Page 1

Bravo
®
pH Monitoring System
User Guide
DOC-2033-07
March 2016
Page 2

Copyright & Trademarks
Rx Only
Copyright © 2001–2016 Given Imaging Ltd. All rights reserved. Medtronic, Medtronic
logo and Further, Together are trademarks of Medtronic.
TM*
Third party brands are
trademarks of their respective owners. All other brands are trademarks of a Medtronic
company.
FCC Compliance Statement
This device complies with Part 15 of the FCC. Operation is subject to the following two
conditions:
1 This device may not cause harmful interference.
2 This device must accept any interference received, including interference that
may cause undesired operation.
Given Imaging
15 Hampshire Street,
Mansfield, MA 02048 USA
supportUS@givenimaging.com
Medtronic B.V.
Earl Bakkenstraat 10,
6422 PJ Heerlen, The Netherlands
Page 3
Table of Contents
Table of Contents
Introduction......................................................................................................... 1
Description ............................................................................................. 1
Indications for Use ................................................................................. 1
Contraindications ................................................................................... 1
Warnings and Precautions ...................................................................... 2
Storage ................................................................................................... 3
Electromagnetic Compatibility .............................................................. 3
Patient Information (Benefits and Risks) ............................................... 4
System Components ............................................................................... 5
System Workflow .................................................................................. 6
Bravo pH Recorder ............................................................................................. 7
Description ............................................................................................ 7
Backlight ................................................................................................ 7
Patient Buttons ....................................................................................... 7
Clinician Buttons and Menus ................................................................. 8
Status LED ............................................................................................. 9
General Guidelines ................................................................................. 9
Charging the Recorder ......................................................................... 10
Turning the Recorder On and Off ........................................................ 10
Setting the Date and Time .................................................................... 11
Choosing Study Settings ...................................................................... 11
Calibrating Capsules ......................................................................................... 13
Existing Data ........................................................................................ 13
Starting Calibration .............................................................................. 13
Performing a Bravo pH Study .......................................................................... 19
Setting up the Vacuum ......................................................................... 19
Starting Recording ............................................................................... 20
Placing the Capsule .............................................................................. 21
Stopping a pH Study ............................................................................ 25
Reviewing Instructions with Patients ................................................... 26
Uploading pH Data ........................................................................................... 28
Recorder Maintenance ...................................................................................... 29
Safety and Technical Checks ............................................................... 29
Cleaning the Recorder .......................................................................... 29
Cleaning the Case and Strap ................................................................ 29
Servicing the Battery ............................................................................ 29
Bravo pH Monitoring System i User Guide
Page 4
Table of Contents
Troubleshooting ................................................................................................ 30
Delivery Device Disassembly Procedure ............................................. 30
Recorder Troubleshooting .................................................................... 32
Appendix A: Technical Data ............................................................................ 35
Bravo pH Recorder .............................................................................. 35
Recorder Servicing ............................................................................... 35
USB Cable ........................................................................................... 36
Charger ................................................................................................ 36
Battery ................................................................................................. 36
FCC Compliance Statement ................................................................. 36
Declaration of Conformity ................................................................... 36
Essential Performance of Bravo Recorder ........................................... 36
Electromagnetic Compatibility Declaration (EN / IEC 60601-1-2) .... 37
Bravo pH Capsule Specifications ........................................................ 41
Bravo pH Delivery Device Specifications ........................................... 41
Appendix B: Symbols on Package Labeling .................................................... 42
Bravo pH Monitoring System ii User Guide
Page 5

Introduction
!
Description
The Bravo® pH Monitoring System is intended to be used for gastroesophageal
pH measurement and monitoring of gastric reflux:
•
First, a Bravo pH capsule is calibrated and the Bravo pH recorder (an ambulatory,
programmable data recorder) is prepared.
•
Using the delivery device, the capsule is positioned and attached in the patient’s
esophagus, following either endoscopy or manometry.
•
The data is collected by the capsule and transmitted to the recorder for the duration
of the study.
•
The data is then uploaded from the recorder to the software application on the PC or
workstation. The software application is used to record, store, view, and analyze
gastroesophageal pH data, enabling physicians to interpret study results.
Indications for Use
The Bravo pH Monitoring System is intended to be used for gastroesophageal pH
measurement and monitoring of gastric reflux in adults and children from 4 years of age.
The Bravo pH capsule can be attached following either endoscopy or manometry. The
AccuView and Reflux software applications are intended to record, store, view, and
analyze gastroesophageal pH data.
Contraindications
Patients with bleeding diathesis, strictures, severe esophagitis, varices, obstructions,
pacemakers or implantable cardiac defibrillators are contraindicated.
Warning
Patients are restricted from undergoing an MRI study for 30 days from
the start of a pH study. The Bravo pH Monitoring System is not
compatible for use in an MRI magnetic field. Use of the Bravo pH
Monitoring System in an MRI magnetic field will result in damage to the
system and possible patient injury.
Bravo pH Monitoring System 1 User Guide
Page 6
Warnings and Precautions
•
Bravo pH capsule with delivery device: Potential complications include, but are
not limited to:
• aspiration of the capsule if inadvertently pulled back up into the upper
esophagus by the delivery device. There is a possibility that this may occur in a
procedure in which the capsule did not attach to the esophageal mucosa.
• tears or perforations in the mucosal and submucosal layers of the esophagus
causing bleeding and requiring possible medical intervention.
• gastrointestinal endoscopy: Potential complications include, but are not
limited to: perforation, hemorrhage, aspiration, fever, infection, hypertension,
respiratory arrest, and cardiac arrhythmia or arrest.
• nasal intubation: Potential complications include, but are not limited to: sore
throat, discomfort, and nasopharyngeal damage resulting in bleeding and soft
tissue damage.
•
Bravo pH capsule: Potential complications include, but are not limited to:
• discomfort associated with the capsule, or failure to detach from the esophagus
within several days after placement, either of which may necessitate endoscopic
removal.
• premature detachment of the capsule.
•
The safety and efficacy of the Bravo pH capsule with delivery device has not been
established for pediatric use on patients below the age of 4.
•
The Bravo pH capsule with delivery device is a single-use, disposable device. Reuse
or any other misuse of a Bravo pH capsule with delivery device (such as sharp
bending or kinking) results in an increased potential for damage to the delivery
device and capsule, and possible patient injury.
•
Prior to use, all equipment for the pH study should be examined carefully to verify
proper function.
•
Unauthorized maintenance by inadequately trained personnel would result in an
unacceptable risk (e.g., excessive temperatures, fire, or explosion).
•
A thorough understanding of the technical principles, clinical applications and risks
associated with the Bravo recorder is necessary before using this product. Read the
entire manual before using the system for the first time.
•
No modification of this equipment is allowed.
•
Patients are restricted from undergoing an MRI study within 30 days of the pH
study.
•
The Bravo capsule contains a trocar needle that is made of stainless steel. Use
caution in patients with known sensitivities or allergies to the metals that are
contained including chromium, nickel, copper, cobalt, and iron. The Bravo pH test
lasts from 48 to 96 hours.
Br
avo pH Monitoring System 2 User Guide
Page 7
•
Prior to the pH study, the patient should not eat or drink for a minimum of 6 hours.
•
If excretion of the Bravo pH capsule from the patient has not been positively
verified, and the patient develops unexplained postprocedure abdominal pain,
vomiting, or other symptoms of obstruction, the patient should contact the physician
for evaluation and possible abdominal X-ray.
•
Undergoing an MRI while the Bravo pH capsule is inside the patient’s body may
result in serious damage to the patient’s intestinal tract or abdominal cavity. If the
patient did not positively verify the excretion of any Bravo pH capsule, the patient
should contact the physician for evaluation and possible abdominal X-ray before
undergoing an MRI examination.
Storage
Store all components in a controlled room temperature environment:
•
capsules at 15–45 °C (59–113 °F)
•
recorder at 0–40 °C (32–104 °F)
Electromagnetic Compatibility
Electrical equipment for medical use requires special electromagnetic compatibility
(EMC) precautions and should be installed and serviced according to the documentation
of device. Portable and mobile communication equipment can affect electrical equipment
for medical use. For additional information on electromagnetic compatibility, see
Electromagnetic Compatibility Declaration (EN / IEC 60601-1-2) on page 37.
Bravo pH Monitoring System 3 User Guide
Page 8

Patient Information (Benefits and Risks)
֠
Benefits
Bravo pH monitoring system provides a more tolerable and convenient way to
evaluate your reflux symptoms when compared to catheter-based pH monitoring
systems.
The capsule is temporarily attached to the wall of your esophagus. The capsule
transmits pH information wirelessly to a small recorder that you wear. Data can be
transmitted approximately 2 meters (6 feet), which means that you can take the
recorder off to shower and sleep without interrupting the test.
You can engage in your usual activities during the test, which can provide your
doctor with a more accurate picture of your acid exposure compared to data collected
using catheter-based systems.
Risks
The Bravo pH test is not for everyone. If you have bleeding diathesis, strictures,
severe esophagitis, varices, obstructions, a pacemaker, or an implantable cardiac
defibrillator, you should not undergo a Bravo pH test. Additionally, because the
capsule contains a small magnet, you should not have an MRI study within 30 days
of undergoing the Bravo pH test.
Potential complications include, but are not limited to, the following events:
• perforation
• premature detachment of the pH capsule
• failure of the pH capsule to detach from the esophagus within several days after
placement or discomfort associated with the pH capsule, requiring endoscopic
removal
• tears in the mucosal and submucosal layers of the esophagus, causing bleeding
and requiring possible medical intervention
Potential complications associated with gastrointestinal endoscopy include:
• perforation or hemorrhage
• aspiration
• fever or infection
• hypertension
• respiratory arrest
• cardiac arrhythmia or arrest
Note
All pH testing procedures carry some risks. This information should not
be used as a substitute for talking with your doctor about diagnosis and
treatment.
Bravo pH Monitoring System 4 User Guide
Page 9
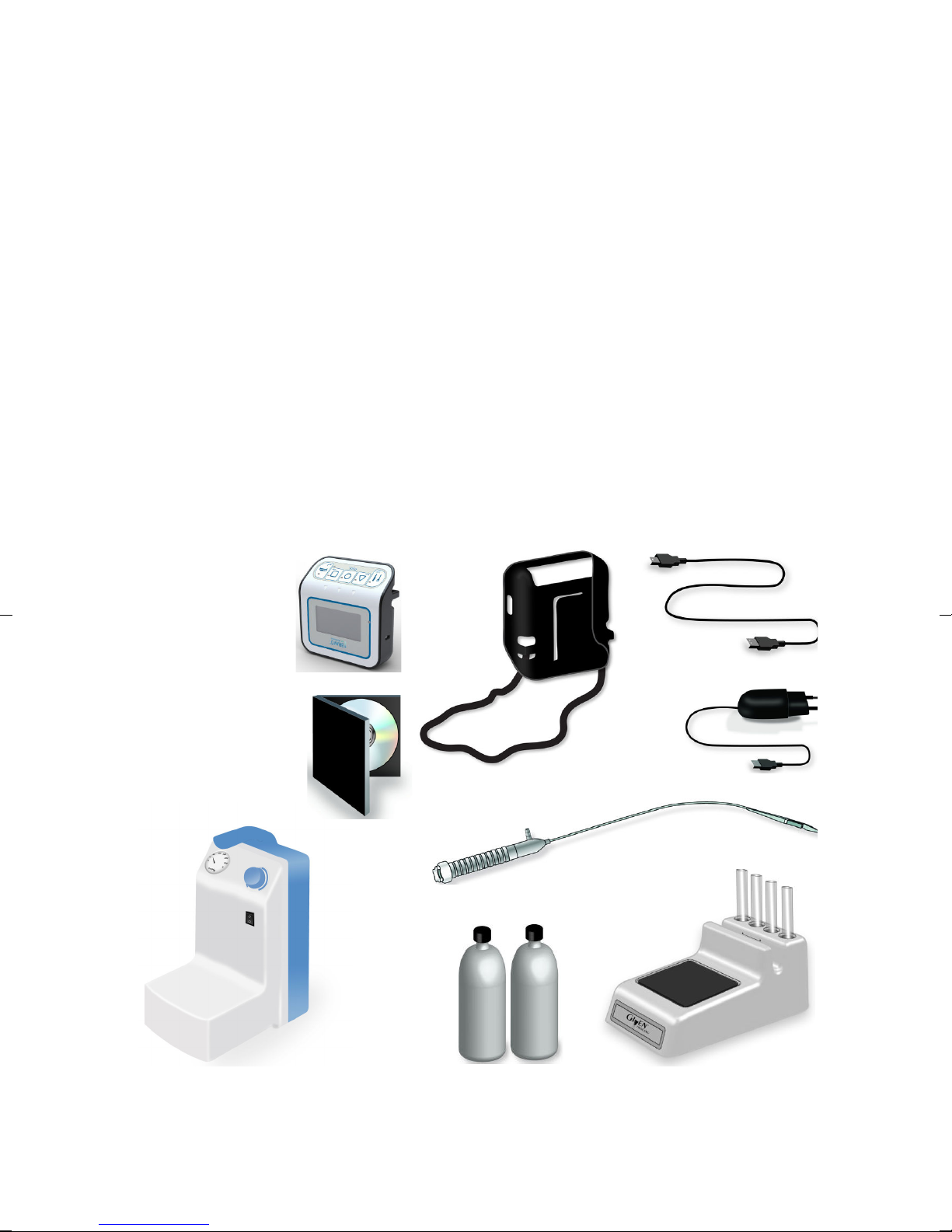
System Components
2
1
3
4
5
6
7
8
9
The Bravo pH Monitoring System consists of the following items:
1
Bravo pH recorder (referred to as recorder in this user guide)
2
case and shoulder strap
3
USB cable
4
charger
5
AccuView or Reflux software, delivered separately either on media or
pre-installed on a bundled PC workstation (referred to as PC in this user guide)
6
Bravo pH capsule with delivery device (referred to as capsule in this user guide)
7
vacuum pump
8
pH 1.07 and pH 7.01 calibration buffer solutions
9
calibration stand
10
sterile water (to be supplied by the user)
Bravo pH Monitoring System 5 User Guide
Page 10

System Workflow
֠
When using the Bravo pH Monitoring System, you follow this general workflow:
1
2
3
4
5
6
Charging the battery: you can charge the recorder by connecting the supplied
charger to an electrical outlet or by connecting the supplied USB cable to your PC.
Recorder setup: this includes setting the date and time and defining the default
settings for studies. You only need to do it once (though values can be changed later
as needed). See Setting the Date and Time on page 11.
Calibration: this includes making sure that the capsule is correctly reading the pH
levels. You do this for every study. See Calibrating Capsules on page 13.
Capsule placement: this includes performing the procedure of positioning and
attaching the capsule in the patient. See Placing the Capsule on page 21.
Patient instructions: this includes reviewing information about the study with the
patient, such as instructions on using the recorder and filling out the patient diary.
See Reviewing Instructions with Patients on page 26.
Study duration: this includes the patient wearing the recorder for the study
duration.
7
You must also become familiar with the basic workings of the recorder, including normal
maintenance functions such as recharging and cleaning. See Bravo pH Recorder on page 7
and Recorder Maintenance on page 29.
Data upload: this includes transferring the study data from the recorder to the PC
for analysis in the application software. See Uploading pH Data on page 28.
Note
The recorder always defaults to the expected action in the workflow. For
example, if the calibration process has been completed successfully, the
recorder menu cursor automatically points to Start Study. This simplifies the
process and reduces the risk of errors. However, you can manually select
another menu option at any time.
Bravo pH Monitoring System 6 User Guide
Page 11
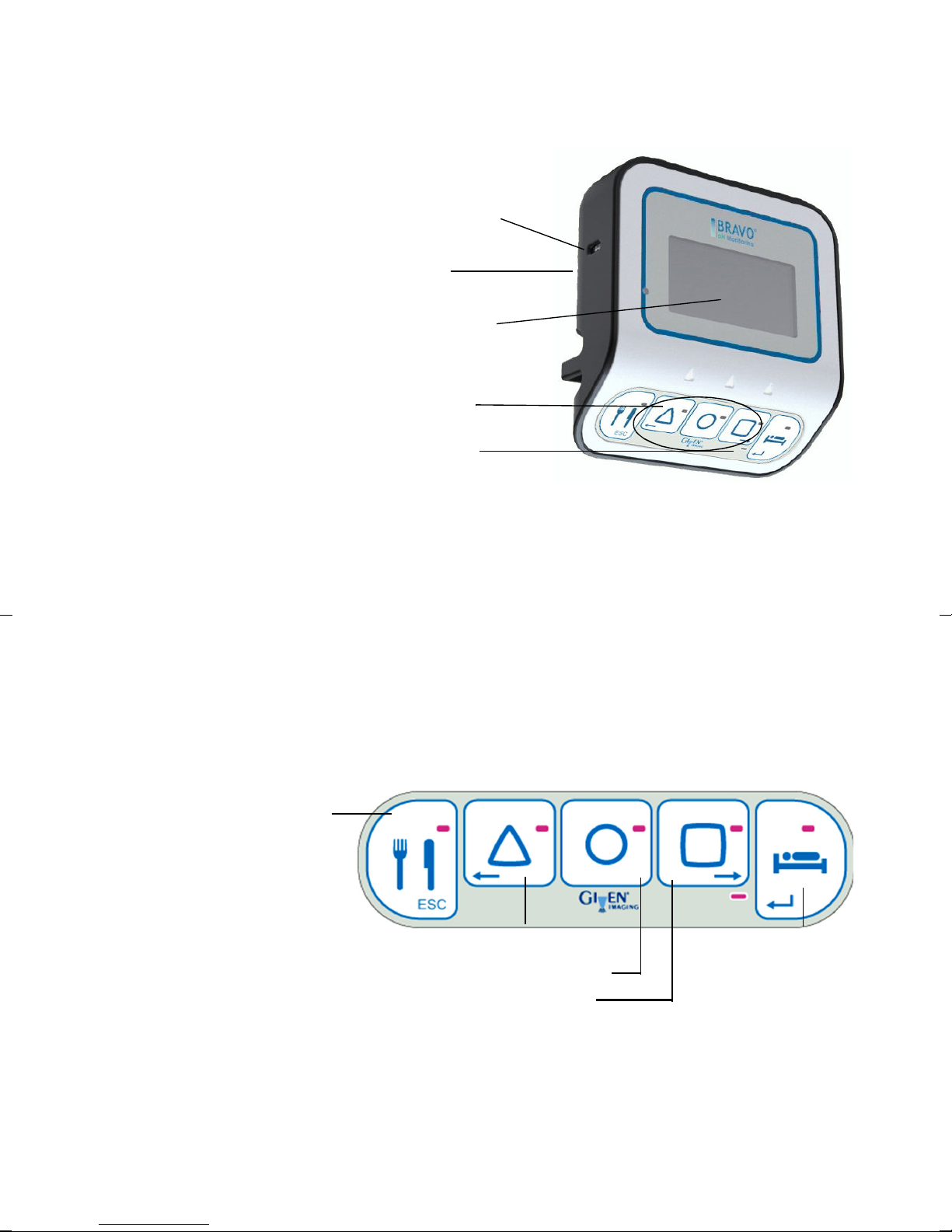
Bravo pH Recorder
on/off button
LCD screen
symptom
buttons
recorder
status LED
Figure 1. Bravo recorder, front
case
3
meal
supine
heartburn*
regurgitation*
chest pain*
* The default values of these buttons can be
set in the software application.
Description
The Bravo pH recorder is
lightweight and compact. It
fits into a case that comes
with a strap and a belt clip.
Patients wear the recorder
(over the shoulder or
attached to a belt)
throughout the study period.
Backlight
The recorder has a backlit
LCD screen and a row of
symptom buttons. The
backlight turns off
automatically (select the
backlight duration in
Preferences
any of the recorder functions be used (for example, menu access for recorder setup, or
symptom buttons for patient use).
). Pressing any key turns on the backlight. Only when the backlight is on can
Patient Buttons
When the recorder is placed in the case and is in record mode (that is, during a study), the
on/off button and USB port are covered. The patient can use the three symptom buttons
(Chest Pain, Regurgitation, and Heartburn) and the two event buttons (Meals and Supine)
to record events during a study (see Figure 2).
Figure 2. Symptom and event buttons.
Bravo pH Monitoring System 7 User Guide
Page 12
During a study, the patient pushes
֠
regurgitation icon
Figure 3. Regurgitation symptom icon as it
appears on the recorder screen
before and after pressing the
button.
after pressing button
Calibrate
Start Study
Settings
►
any button to turn on the backlight.
Once the backlight is on, pressing
a symptom button causes a beep to
occur, the button’s LED to light up
briefly, and its icon is inverted
briefly on the screen (see
Figure 3). If the button is one of
the event buttons, a beep occurs
and the button’s LED starts
blinking, indicating the event’s
start time. The blinking continues until the patient presses the button again when the event
ends. (That is, the patient presses any button to first turn on the backlight, and then presses
the event button to signal the end of the event.)
Note
Meal and Supine buttons can be used for patient input during the
study.
When the recorder’s PC Software preference is set to RAPID pH,
the Meal and Supine features are not supported. These buttons are
located on the left and the right of the symptom buttons and are
used only to navigate while setting up a study. Once a study
recording has begun, the Meal and Supine buttons are disabled for
patient use.
For details about selecting the software application, see Choosing
Study Settings on page 11.
Clinician Buttons and Menus
You, the clinicians, have access to the menu to program the recorder
for a study. The main menu appears after the welcome screen when
the recorder is turned on.
While in the menu, you use the symptom buttons to navigate. The
buttons have different meanings depending on the screen. For
example, you may be prompted to press
shows an arrow pointing to the appropriate button:
Escape/back. Goes back (returns to previous level in the menu).
For the purpose of this document, ESC is used to indicate either escape/back
button.
Yes, No, Skip, Cancel
, etc. The recorder screen
Escape/back: same functionality as the above.
In addition, it is also used to set the date and time.
For the purpose of this document, ESC is used to indicate either escape/back
Bravo pH Monitoring System 8 User Guide
button.
Page 13
Scroll. Moves to the next option for a setting.
֠
Enter/next. Saves the settings on that screen and returns to the previous location
in the menu.
For the purpose of this document, Enter is used to indicate either Enter/next
button.
Enter/next: same functionality as the above.
For the purpose of this document, Enter is used to indicate either Enter/next
button.
Note
Instructions are provided using the name of the function (such as Yes).
Button positions change, so always refer to the recorder screen to see
which buttons are used.
Once a recording has started during a study, the menu is no longer
accessible.
Status LED
There is a small LED below the symptom buttons. The LED
indicates the status of recorder by the color and duration or
frequency of the flash.
LED Status Meaning
off (no light)
blinking blue
blinking or steady red
steady green
blinking green
steady orange
Not recording any capsule transmissions. Data
from previous study has been uploaded.
Receiving transmission from the paired capsule
(recording).
A transmission error has occurred.
Study completed but data has not yet been
uploaded to the software application.
Data is being uploaded.
User has pressed OK after
completed
message appeared on the screen.
calibration is
General Guidelines
When working with the recorder:
•
All values in
Preferences
Settings
(for example, date and time format and interface language) stay in
effect for all studies until you change them.
Bravo pH Monitoring System 9 User Guide
(for example, study duration, number of capsules) and
Page 14

•
֠
֠
Fully recharge the battery before each study (see Charging the Recorder on
page 10).
•
Clean the recorder after each study (see Cleaning the Recorder on page 29).
Charging the Recorder
The recorder is delivered with the battery fully discharged. It must be recharged before
using. A fully discharged recorder battery may take up to 7 hours to charge.
•
Connect the recorder to the charger and plug it into an electrical outlet, or
•
Connect the recorder to a USB cable and connect it to your PC. Do not use this
method for charging more than one recorder simultaneously.
Turning the Recorder On and Off
1. Press and hold the on/off button (see Description on page 7) for 5 seconds until the
recorder screen turns on.
The recorder automatically performs an internal diagnostic check, which includes
checking the batteries and verifying the time and date.
• The recorder screen displays a brief welcome message showing the software and
hardware versions.
• If the battery is sufficiently charged and the time and date are available, the main
menu is displayed.
• If the date or time is not available, the recorder automatically displays the screen
to set the date and time.
• If the main battery is low, the recorder displays:
Note
The cursor’s default position shows the next logical step in your
workflow. For example, if you have completed calibration, the cursor
appears at Start Study. (You can move the cursor to select something
else.)
Charge battery!
2. To turn off the recorder, press and hold the on/off button for 2–3 seconds until
OFF the Recorder?
Note
The backlight remains on during Settings and Calibration processes.
When recording or the main screen is displayed, the backlight turns off
after the predefined time (default 30 seconds; see Choosing Study
Settings on page 11). Press any key to turn it back on. If you are not
sure if the recorder is turned on, press the on/off button once.
appears on the recorder screen.
Turn
Bravo pH Monitoring System 10 User Guide
Page 15

Setting the Date and Time
֠
Set Date/Time
DD-MM-YY
hh:mm AM/PM
►
pH Capsules #1
Study Duration: 48hr
Preferences:
►
You must set the date and time the first time you turn on the recorder or if the battery has
fully discharged. Once the date and time are set (and as long as the battery does not fully
discharge), the recorder maintains the correct date and time, even when it is turned off.
However, if the battery was fully discharged before turning the recorder on, the screen will
automatically display the
To set the date and time:
1. The first part of the date (for example, the day field) is
highlighted. Use below the icon displayed on
the screen and ( on the screen) to change it to
the correct date. Press ( on the screen) to move to the next field.
2. Repeat this process for the rest of the date (for example, month and year).
Set Date/Time
screen.
3. Repeat this process to set the time. When you are done, press
returned to the main menu.
If time and date are correct, press
ESC
and return to the main menu.
Choosing Study Settings
1. From the main screen, select
appears:
2. Set the number of capsules as follows:
a. With the cursor on
Enter
.
pH Capsules #1
b. Use to select the number of capsules.
c. Press
Enter
. You move on to the next setting:
3 Set the study duration as follows:
a. With the cursor on
Study Duration
Settings
. This screen
, press
Study Duration
, press
Enter
.
Enter
.
. You are
b. Use to select the study duration (24, 48, or 96 hours).
Bravo pH Monitoring System 11 User Guide
Note
The 96-hour option is only available if you are using AccuView or
Reflux software as your PC software application.
Page 16

֠
֠
c. Press
value
Enter
. You move on to the first screen of the next setting:
of
Preferences
Note
At any time you may select Settings from the Main screen and
access Preferences to review and set the parameters.
.
Show pH
4. The Preferences screen allows you to define setting that affect all studies.
• Show pH Value: If
Yes
, the current pH value appears on the recorder screen
during studies. If No, pH values are only displayed for the first 30 minutes of a
study. The factory default is No.
• Button Beep: If
button. The factory default is
• Capsule LED Blink: If
signals are received. The factory default is
Yes
, the recorder beeps when the patient presses a symptom
Yes
.
Yes
, the recorder capsule LED blinks when the capsule
Yes
.
• Set Date/Time: Once set, the recorder maintains the correct date and time. You
should only have to change this again if there is a time change (for example,
going on or off of Daylight Savings), or if the recorder battery is allowed to fully
discharge.
• Date Format: You can set the date format to
factory default is
MM-DD-YY
.
MM-DD-YY
or
DD-MM-YY
. The
• Time Format: You can set the time format to
(military)
. The factory default is
12-hour
• PC Software: Select the software used to record, view, and analyze the data
from studies. The choices are
software, as well) and
RAPID pH
AccuView
.
• Language: Select the language for the recorder interface. The choices are
English, Danish, Dutch, Finnish, French, German, Italian, Norwegian,
Portuguese, Spanish, and Swedish. The factory default is
• Backlight Duration: Set the time that the recorder screen backlight stays on
after a button is pressed. The choices are 15, 30 (factory default), 45, or 60
seconds.
5. To return one level up in the menu tree, press
Note
Once you have set preferences, you will not need to reset them
unless:
• you want to change something, or
• the recorder battery is fully discharged.
12-hour (AM/PM)
or
24-hour
.
(factory default applies to Reflux
ESC
English
.
.
Bravo pH Monitoring System 12 User Guide
Page 17

Calibrating Capsules
֠
֠
Calibration is the process of making sure that the capsule is reading pH levels properly.
You must go through calibration for each capsule.
Note
If you need to change the number of capsules or the study duration,
select Settings from the main menu and make the necessary
changes.
Existing Data
If data exists from a previous study and has not yet been uploaded, you must first do so
before you can start calibration. The record screen shows the message:
not uploaded! To upload, connect to PC.
•
To stop calibration and upload the existing data, press
Cancel
for your software application. (If you are using AccuView or Reflux software, see
Uploading pH Data on page 28.) When the data is uploaded, start the new study
again by selecting
Calibrate
from the main menu.
Last study data
. Follow the directions
•
To continue (overwrite the existing data without uploading it), press
press
Yes
to confirm that you want to overwrite the last study data. The message
Clearing data...
appears, and then the recorder continues with the calibration
Next
and then
process.
Starting Calibration
If the message about existing data does not appear, begin calibration as follows:
1. Select
The current settings (
screen. Press
This message appears:
calibrate.
2. Position the calibration stand on a level surface and place a clean calibration tube
into each of the four holders. Place the recorder on the calibration stand (Figure 7).
Calibrate
OK
Note
The backlight stays on during calibration, except during the 10-minute
pre-soak stage.
from the main menu.
Date/Time, # of Capsules
to confirm or
Place pH Capsule #1 in pH 7.01 and press Start to
Cancel
, and
Study Duration
to return to the main screen.
) appear on the
3. Check the expiration date on the buffer fluid bottles (next to on the label).
4. Fill each of the four tubes in the calibration stand halfway (enough to be able to
cover the capsule when it is inserted) as follows. The buffer solutions should be at
room temperature (20–25 °C, 68–77 °F).
Bravo pH Monitoring System 13 User Guide
Page 18

• tube 1: pH 7.01 buffer solution
!
֠
• tube 2: sterile water
• tube 3: pH 1.07 buffer solution
• tube 4: sterile water
5. Check the expiration date on the capsule (next to on the label).
6. Without bending or kinking the delivery device, carefully remove the Bravo pH
capsule with delivery device from the external shipping box and then from the inner
pouch (Figure 4).
Caution
Avoid bending or kinking the delivery device. Sharp bending or kinking
can damage the delivery device, which will require the device to be
discarded before use.
Figure 4. Remove Bravo pH capsule with delivery device from pouch.
7. Remove the capsule’s plastic cover, the reference sensor cover (soaker bulb cover),
and the magnetic clip (Figure 5). Set the magnetic clip aside.
Note
Keep the magnetic clip at least 2 meters (6 feet) away from the delivery
device so that it will not interfere with the capsule. If a procedure is
delayed, you can replace the magnetic clip on the capsule to return it
to an inactive state until needed. Do not discard the magnetic clip until
after the procedure has been performed.
Bravo pH Monitoring System 14 User Guide
Page 19

Figure 5. Remove Bravo pH capsule shipping components.
֠
֠
Open the plastic cover.
Remove magnetic clip.
Remove soaker bulb.
.
8. Check the soaker bulb for fluid and set aside.
Note
The capsule automatically turns on when the magnetic clip is removed.
In addition, if the recorder is turned on, you can see the capsule status
LED blinking on the recorder.
Note
Even if the soaker bulb doesn’t show the presence of liquid, the
capsule can calibrate as usual. Perform the capsule calibration as per
instructions. If the capsule fails to calibrate, contact your customer
support representative.
Bravo pH Monitoring System 15 User Guide
Page 20

9. After opening the capsule package, make sure that the capsule trocar needle has not
trocar
needle
suction
chamber
calibration tube
delivery device
handle
advanced (Figure 6).
Figure 6. Make sure that the trocar needle has not advanced too far into the
chamber.
10. Without bending or kinking the delivery device, place the delivery device handle
into the calibration stand (Figure 7).
Figure 7. Delivery device in calibration stand.
11. Carefully place the capsule into the pH 7.01 buffer solution calibration tube as
indicated on the recorder screen.
Bravo pH Monitoring System 16 User Guide
Page 21

!
Caution
!
֠
When placing the capsule into the calibration tube, do not allow either
part of the pH sensor (the short silver-colored antimony and long clear
reference sensor) to catch on the edge of the calibration tube, as this
can damage the pH sensor. Avoid the tube edge while carefully
lowering the capsule into the solution. Any damage to the pH sensor
will require the delivery device to be discarded before use.
12. Make sure that the capsule is completely covered with the buffer solution. Gently
agitate the capsule to remove any air bubbles.
Caution
After agitating to remove air bubbles, do not move the capsule again or
move the calibration stand during the calibration process.
13. On the recorder, press
This message appears:
14. When the recorder identifies the capsule, this message appears:
capsule ID number match the delivery device number?
• The capsule ID is printed on the packaging label. If the ID matches, press
not, press No and the search begins again.
• If the recorder still does not recognize the capsule or displays an error message,
repeat the procedure. If the problem persists after three attempts, see Recorder
Troubleshooting on page 32.
15. Press
Yes
. This message appears:
Note
If you press Skip, the recorder goes directly to the next step, and the
action (skipping pre-soak) is written to the recorder log.
If you did not skip (that is, if you pressed Yes), the backlight goes out.
It automatically turns back on at the end of the 10 minute pre-soak
period.
When the pre-soak period ends (or is canceled by pressing
appears:
pH 7.01 calibrating.
Start
. The recorder starts searching for the capsule’s signal.
Waiting for pH capsule 1.
Initiating 10 minute pre-soaking.
Skip
Does this pH
Yes
), this message
. If
At the end of this calibration period, the recorder beeps and this message appears:
Rinse the pH capsule in sterile water and press Next.
16. Rinse the capsule in sterile water. Press
#1 in pH 1.07 and press Start to calibrate.
17. Place the capsule in the pH 1.07 solution and press
Bravo pH Monitoring System 17 User Guide
Next
. This message appears:
Start
.
Place capsule
Page 22

At the end of this calibration period, the recorder beeps and this message appears:
!
֠
֠
pH capsule ID xxxx calibrated.
Caution
If either part of the calibration process (pH 7.01 or 1.07) fails, see
Recorder Troubleshooting on page 32.
18. Press OK. The calibration data is saved and the recorder main menu appears with the
cursor ready at
19. Proceed to Performing a Bravo pH Study on page 19.
Start study
Note
If you are performing a two-capsule procedure, the recorder guides you
through the same calibration process for the second capsule.
After the first capsule is calibrated, it can remain outside the buffer
solutions for up to 60 minutes before use.
.
(where
xxxx
is the capsule ID).
Note
If you are not going to place the capsule in the patient immediately,
return the capsule to the pH 7.01 buffer solution. It may remain there
for up to 8 hours. Rinse the capsule in sterile water before use.
Bravo pH Monitoring System 18 User Guide
Page 23

Performing a Bravo pH Study
֠
Place finger over
suction chamber.
Remove finger.Connect vacuum
hose.
a b c
vacuum
hose
vacuum port
The actions of performing the study (testing the vacuum, placing the capsule, and
instructing the patient) should be performed in one continuous sequence as follows.
Setting up the Vacuum
1. Make sure that the vacuum flow knob is turned to maximum.
2. Connect the vacuum hose (supplied with the vacuum pump) to the vacuum port on
the delivery device handle (Figure 8a).
Figure 8. Verify the vacuum function.
3. With your gloved finger covering the suction chamber (Figure 8b), verify that the
vacuum gauge reading is at least 550 mmHg. Make a note of the gauge reading.
Note
At higher altitudes, the pressure
readings may be lower. For
different altitudes, use this table
for the minimum recommended
vacuum pressure. Contact
customer support for additional
information.
Altitude
feet meters
0 0 550
2000 610 530
4000 1220 510
6000 1830 490
Minimum
Pressure
mmHg
4. Remove your finger from the suction chamber. Verify that the vacuum gauge
reading drops (Figure 8c) by at least 50 mmHg (500 mmHg or lower).
Bravo pH Monitoring System 19 User Guide
Page 24

!
Caution
֠
If the vacuum readings fail these required
minimum levels, remove the delivery device
and perform the same action on the vacuum
tube alone:
• Cover the tube with your finger. The
vacuum gauge should read at least
575 mmHg. Remove your finger. The
vacuum gauge should drop drastically (to
zero or close to zero). If so, the vacuum
unit is functioning properly. Perform the
procedure with a different delivery device
(after performing calibration).
• If the vacuum fails to reach 575 mmHg or
fails to drop to close to zero, the vacuum
unit may require service. Contact customer support.
5. Turn off the vacuum and detach the tubing from the delivery device.
6. Proceed to Starting Recording.
Starting Recording
1. If the recorder is turned off or in sleep mode, turn it on.
2. From the main menu, select
If the recorder detects a conflict with the capsule ID, this message appears:
ID mismatch. Use pH capsule ID: xxxxx.
page 32.
3. Verify that the recorder is recording pH values.
Note
The recorder screen shuts off after 60 seconds to conserve battery
power. To turn on the recorder screen during a study, press any button.
4. Proceed to Placing the Capsule on page 21.
Start Study
and press
Enter
See Recorder Troubleshooting on
.
Capsule
Bravo pH Monitoring System 20 User Guide
Page 25

Placing the Capsule
!
pH sensor
pH capsule
suction chamber
depth markings
vacuum
hose
1. Oral placement of the Bravo capsule can be performed either using:
a. endoscopy: using an endoscope, determine the desired location for the capsule
in the esophagus. Typically, the capsule is placed 6 cm (2.4 inches) above the
squamo-columnar junction. Measure and record the distance traveled by the
endoscope to the desired location.
b. manometry: using a transnasal manometry catheter, determine the desired
location for the capsule in the esophagus. Typically, the capsule is placed 5 cm
(2 inches) above the proximal aspect of the landmarks (LES). Use a correction
factor of approximately 4 cm to account for the longer pathway that the
manometry catheter has to travel through the nasopharynx.
2. Remove the endoscope or manometry catheter from the patient.
3. With the vacuum off, complete the following steps:
a. Remove the capsule from the buffer solution and rinse it in sterile water.
b. Mark the distance determined in step 1 on the delivery device. The depth
markings on the delivery device are indexed from the capsule’s pH sensor
(Figure 9).
c. Carefully advance the delivery device through the mouth (with the capsule
facing the patient’s tongue) to the desired location in the esophagus.
Caution
If lubricants are used to ease placement insertion, do not cover
the suction chamber with lubricant. This could interfere with the
attachment of the capsule.
Figure 9. Capsule depth markings are indexed from pH sensor.
Bravo pH Monitoring System 21 User Guide
Page 26

d. Holding the delivery device as straight as possible in a relaxed horizontal
!
!
!
position, stabilize it by the patient’s mouth to make sure that it does not move.
Warning
Do not advance the delivery device into the trachea or lungs.
Advancing the delivery device into the trachea or lungs can cause
possible injury to the patient.
4. Endoscopically check the esophageal inlet to verify the desired placement of the
delivery device in the esophagus. Carefully remove the delivery device immediately
if it has entered the trachea.
5. After proper positioning of the delivery device:
a. Attach the vacuum hose to the handle (Figure 8).
b. Turn on the vacuum source and verify that the gauge reading is the same as
you noted during vacuum setup.
Caution
If the minimum vacuum level (550 mmHg) is not obtained,
reposition the Bravo delivery device to achieve proper vacuum.
Do not allow the delivery device to move during vacuum level
acquisition. Failure to immobilize the delivery device can result in
less than optimal attachment or non-attachment that will require the
vacuum level acquisition process to be repeated.
6. After the vacuum level of at least 550 mmHg has been reached and the vacuum
stabilizes, allow 30 seconds for the tissue to fill the suction chamber.
Caution
Do not proceed without waiting a full 30 seconds. Failure to wait a
full 30 seconds may result in insufficient or no tissue filling the
suction chamber. This can result in the capsule not being securely
attached or not attached to the patient’s esophagus.
7. Remove the safety tab:
Bravo pH Monitoring System 22 User Guide
Page 27

8. Swiftly press the plunger on top of the handle all the way down until it stops at its
!
֠
Press the plunger down to the locking position and release it.
Rotate the plunger clockwise until the white line on the plunger lines up with the white upward
arrow.
locking position. This advances the trocar needle into the suction chamber
(Figure 10).
Warning
Press down on the plunger with a swift and smooth motion to actuate
the delivery device mechanism. Pressing down on the plunger too
slowly may result in the capsule not properly attaching to the patient’s
esophagus or not detaching from the delivery device.
Do not rotate the plunger while depressing it!
Note
• Your hand may feel the delivery mechanism actuate, and you may
hear a click when this occurs.
• Remove your thumb after the plunger locks and before you begin
rotating it 1/8 of a turn.
Figure 10. Attach the capsule to the patient’s esophagus.
9. Using your thumb, rotate the plunger from the side one-eighth (1/8) of a turn
clockwise to release the capsule from the delivery device (Figure 11). The plunger
springs up so that a white line is visible on the sixth rib of the plunger (Figure 12).
Figure 11. Release the capsule from the delivery device.
Bravo pH Monitoring System 23 User Guide
Page 28

If the plunger does not automatically spring up, push it up gently with your thumb.
!
֠
!
!
Do not cover the hole
at the bottom of the
handle with your
hand.
The plunger should spring back up
automatically. Verify that the
plunger springs back to the white
line marking the plunger’s sixth rib.
If the plunger does not automatically
spring up, use your thumb to lightly
push it up.
a
b
These steps release the capsule from the delivery device.
Figure 12. Release the capsule from the delivery device, cont.
Warning
Do not rotate or otherwise force the plunger beyond the white line on
the barrel. Rotating or forcing the plunger beyond this may result in
possible damage to the delivery device. This may also interfere with
the detachment of the capsule from the delivery device, and cause
possible injury to the patient.
.
Note
Following correct rotation of the plunger, the white marking should now
be visible on the sixth rib of the plunger. If not, use your thumb to raise
the plunger until the white marking is visible.
Warning
If problems occur with capsule detachment, see Delivery Device
Disassembly Procedure on page 30.
.
10. Turn off the vacuum source. Remove the delivery device and discard it according to
local waste management regulations.
Warning
Bravo pH Monitoring System 24 User Guide
Do not remove the delivery device from the patient with the vacuum
source on. Removing the device with the vacuum source on can result
in dislodgement of the capsule or possible injury to the patient’s
esophagus.
Page 29

11. At the clinician’s discretion, endoscopically confirm the capsule’s attachment.
!
֠
Caution
Avoid contacting the capsule with the endoscope. Contact between the
endoscope and capsule may result in the dislodgement of the capsule.
12. Confirm that the recorder is recording pH values and that the capsule status LED is
blinking in blue.
13. Proceed to Reviewing Instructions with Patients on page 26.
Note
The recorder's display turns off after 60 seconds to conserve battery
life. Press any key to activate the recorder's screen during a study.
Stopping a pH Study
The study completes automatically when the recorder no longer collects pH data and the
recorder screen turns off. The study data is stored in the recorder until it is cleared.
To stop a study manually (before its intended completion):
1. Press the on/off button for several seconds until this message is displayed:
sure you want to stop the pH study?
2. Select your action:
• If you select No, the study continues uninterrupted.
• If you select
upload, connect recorder to PC.
Remember: if you stop the study, you must upload the data to unlock the recorder
before you can continue.
Yes
, this message is displayed:
Last study data not uploaded! To
Are you
Bravo pH Monitoring System 25 User Guide
Page 30

Reviewing Instructions with Patients
֠
meal
supine
regurgitation
capsule LED
chest pain
heartburn
When this
button is
pressed...
This icon
appears
on the
screen
Review the following information with the patient.
1. Familiarize the patient with the recorder. Instruct the patient to press the appropriate
button at the first sensation of the symptom (chest pain, regurgitation, or heartburn).
Note
The default meaning of recorder symptom buttons can be changed in
the software application (AccuView or Reflux software). Refer to the
software user guide for details. If you are using the buttons for other
symptoms, make sure to explain the function of each symptom button
to the patient.
Figure 13. Default values of symptom buttons.
2. Explain that the patient must first press any button to turn on the backlight, and then
press the appropriate symptom or event button.
3. Show the patient that the indicator light on the symptom button (Figure 13)
illuminates for 3 seconds confirming that a symptom button was pressed.
4. Explain the beep sound (if the recorder was programmed to beep when a symptom
button is pressed).
5. Explain the use of the Patient Diary. Patients need to write down eating, lying down
(supine), and other user-defined periods, using the time on the recorder’s screen for
the start and end times.
User-defined periods allow the patient to record a period of time when they are
engaged in an activity that the physician determined may affect pH readings, such as
smoking, exercising, or wheezing.
6. Instruct the patient to make sure that the recorder is always monitoring the capsule:
Bravo pH Monitoring System 26 User Guide
Page 31

• The patient must stay within 2 meters (6 feet) of the recorder during the study
!
except, as necessary, for bathing. The recorder is not water resistant and should
not be worn in the shower or in other wet environments.
• When resting or lying down, place the recorder on a night stand near the bed, the
buttons turned toward the patient both for convenience of reaching it for
possible symptom recording as well as for optimal reception.
• If a night stand is not near the bed, the patient should clip the recorder to the
pillow, making sure the buttons face the patient.
• If the recorder is too far from the capsule, it beeps for up to 30 seconds and the
capsule number icon disappears from the screen (
for single-capsule
procedures, for double-capsule procedures). This indicates that the
transmission from the capsule to the recorder has been interrupted. The patient
should hold the recorder on the chest until
or
appears.
• This beep can also occur if there are other electronic or electrical devices
operating at the same frequencies as the recorder. Instruct the patient to move
away from these other devices; if the capsule number icon appears, the problem
was caused by interference from another device.
• Instruct the patient to contact the doctor if or
disappears from the
recorder screen or if there are any problems or questions during the study.
7. Place the recorder in the case and show the patient how to adjust the shoulder strap.
Warning
The recorder must be worn over clothing.
If wearing the recorder with the shoulder strap, the patient must stay
clear of moving equipment or machines that are potentially hazardous.
If the strap becomes entangled with a moving part, it could cause the
patient to be pulled into a dangerous position. This could result in
possible patient injury.
Do not allow children to wear or play with the recorder shoulder strap
either with or without the case. It is intended only for the prescribed use
by an adult. Use of the shoulder strap by a child could result in possible
injury. Instead, it is recommended to use the belt clip for children.
8. Instruct the patient about what to expect when the capsule detaches.
9. Instruct the patient to return the recorder at the completion of the study.
Bravo pH Monitoring System 27 User Guide
Page 32

Uploading pH Data
֠
Note
To perform multiple uploads from the same recorder, disconnect the
recorder from the USB and reconnect it again between uploads.
The Bravo recorder supports both AccuView and Reflux software.
1. Connect the Bravo recorder to your PC using the supplied USB cable.
2. Open AccuView or Reflux software by double-clicking the AccuView or Reflux
software icon on your desktop.
3. Click
bar appears. Once the
upload is complete, the
Edit Information & Diary
screen appears.
4. If more than one protocol
is available for the Bravo
pH capsule, select the
desired one from the
Protocol
5. Click OK.
Upload
list.
. A progress
Bravo pH Monitoring System 28 User Guide
Page 33

Recorder Maintenance
!
Safety and Technical Checks
There are no required safety or technical checks, and no periodic maintenance for the
recorder.
The recorder contains no serviceable components apart from the battery. If the recorder
requires repair or is nonfunctional, contact customer support.
Cleaning the Recorder
Clean the recorder after each study.
1. Turn off the recorder.
2. Wipe the exterior surface of the recorder with 70–90% isopropyl alcohol.
3. Allow any alcohol to dry thoroughly before using the recorder.
Caution
Do not allow liquid to get into the recorder body or inside the front
cover. The recorder is not fluid resistant. Allowing any fluid (alcohol,
water, etc.) inside of the recorder can damage the recorder and cause
it to malfunction.
Cleaning the Case and Strap
Wipe the case and strap with any commonly-used disinfectant.
Servicing the Battery
The recorder operates on one internal rechargeable lithium battery. When the battery has
been recharged 275 times, a message appears on the screen reminding you to replace the
battery.
Contact customer support to have the battery replaced.
Bravo pH Monitoring System 29 User Guide
Page 34

Troubleshooting
!
֠
!
Delivery Device Disassembly Procedure
If the capsule is attached to the patient’s esophageal tissue, but will not release from the
delivery device, we recommend this procedure.
Caution
When performing the following steps to remove the capsule, it is
extremely important to minimize movement of the capsule and the
patient. Any movement could cause tissue injury at the site of the
capsule attachment.
1. If available, insert an endoscope and confirm tissue has been pinned and the capsule
has not been released from the delivery device. Use care when inserting the
endoscope, and avoid force upon the capsule and the delivery device. Remove
endoscope. If an endoscope is not available, proceed to step 2.
2. See Figure 14. Secure delivery device near biteblock or near nasal passage using a
hemostat clamp. An assistant should continue to firmly hold the shaft of the delivery
device with a hemostat until the release procedure (described below) is completed.
Note
For the following steps, moderate force may be needed to
disassemble the Bravo delivery device handle.
.
Warning
During the disassembly of the handle, position the handle away from
the patient’s face while protecting the patient’s mouth and nose, and
away from the eyes of the patient and nearby staff. Plastic parts may
break off during handle disassembly, resulting in possible injury to the
patient and clinical staff.
Figure 14. Delivery device secured at mouth (or nose, if applicable) using a
hemostat with hand. Keep a firm grip on the hemostat throughout the
release procedure.
Bravo pH Monitoring System 30 User Guide
Page 35

