
PROFESSIONAL MEDICAL PRODUCTS
ASPIRATORE CHIRURGICO VEGA
VEGA SUCTION ASPIRATOR
ASPIRATEUR VEGA
CHIRURGISCHER ABSAUGER VEGA
ASPIRADOR QUIRÚRGICO VEGA
APARELHO DE SUCḈÃO CIRÚRGICO VEGA
M28210-M-Rev.7-12.20
28210
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
Made in Italy
0476

13
VEGA SURGICAL ASPIRATOR is a medical device powered by 230V ~ 50Hz electricity, to be used for nasal,
oral, tracheal aspiration of body uids, such as mucus, phlegm and blood, in adults or children. Luminaire
designed to offer ease of transport and continuous use.
Thanks to these features and its performance, this product is particularly suitable for use in hospital wards, for
minor surgery applications and post-operative treatments at home. Built with a plastic body with high thermal
and electrical insulation in compliance with European safety regulations, the appliance is supplied with a com-
plete suction tank in sterilizable polycarbonate, with overow valve, and is equipped with a suction regulator
and vacuum gauge placed on the front panel.
ENGLISH
GENERAL WARNING
Read instruction manual carefully before use.
The device is for use by qualied personnel (surgeon / professional nurse / assistant)
The use of the device at home is restricted to an adult in full possession of mental faculties and / or home
carers
The instrument must not disassembled. For technical service always contact Gima S.p.A.
IMPORTANT SAFETY RULES
1. Check the condition of the unit before each use. The surface of the unit should carefully be inspected for
visual damage. Check the mains cable and do not connect to power if damage is apparent;
2. Before connecting the appliance always check that the electric data indicated on the data label and the type
of plug used, correspond to those of the mains electricity to which it’s to be connected;
3. Respect the safety regulations indicated for electrical appliances and particularly:
- Use original components and accessories provided by the manufacturer to guarantee the highest efciency and safety of the device;
- The device can be used only with the bacteriological lter;
- Never immerge the appliance into water;
- Do not place or store the aspirator in places where it may fall or be pulled into the bathtub or washbasin.
In the event it is accidentally dropped, do not attempt to remove the device from the water whilst the plug
is still connected: disconnect the mains switch, remove the plug from the power supply and contact the
GIMA technical service department. Do not attempt to make the device work before it has been thoroughly checked by qualied personnel and/or the GIMA technical service department.
- Position the device on stable and at surfaces in a way that the air inlets on the back aren’t obstructed;
- To avoid incidents, do not place the aspirator on unstable surfaces, which may cause it to accidentally fall
and lead to a malfunction and/or breakage. Should there be signs of damage to the plastic parts, which
may expose inner parts of the energised device, do not connect the plug to the electrical socket. Do not
attempt to make the device work before it has been thoroughly checked by qualied personnel and/or the
GIMA technical service department.
- Don’t use in the presence of inammable substances such as anaesthetic, oxygen or nitrous oxide;
- Don’t touch the device with wet hands and always prevent the appliance coming into contact with liquids;
- Don’t leave the appliance connected to the power supply socket when not in use;
- Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly;
- Store and use the device in places protected against the weather and far from any sources of heat. After
each use, it is recommended to store the device in its own box away from dust and sunlight.
- In general, it is inadvisable to use single or multiple adapters and/or extensions. Should their use be
necessary, you must use ones that are in compliance with safety regulations, however, taking care not to
exceed the maximum power supply tolerated, which is indicated on the adapters and extensions.
- Prevent children from using the device without proper supervision;
4. For repairs, exclusively contact technical service and request the use of original spare parts. Failure to
comply with the above can jeopardise the safety of the device;
5. Use only for the purpose intended. Don’t use for anything other than the use dened by the manufacturer.
The manufacturer will not be responsible for damage due to improper use or connection to an electrical
system not complying with current regulation.
6. The medical device requires special precautions regarding electromagnetic compatibility and must be
installed and used in accordance with the information provided with the accompanying documents: the
VEGA SUCTION UNIT device must be installed and used away from mobile and portable RF communica-

