G-Lab MD2220, MD2222, MD2223 Instruction Manual
DIGITAL AUTOMATIC
WRIST BLOOD PRESSURE MONITOR
INSTRUCTION MANUAL
MODEL No.: MD2220/MD2222/MD2223
Version: 1
TABLE OF CONTENTS
INTRODUCTION...............................................................................1
COMPLIANCE...................................................................................1
SYMBOLS........................................................................................2
IMPORTANT NOTES.....................................................................3-5
DEVICE DESCRIPTION.....................................................................6
BATTERY INSTALLATION.................................................................7
SETTING DATE AND TIME................................................................8
BEFORE TAKING A MEASUREMENT................................................ 9
APPLYING WRIST CUFF...................................................................9
SELECT USER..................................................................................10
PERFORMING BLOOD PRESSURE MEASUREMENT......................11
RECALL AVERAGE AND PREVIOUS MEASUREMENT DATA...........12
DELETE MEASUREMENT DATA......................................................13
WHO CLASSIFICATION INDICATOR............................................... 14
ABOUT IRREGULAR HEARTBEAT ...................................................15
ABOUT BLOOD PRESSURE............................................................15
TROUBLESHOOTING......................................................................16
TECHNICAL SPECIFICATION..........................................................17
APPENDIX I...............................................................................18-19
APPENDIX II..............................................................................20-22
APPENDIX III.............................................................................23-24
APPENDIX IV.................................................................................. 25
INTRODUCTION
Thank you for purchasing the G.LAB MD2220/MD2222/MD2223
Wrist Blood Pressure Monitor.
The device is easy-to-use and good for home users and healthcare
professionals. It applies non-invasive oscillometric method which
can measure your blood pressure and pulse rate quickly and easily,
and it saves the data automatically to let you review the average and
measured data at any time.
Indication for use
Digital Automatic Wrist Blood Pressure Monitor WBPM22 Series is
for use by medical professional or home user. The WBPM22 Series
is intended to measure the systolic and diastolic blood pressure,
and pulse rate of an adult individual by using a non-invasive
technique, in which an inflatable cuff is wrapped around the wrist.
COMPLIANCE
This device conforms to European Medical Device Directive
93/42/EEC.
This device complies with:
• EN ISO 81060 standard relating to non-invasive sphygmomanom eters
Part 1: Requirements and test methods for non-automated
measurement types and EN 1060 standard relating to non-invasive
sphygmomanometers.
Part 3: Supplementary requirements for electro-mechanical blood
pressure measuring systems.
• EN 60601 standard relating to medical electrical equipment
Part 1-2: General requirements for basic safety and essential
performance and essential performance – Collateral standard:
Electromagnetic compatibility – Requirements and tests.
• EN 1060-4:2004 standard relating to non-invasive sphygmoma nometers
Part 4: Test procedures to determine the overall system accuracy
of automated non-invasive sphygmomanometers.
• ISO 81060-2:2013 standard relating to non-Invasive
sphygmomanometers
Part 2: Clinical validation of automated measurement type.
• IEC 80601-2-30:2009+A1:2013 standard relating to medical
electrical equipment
Part 2-30: Particular requirements for the basic safety and
essential performance of automated type non-invasive
sphygmomanometers.
- 1 -

SYMBOLS
The following symbols are used in this instruction manual, or
appear on the device, accessories and packaging.
To assure correct use of the device, basic safety measures should
always be followed including the warnings and cautions listed in
this instruction manual.
- 2 -
Symbols Function / Meaning
WARNING / ATTENTION
Indicates a potentially hazardous situation
which, if not avoided, could result in death
or serious injury.
PRECAUTION / IMPORTANT INFORMATION
SN Serial Number
Manufacturer
Type BF: Device, cuff and tubing are designed to
provide special protection against electrical
shocks.
SYS Systolic Blood Pressure in mmHg
DIA Diastolic Blood Pressure in mmHg
PUL Pulse
Symbols Function / Meaning
EC Directive Medical Device Label
WEEE Label
Refer to instruction manual / booklet
Keep dry

IMPORTANT NOTES
DO NOT use this device on newborns, infants, children,
toddlers or persons who cannot express their intentions. The
device is designed for use on adults only.
DO NOT self-diagnosis from the measurement results and start
treatment by yourself.
DO NOT adjust medication based on the measurement results.
Consult your physician for specific information about your
blood pressure.
The Irregular Heartbeat detection function may help to detect
potential cardiac arrhythmia at an early stage but it is not
intended to replace cardiac examination.
The “WHO Blood Pressure Classification” chart is a guide for
reference and is not intended to replace medical diagnosis.
Use the device only as intended. Do not use the device for any
other purpose.
Do not apply the device on a wrist with an unhealed wound or
under medical treatment.
Do not take measurements more than necessary. High
measurement repetition rates may cause pain, numbness,
temporary red marks or bruising to the arm/wrist due to blood
flow interference.
If you have any of the following medical conditions, you may
get an inaccurate reading with the device. Please consult your
physician before using the device.
• Patients in shock
• Cardiac arrhythmias
• Atrial or ventricular premature beats
• Atrial fibrillation
• Arterial sclerosis
• Poor perfusion
• Vessel anomalies
• Very low blood pressure
• Pregnancy
• Diabetes
• Pre-eclampsia
• Renal diseases
• Underwent breast or axillary lymph node removal operation
• With an arteriovenous shunt.
• With an intravenous drip or blood transfusion.
• With implanted electrical device such as cardiac pacemaker
• With other medical electrical equipment attached
• With condition that may compromise circulation
• Severe blood flow problems or blood disorders, as cuff
inflation can cause bruising.
• Trembling or shivering
Do not use the device with other medical electrical equipment
simultaneously.
- 3 -
IMPORTANT NOTES
Do not use the device where high frequency surgical
equipment, magnetic resonance imaging (MRI), computerized
tomography (CT) scanner or X-ray machine is operating.
Do not use the device near electromagnetic fields emission
equipment such as cellular phones, microwave ovens or
televisions.
Do not use the device where flammable gases (e.g. anesthetics
gas, oxygen and hydrogen) or flammable liquids (e.g. alcohol)
are present.
Do not use the device in a moving vehicle such as car or
airplane.
Do not use the device outside the specified environment. It may
cause an inaccurate reading.
The product contains small parts that may cause a choking
hazard to infants and children. Keep the device and its parts out
of reach of infants and children.
Do not attempt to open, disassemble, repair, modify or adjust
the device by yourself. It may cause accident, damage the
device, cause inaccurate measurement and void the user
warranty.
Do not subject the device to strong knocks (e.g. dropping the
unit on the floor), extreme in temperature, high humidity, direct
sunlight, dust or chemicals. This may damage the device.
The device is not water resistant. Avoid water, rain or sweat
from infiltrating the device.
Clean the device and cuff carefully with a dry, soft cloth or a
cloth dampened with water. Do not use aggressive solvents
such as alcohol, benzene, thinner or other strong chemicals to
clean the device.
Do not fold the cuff tightly for a long period. Such condition
may shorten the life of the part.
Dispose used equipment, parts, batteries and optional
accessories according to applicable local regulations. Unlawful
disposal may cause environmental pollution.
Do not use any cuffs and accessories other than those
explicitly recommended by the manufacturer for use with this
product. Cuffs and accessories not approved for use with this
device may cause damage to your health and to the product.
In case the cuff does not stop inflating, interrupt the
measurement by pressing the ON/OFF button and open the cuff
at once.
- 4 -

IMPORTANT NOTES
Do not wrap the cuff around body parts other than your upper
left arm. Misuse represents a risk to your health.
Packaging materials are a deadly hazard for children and can
cause suffocation. Remove all packaging materials immediately
and keep them away from children at all times.
Proper cuff size is important for accurate measurements. Only
use the device on adults who have the right upper arm
circumference for this unit. See “TECHNICAL SPECIFICATION”
for suitable arm circumferences.
Batteries should not be charged or reactivated by any other
means. The batteries may explode.
Take extra precaution to keep a leaking battery away from fire
as there is a risk of ignition or explosion.
The tubing presents a strangulation hazard. Keep this product
away from children and those who require close supervision,
e.g. people with mental disorders.
Do not drape tube around neck. This presents a strangulation
hazard.
Remove any kind of arm jewellery or the like before taking a
measurement. This could cause bruises.
When storing the device, make sure that no heavy objects are
placed on top of it.
Do not place the arm cuff over heavy clothing (e.g. a jacket or
sweater sleeve) as the blood pressure monitor will not be able
to take a proper measurement and there is an elevated danger
of acquiring hematoma or skin marks during the course of the
measurement.
When applying the cuff, make sure there are no wrinkles in the
cuff as this could cause bruises.
Blood pressure measurements can lead to temporary marks on
the skin at the site of the cuff placement. This is especially the
case in high repetition rates, in hypertonic patients and in
patients with weak heart rates. In rare cases a mark may
persist for couple of days. Please contact your physician about
these specific risks of cuff pressure in your specific case.
Do not exert any kind of pressure on the hose during
measurement, e.g. laying your arms or any other object on
the hose. This could cause incorrect measurements.
The device is designed and manufactured for a long service life.
However it is generally recommended to have the monitor
inspected every 2 years to ensure proper functioning and
accuracy. Please contact your dealer for maintenance.
Do not drop or insert any object into any openings or hoses.
This may damage the unit.
Do not press the buttons with excessive force or with pointed
objects.
- 5 -
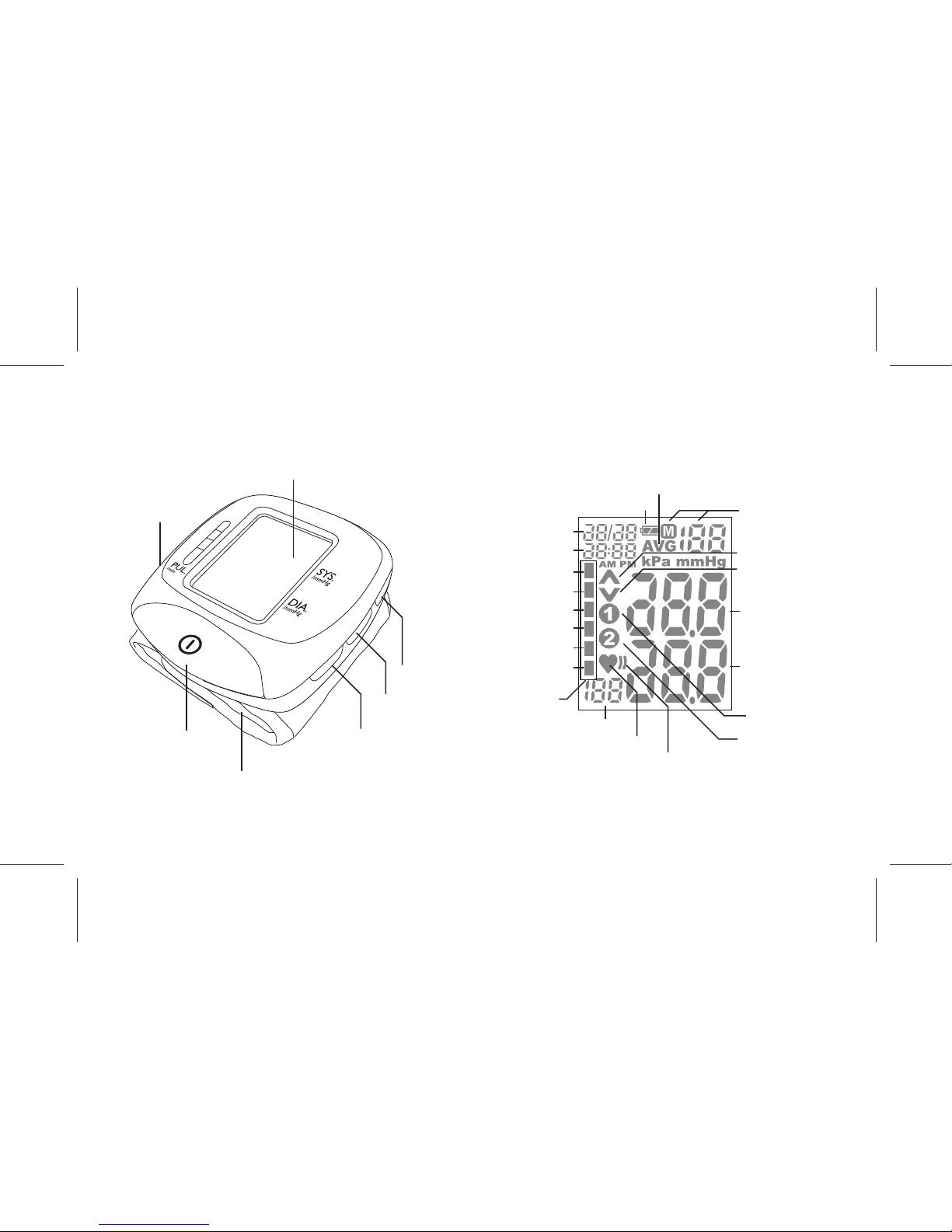
DEVICE DESCRIPTION
Information on the Display :
- 6 -
Heart Mark
Irregular Heartbeat
Indicator
Battery Indicator
Pressurizing
Releasing Air
Average Data
User 1
User 2
Memory