3. With gloved hands, place one hand onto the gray handle portion and one hand on the
֠
֠
blue suction port part. Firm force may be needed to induce separation of the gray
and blue parts. When the two parts have separated, go to step 4.
Note
Be careful not to transfer force or motion to the capsule portion of the
delivery device.
.
Figure 15. Break handle as indicated.
4. See Figure 16. Withdraw the gray handle a minimum of 4 cm (about 1.5 in.). When
performing this action, the wire that secures the capsule is also withdrawn and will
automatically release the capsule.
Note
If it is difficult to withdraw the handle and you feel resistance, release
the hemostat to allow the gray handle to be retracted. Make sure not to
pull on the shaft to avoid transferring force to the capsule.
Figure 16. Withdraw the gray handle 4 cm minimum. THIS ACTION WILL
RELEASE THE CAPSULE. If the wires are difficult to withdraw, remove
the hemostat. This will release the capsule at distal end.
5. When the capsule releases from the delivery device, remove the delivery device
from the patient.
6. If possible, use an endoscope to confirm that the capsule remained attached to the
esophageal tissue.
Bravo pH Monitoring System 31 User Guide
Page 36

Recorder Troubleshooting
Following is a list of problems you may encounter while operating the recorder. If you
cannot resolve the problem with the solution provided, or you do not see the problem
listed, contact your product customer support.
Problem Cause Solution
Initial Startup
Recorder displays message:
ERROR
Recorder displays message:
REPLACE BATTERY
Recorder is locked (no buttons
work) and displays message:
Last study data not uploaded!
To upload, connect recorder to
PC.
Calibration
Recorder displays message:
Calibration done. To use,
press Cancel. To start
new, press Yes.
Recorder displays message:
Charge battery.
Recorder displays message:
Capsule 1 Calibration Error
Error 1: Unexpected pH
value
General recorder problem. Contact customer support to
arrange servicing for recorder.
Battery voltage low. Contact customer support to
arrange battery replacement
for recorder.
A study is done but the data
has not yet been uploaded
from the recorder.
Calibration has already been
performed but the calibration
data has not yet been used in a
study.
Recorder battery is below 30%
capacity.
(various) Press
Capsule sends what appears
to be a pH value, but it is
outside expected range (pH
7.01 to 1.07).
Connect the recorder to the PC
and follow the instructions on
the recorder screen.
Perform the study with this
calibration data or redo
calibration.
Connect recorder to charger.
Allow to fully recharge (may
take several hours).
Help
to display error
message.
Error 2: Slope too low Difference in mV between
Error 3: Slope too high Difference in mV between
Error 4: Signal unstable Recorder cannot detect stable
Bravo pH Monitoring System 32 User Guide
signals read by capsule during
calibration (that is, difference
between pH 7.01 and pH 1.07
solutions) is lower than
expected.
signals read by capsule during
calibration is greater than
expected.
signal from capsule within 60
seconds during calibration.
For each of these calibration
messages, try:
1. Replace buffer solution.
2. Try calibrating a new pH
capsule.
If error persists, contact
customer support.
Page 37

Problem Cause Solution
Recorder displays message:
Listening for caps... and does
not progress further.
Start Study
Recorder displays message:
Charge battery.
Recorder displays message:
Settings for two capsules but
only capsule xxxx found. Start
recording?
Recorder displays message:
Capsule ID mismatch.
Use capsule ID: xxxx.
Capsule out-of-range. Try each of these:
1. Move recorder closer to
capsule.
2. Replace buffer solution.
3. Try calibrating a new pH
capsule.
If error persists, contact
customer support.
Recorder battery is below 90%
capacity.
Recorder was configured for
two-capsule study, but is now
only detecting signal of one
capsule.
Capsule was not calibrated
using this recorder, or there
may be interference with
another capsule in the area.
Connect recorder to charger.
Allow it to fully recharge (may
take up to 7 hours).
If you continue recording, you
will only collect data for the
capsule identified in this error
message.
Otherwise, press
perform calibration with
another capsule, and continue.
Try each of these:
1. Select the recorder that
was calibrated to the
capsule being used
2. Move recorder closer to
capsule (place recorder on
patient’s chest).
3. Remove patient from the
area (100 meters) and try
again.
ESC
,
Recorder displays message:
Capsule ID mismatch.
Use capsule ID: 0000.
No pH display on recorder screen Capsule out-of-range. Move recorder closer to
Data Transfer
Recorder displays the
disconnect
Data upload fails. Communication error. Disconnect the recorder from
icon
do not
Bravo pH Monitoring System 33 User Guide
Calibration data is missing or
invalid.
Capsule not in contact with
fluid.
Recorder is connected to PC
software application and data
is being transferred.
Recalibrate the capsule. Make
sure that you get the message
pH capsule #1 calibrated.
capsule.
Place capsule in buffer
solution.
This is normal. Do nothing (do
not disconnect recorder from
USB cable until this icon is no
longer displayed, indicating
that data transfer has finished).
the USB cable and then
reconnect it.
Page 38

Problem Cause Solution
Patient Interface
Long beep and patient display
flashes battery symbol
Long beep (up to 30 seconds)
and the capsule indicator or
disappears from the
display
Indicator light does not blink
when a symptom button is
pressed
Indicator light blinks but recorder
does not beep when a symptom
button is pressed
Patient display does not show
time
Patient display shows time, but
not pH reading
Patient display shows Hi (H) pH level momentarily out-of-
Battery low. Recharge.
Signal from capsule lost. Move recorder closer to
Interference from 433 MHz
electromagnetic sources.
Indicator light or button not
functioning.
Recorder was programmed not
to beep when symptom button
is pressed.
Battery is depleted. Contact customer support to
Recorder was programmed not
to display pH reading.
range (too high).
capsule until capsule indicator
reappears.
Move recorder away from all
possible sources of radio
waves.
Manually record symptoms.
Contact customer support to
arrange servicing for recorder.
Program recorder to beep
when symptom button is
pressed. See Choosing Study
Settings on page 11.
arrange battery service.
Reprogram recorder to display
pH reading. See Choosing
Study Settings on page 11.
Continue with study (no action
required).
Patient display shows Lo (L) pH level momentarily out-of-
range (too low).
Continue with study (no action
required).
Bravo pH Monitoring System 34 User Guide
Page 39

Appendix A: Technical Data
Bravo pH Recorder
Power Supply: One lithium polymer battery (3.7 volt)
Size: Height: 90 mm (3.5 in.)
Weight: 150 g (5.3 oz)
Width: 100 mm (3.9 in.)
Depth: 30 mm (1.2 in.)
Operating, Transporting, and Storage
Temperature:
Operating and Storage Pressure: 520–790 mmHg
Operating, Transporting, and Storage
Humidity:
Number of Channels: 1 or 2 capsules
Symptom Buttons: Chest pain
Memory: Code: 128 Kilobytes, 16 K Sram
Recording Time (study duration): 24, 48, or 96 hours (selectable)
Capsule Sampling Interval: 6 seconds, transmits every 12 seconds
Measuring Range: pH level 1.0–8.0
Transmission of Data: USB
Communication Frequency: 433.9 MHz
Bandwidth of the Receiving Section: Maximum 600 KHz
0–40 °C (32–104 °F)
Up to 85% relative humidity (noncondensing)
Regurgitation
Heartburn
Data: 8 Mbytes
Protection from Electric Shock: Internally powered BF equipment
Mode of Operation: Continuous
Water Ingress Protection: Ordinary
Capsule Transmission Duty Cycle: Every 12 seconds
Recorder Servicing
The recorder does not require routine servicing of internal components, unless the
recorder becomes damaged. For service, contact the appropriate customer support
representative.
Bravo pH Monitoring System 35 User Guide
Page 40

USB Cable
Length: 1
meter
Charger
Input: 120 to 240 V (0.2 Amp, 50/60 Hz)
Output: 5 V (1 Amp max, 5 W max)
Battery
Weight
Rated capacity
Nominal voltage 3.7 V
Max. operating voltage range 2.75 V to 4.2 V
Expected cycle life 500 cycles
Temperature range charge 0°C to 45°C
Humidity 65 +
+26 g
1150 mAh min, 1200 mAh typical
discharge -20°C to 60°C
20% RH
FCC Compliance Statement
This device complies with Part 15 of the FCC Rules. Operation is subject to the following
wo conditions:
t
• This device may not cause harmful interference, and
• This device must accept any interference received, including interference that may
ause undesired operation.
c
Declaration of Conformity
Given Imaging declares that this product is in conformity with the essential requirements
of Directive 1999/5/EC on Radio and Telecommunications Terminal Equipment and
Directive 93/42/EEC as amended by 2007/47/EC on Medical Devices.
For additional information, contact Given Imaging.
Essential Performance of Bravo Recorder
Essential performance: < 0.5% missing frames (two-capsule procedure at 1 meter radius in
a RF shielded room up to 96h recording).
Br
avo pH Monitoring System 36 User Guide
Page 41

Electromagnetic Compatibility Declaration (EN / IEC 60601-1-2)
!
This equipment has been tested and found to comply with EN / IEC 60601-1-2.
Compliance with EN / IEC 60601-1-2 shows the equipment is reasonably protected
against harmful interference in a typical medical installation. Tables 1, 2, and 3 apply to
Given Imaging in-line powered and battery-powered external devices.
Caution
Bravo recorder needs special precautions regarding EMC and needs to be
installed and put into service according to the EMC information provided in
the accompanying documents.
Portable and mobile RF communications equipment can affect Bravo
recorder.
The use of accessories, transducers and cables other than those specified,
with the exception of transducers and cables sold by the manufacturer of the
Bravo recorder as replacement parts for internal components, may result in
increased emissions or decreased immunity of the Bravo recorder.
The Bravo recorder should not be used adjacent to or stacked with other
equipment. If adjacent or stacked use is necessary, the Bravo recorder
should be observed to verify normal operation in the configuration in which it
will be used.
Use of accessories, transducers, and cables with the Bravo recorder other
than those specified may result in increased emissions or decreased
immunity of the Bravo recorder.
The Bravo recorder may be interfered with by other equipment, even if that
other equipment complies with CISPR emission requirements.
Table 1. Electromagnetic emissions
Bravo recorder is intended for use in the electromagnetic environment specified below. The customer or
the user of Bravo recorder should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment – guidance
Non-ionizing
electromagnetic
emissions
CISPR 11
Non-ionizing
electromagnetic
emissions
CISPR 11
Harmonic
emissions
EN 61000-3-2
Voltage
fluctuations/flicker
emissions EN
61000-3-3
Group 1 (Bravo
pH recorder)
Class B (Bravo pH
recorder)
Not Applicable
(Battery-powered
devices)
Not Applicable
(Battery-powered
devices)
The Bravo recorder uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
The Bravo recorder is suitable for use in all establishments, including
domestic establishments and those directly connected to the public lowvoltage power supply network that supplies buildings used for domestic
purposes.
Bravo pH Monitoring System 37 User Guide
Page 42

Table 2. Electromagnetic immunity
Bravo recorder is intended for use in the electromagnetic environment specified below. The customer or the user of
Bravo recorder should assure that it is used in such an environment.
Immunity test EN 60601 test
Electrostatic discharge
(ESD): EN 61000-4-2]
±6 kV contact
±8 kV air
±2 kV for power
Electrical fast transient/
burst: EN 61000-4-4
supply lines
±1 kV for input/
output lines
±1 kV differential
Surge: EN 61000-4-5
mode
±2 kV common
mode
<5% U
(>95% dip in UT)
for 0.5 cycle
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines: EN 61000-4-11
40% U
(60% dip in UT)
for 5 cycles
70% U
(30% dip in UT)
for 25 cycles
<5% U
(>95% dip in UT)
for 5 sec
Power frequency (50/
60 Hz) magnetic field:
EN 61000-4-8
[All devices]
level
level
±6 kV contact
±8 kV air
N/A
N/A
Compliance
T
T
T
T
N/A
N/A
N/A
N/A
3 A/m 3 A/m
Electromagnetic environment – guidance
Floors should be wood, concrete, or ceramic
tile. If floors are covered with synthetic material,
the relative humidity should be at least 30%.
Not Applicable. Battery-powered device with
signal line not longer than 1 meter.
Not Applicable. Battery-powered device with
signal line not longer than 1 meter.
Not Applicable. Battery-powered device.
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
Bravo pH Monitoring System 38 User Guide
Page 43

Table 2. Electromagnetic immunity (continued)
Bravo recorder is intended for use in the electromagnetic environment specified below. The customer or the user of
Bravo recorder should assure that it is used in such an environment.
Immunity test EN 60601 test
level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80
MHz
3 V/m 80 MHz to
2.5 GHz
Compliance
level
N/A (Battery-
powered device
with signal line
not longer than 1
meter.)
10 Vrms
150kHz to
80 MHz in
ISM bands
[E1] = 3 V/m
Electromagnetic environment – guidance
Recommended separation distance
Portable and mobile communication equipment
should be used no closer to any part of the
Bravo System, including cables, than the
recommended separation distance (d)
calculated from the equation applicable to the
frequency of the transmitter.
d = 1.2 P
d = 1.2 P
412.224 - 455.616 MHz range is exclusion
band for the Bravo recorder in Rx mode.
The Bravo recorder in Rx mode has no
immunity against electromagnetic energy in this
band in order to perform its intended function.
The nearby electronic equipment may affect
the system.
Recommended separation distance:
d = 1.2 P, 80–800 MHz range
d = 2.3 P, 800–2500 MHz range
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer
and d is the recommended separation distance in meters (m).
Field strengths from fixed transmitters, as determined by an electromagnetic site survey,a should be less than the
compliance level in each frequency range.
Interference may occur in the vicinity of equipment marked with
the following symbol:
NOTE 1: At 80 and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the device is used exceeds the applicable
compliance level above, the device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating the Bravo System.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 1 V/m.
b
Bravo pH Monitoring System 39 User Guide
Page 44

Table 3. Recommended separation distances between portable and mobile electromagnetic communication
equipment and Bravo recorder
Bravo recorder is intended for use in an electromagnetic environment in which radiated
electromagnetic disturbances are controlled. The customer or the user of the Bravo recorder can help
prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile communication equipment (transmitters) and the Bravo recorder as recommended below,
according to the maximum output power of the communication equipment.
Rated maximum output
power of Transmitter W
80 to 412.224
MHz
412.224 to
455.616 MHz
455.616 to 800
MHz
800 MHz to 2.5 GHz
d = 1.2 P d = 1.2 P d = 1.2 P d = 2.3 P
0,01
0,1
1
10
100
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters
(m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output
power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
NOTE 3: In the band 412.224 to 455.616 MHz, Bravo recorder in Rx mode has no immunity against electromagnetic
energy in order to perform its intended function. Nearby electronic equipment may affect the system. Immunity within
this band is provided in upload mode of operation only.
0.12 0.12 0.12 0.23
0.38 0.38 0.38 0.74
1.2 1.2 1.2 2.3
3.8 3.8 3.8 7.4
12 12 12 23
Table 4. Transmitter requirements
Description Specification
Operating frequency
Transmission type
Effective radiated power
433.9 MHz
Amplitude shift key (ASK)
29.7 µW
Bravo pH Monitoring System 40 User Guide
Page 45

Bravo pH Capsule Specifications
Materials
Dimensions
Output &
Transmission
Storage & Operation
Cap Makrolon®
Shell Makrolon®
Reference sensor Polyethylenterephthalat
Filler material 2 component epoxy
Length 28 mm
Width 6.5 mm
Height 6.0 mm
EIRP 17.6 µW (-47.53 dBm) at a 3-meter distance
Format Amplitude-shift keying
Frequency 433.92 MHz
Rate 60 ms every 12 seconds
Storage temperature 15–45°C (59–113 °F)
Operation temp. 20–45°C (68–113 °F)
Storage humidity Up to 85%
Operation humidity N/A
Storage & operation
pressure
508–635 mmHg
Bravo pH Delivery Device Specifications
Materials
Proboscis & Boot Aliphatic, polyether-based TPU
Nest Acrylonitrile Butadiene Styrene
Foam Polyethelyne (PE)
Tube Polyamide (PA)
Cable gauge 2.4 mm (7 Fr)
Bravo pH Monitoring System 41 User Guide
Page 46

Appendix B: Symbols on Package Labeling
Refer to the device to see which symbols apply to this product.
Conformité Européenne
(European Conformity).
Fragile Temperature limits
Caution CSA mark
Consult the instructions for use Expiration date
Lot number Do not re-use
Serial number FCC
Manufacturer address IEC 60601-1/EN60601-1, Type
Keep dry
BF Equipment
Direct current Product number
Do not dispose of this product
in the unsorted municipal
waste stream. Dispose of this
product according to local
regulations.
Bravo pH capsules are MR
unsafe
Non-ionizing radiation (from
wireless transmission)
Bravo pH Monitoring System 42 User Guide
 Loading...
Loading...