ENGLISH
14
tion devices (mobile phones, transceivers, etc.) that may interference with the said device.
7. Instrument and accessory discharging must be done according to current regulations in the country of use.
8. WARNING: Do not change this equipment without the permission of the manufacturer GIMA S.p.A. None
of electric or mechanical parts has been designed to be repaired by customers or end-users. Don’t open
the device, do not mishandle the electric / mechanical parts. Always contact technical assistance
9. Using the device in environmental conditions different than those indicated in this manual may harm seriously the safety and the technical characteristics of the same.
10. The medical device is in contact with the patient by means of a disposable probe (supplied with the device)
furnished with the relative CE compliance certication according to the requirements of regulation ISO
10993-1: thus, no allergic reactions and skin irritations may occur.
11. The product and its parts are biocompatible in accordance with the requirements of regulation EN 60601-1.
12. Operation of the device is very simple and therefore no further explanations are required other than those
indicated in the following user manual.
13. Use in Home-Care: Keep all accessories of the device out of reach of children under 36 months of age
since they contain small parts that may be swallowed.
14. Do not leave the device unattended in places accessible to children and/or persons not in full possession
of mental facultiesas they may strangle themselves with the patient’s tube and/or the power cable.
The manufacturer cannot be held liable for accidental or indirect damages should the device
be modied, repaired without authorization or should any of its component be damaged due to accident or misuse. Any minimal modication / repair on the device voids the warranty and does not
guarantee the compliance with the technical requirements provided by the MDD 93/42/EEC (and subsequent changes) and its normatives.
CONTRAINDICATIONS
- Before using the VEGA, consult the instructions for use: failure to read all the instructions in this manual
can be harmful for the patient.
- The device cannot be used to drain chest uids;
- The device must not be used for suction of explosive, corrosive or easily ammable liquids.
- VEGA is not suitable for MRI. Do not introduce the device in MRI environments.
TECHNICAL CHARACTERISTICS
Model VEGA SUCTION ASPIRATOR
Typology (MDD 93/42/EEC) Medical device Class IIa
Classication UNI EN ISO 10079-1 High Vacuum / Low Flow
Main Voltage 230 V ~ / 50 Hz
Power consuption 184 VA
Fuse F 1 x 1.6A L 250 V
Maximum suction aspiration (without jar) -75kPa (- 0.75bar)
Maximum ow (without jar) 16 l/min
Weight 2.5 Kg
Dimension 350 x 210 x 180 mm
Functioning NON-STOP OPERATED
Accuracy of Vacuum Indicator ± 5%
Regolable from -75kPa (-0.75 bar) to -10kPa (-0.10 bar)

15
ENGLISH
Working Condition Room temperature: 5 ÷ 35°C
Conservation condition and Transport Room temperature: -25 ÷ 70°C
Room humidity percentage: 10 ÷ 93% RH
Atmospheric pressure: 700 ÷ 1060 hPa
Room humidity percentage: 0 ÷ 93% RH
Atmospheric pressure: 500 ÷ 1060 hPa
The technical specications may change without notice.
CLEANING OF THE DEVICE
Use a soft dry cloth with not – abrasive and not – solvent detergents. To clean the device external parts always
use a cotton cloth dampened with detergent. Don’t use abrasive or solvent detergents. Before carrying out
any cleaning and / or maintenance operation, disconnect the appliance from the power supply, unplugging it
or turning off the switch on the device
PARTICULAR CARE SHOULD BE TAKEN TO ENSURE THAT THE INTERNAL PARTS OF THE
EQUIPMENT DO NOT GET IN TOUCH WITH LIQUIDS. NEVER CLEAN THE EQUIPMENT WITH WATER.
During all clearing operations use protection gloves and apron (if need be, also wear a face mask and glasses) to avoid getting in contact with contaminating substances (after each utilization cycle of the machine).
ACCESSORIES SUPPLIES
• Complete aspiration jar 1000ml
• Conical tting
• Tubes set 6mm x 10mm (trasparent silicon)
• Aspiration probe CH20
• Antibacterial and hydrophobic lter
Available under request with different versions with complete jar 2000ml.
Anti-bacterial and hydrophobic lter: designed for the individual patient to protect patient and machine from
cross-infections. Prevents the liquids, that come into contact with it, from passing through it. Replace it whenever you suspect that it may be contaminated and/or it becomes wet or discoloured. Replace the lter every
time it is used if the suction pump is used on patients in unknown pathological situations and where an assess-
ment of indirect contamination is not possible. The lter is not manufactured to be decontaminated, dismantled
and/or sterilised. If, however, the patient’s pathology is known and/or there is no risk of indirect contamination,
the lter should be replaced after every work shift or once a month even if the device is not used.
Suction catheter: Single-use device to be used on a single patient. Do not wash or re-sterilize after use.
Reuse may cause cross-infections. Don’t use after lapse of the sell-by date
WARNING: Suction tubes for insertion in the human body purchased separately from the machine
should comply with ISO 10993-1 standards on material biocompatibility.
Aspiration jar: The mechanical resistance of the component is guaranteed up to 30 cycles of cleaning and
sterilization. Beyond this limit, the physical-chemical characteristics of the plastic material may show signs of
decay. Therefore, we recommend that you to change it.
Silicone tubes: the number of cycles of sterilization and/or cleaning is strictly linked to the employment of the
said tube. Therefore, after each cleaning cycle, it is up to the nal user to verify whether the tube is suitable
for reuse. The component must be replaced if there are visible signs of decay of the material constituting the
said component.

ENGLISH
Conical tting: the number of cycles of sterilization and the number of cleaning cycles is strictly linked to
the employment of the said component. Therefore, after each cleaning cycle, it is up to the nal user to verify
whether the tting is suitable for reuse. The component must be replaced if there are visible signs of decay in
the material constituting the said component.
Service life of the device: More than 850 hours of operation (or 3 years) in accordance with the standard
conditions of testing and operation. Shelf life: maximum 5 years from the date of manufacture.
16
CLEANING OF ACCESSORIES
Before using the device, the manufacturer advises you to clean and/or sterilize the accessories. Washing and
/ or cleaning the autoclavable jar is to be carried out as follows:
• Wear protection gloves and apron (glasses and face mask if necessary) to avoid contact with contaminat-
ing substances.
• Disconnect the tank from the device and remove the said container from the support of the device.
• Separate all the parts of the cover (overow device, washer).
• Disconnect all tubes from the jar and the protection lter.
• Empty and dispose of the contents of the suction vessel (also comply with regional regulations);
• