defective, perform a maintenance check immediately.
Note: Switch off the Surface Pro 4, and switch off and unplug the TES
Enterprise before performing any maintenance.
2/14/18 Doc. No. MAN-600087 Rev. D | 26 |
Confidential

Maintenance and Troubleshooting
Table 5 Component Maintenance
Component Maintenance/Replacement Procedure
Power and device cords • Inspect the cords for damage and bent or cracked prongs
or connectors.
• If any of these conditions exist, stop using the device or
power cord and contact GlobalMed for a repair or
replacement.
Battery Contact GlobalMed for new lithium polymer battery. Battery
replacement must be done by GlobalMed.
Caution: Do not incinerate or dispose of lithium polymer
batteries in general trash collection. They may
explode or rupture violently. Check state and local
regulations dealing with the disposal of these
materials. You are legally responsible for hazards
created while your battery is being disposed.
2/14/18 Doc. No. MAN-600087 Rev. D | 27 |
Confidential

Maintenance and Troubleshooting
3.3 Basic Troubleshooting
Table 6 Troubleshooting Solutions
Issue Solution
No power • Using the included charging cable, plug into wall in
case battery is fully discharged.
• Plug another electrical device into the AC wall
outlet to verify that the outlet is working.
No sound • Press Volume + (up) on the Surface Pro 4 or
Speaker/Microphone.
• Make sure MUTE is not selected on the speaker.
• Unplug any headphones from the jack or USB ports
if sound from the speaker is desired.
Device connection issues • Check all cable connections. Check for any dam-
age to the cables.
• Check if the TES Enterprise is powered on and has
a charge.
• Refer to the appropriate software manual for additional troubleshooting.
Laptop/tablet issues • Power down and restart the laptop.
• Refer to
User Guide.
• If this does not rectify the issue contact technical
support.
Surface Pro 4 User Guide Getac
3.4 Cleaning Procedures
The purpose of these procedures are to provide clear direction and
instruction with regard to the cleaning requirements for the TES Enterprise.
These procedures reference the classification scheme found in the Centers
for Disease Control and Prevention (CDC), Guidelines for Disinfection and
Sterilization in Healthcare Facilities, 2008. In order to stratify the relative
degree of risk for infection when utilizing the individual the TES Enterprise
components, the procedures are categorized into three levels. The
categories and their basic definitions are as follows:
• Critical: Items confer a high risk for infection if they are contaminated with any microorganism.
2/14/18 Doc. No. MAN-600087 Rev. D | 28 |
Confidential

Maintenance and Troubleshooting
• Semi-critical: Items that contain mucous membranes or non-intact
skin.
• Non-critical: Items that contact intact skin but not mucous mem-
branes.
Table 7 details the component type, the CDC disinfection and sterilization
procedure, and the CDC classification based on the product’s use.
• ALWAYS use approved disinfecting wipes and/or a soft cloth, lightly
moistened with the approved cleaning solutions per CDC guidelines.
• ALWAYS check with CDC guidelines and product manuals, if in
doubt.
• NEVER spray any liquids directly on the or any of the components.
• NEVER use any abrasive cleaners or volatile solvents.
• NEVER use any alcohol, ammonia, or abrasive products on screens
or monitors as they can etch the screen surface and cause the surface to appear cloudy.
Table 7 CDC cleaning guidelines
Component Procedure
External surface
areas
Outside of case and
wheels
Cables and cords Gently wipe all of the exposed cables and cords with a
Monitor screen(s) Use a soft cloth to gently clean the screen(s). The
Items that may come in contact with non-intact skin for a
brief period of time are usually considered noncritical surfaces and are disinfected with intermediate-level disinfectants such as phenol, iodine solution, alcohol, or chlorine.
Gently wipe the base covering and wheels with a disinfecting wipe and/or soft cloth, lightly moistened with a
facility or CDC-approved cleaning solution.
disinfecting wipe and or soft cloth, lightly moistened with a
facility or CDC approved cleaning solution. All of the electrical cords must be unplugged before cleaning. After
cleaning, check that all of the cables and cords are properly plugged in.
screen(s) are fragile. Do not scrape or tap the screen(s)
with any sharp objects. Upon contamination, use a soft
cloth moistened with an approved spray designed for
monitors and computer screens. Wipe the display with a
soft, dry cloth after cleaning.
CDC
Classification
Semi-critical
Non-critical
Non-critical
Non-critical
2/14/18 Doc. No. MAN-600087 Rev. D | 29 |
Confidential

Table 7 CDC cleaning guidelines (Continued)
Maintenance and Troubleshooting
Component Procedure
Device storage
compartments
Keyboard Gently wipe the keyboard with a disinfecting wipe and/ or
Gently wipe the interior surfaces with a disinfecting wipe
and/or soft cloth, lightly moistened with a facility or CDCapproved cleaning solution.
soft cloth, lightly moistened with a facility or CDCapproved cleaning solution.
CDC
Classification
Non-critical
Non-critical
2/14/18 Doc. No. MAN-600087 Rev. D | 30 |
Confidential
 Loading...
Loading...