Record Number
and Data
Systolic
Pressure
Diastolic
Pressure
Pulse Rate
Severe Hypertension
Moderate Hypertension
Mild Hypertension
High Normal
Normal
Optimal
Date
Time
Pressure Bar
Battery Cover
Cuff
Clock Key
Start / Stop Key
Memory Key
User Selection Key
Display

BATTERY INSTALLATION
1. Remove the battery cover.
2. Remove the used batteries
and insert new batteries.
Use LR03 / AAA alkaline
batteries.
Make sure the battery
polarities (+) and (-) match
the markings on the battery
compartment.
3. Close the battery cover.
Battery Level Indicator
symbol on the display indicates that battery level is normal.
When battery level is getting low, symbol and “E6” will appear
on the display. Replace all used batteries with new batteries.
NOTE:
Batteries may cause a choking hazard to children. Store the
batteries out of the reach of children.
In case battery fluid leaks, do not touch the battery fluid. Avoid
skin contact (e.g. put on protective gloves) and clean the battery
compartment with dry cloth.
Remove the batteries from the battery compartment if the device
will not be used for a long period.
Use only 1.5V alkaline batteries. Do not use other types of
battery such as rechargeable battery. This may damage the
device.
Replace all batteries at the same time. Do not mix used and new
batteries. Use same brand and model of batteries is
recommended.
Battery life may vary with ambient temperature and may be
shorter at low temperature.
- 7 -

SETTING DATE AND TIME
(A) When new batteries are installed
1. “YEAR” will blink on the display.
2. Press M key to set the current Year.
3. Press key to confirm and then "MONTH" will start to blink.
4. Press M key to set the current Month.
5. Press key to confirm and then "DAY" will start to blink.
6. Press M key to set the current Day.
7. Press key to confirm and then "HOUR" will start to blink.
8. Press M key to set the current Hour.
9. Press key to confirm and then "MINUTE" will start to blink.
10. Press M key to set the current Minute.
11. Press key to confirm. Date and time setting is completed.
(B) When device is in OFF status
1. Press key to turn on the device in Clock display.
2. Press and hold key for about 3 seconds until “YEAR” blinks
on the display.
3. Follow the same procedure above to set the date and time.
- 8 -
 Loading...
Loading...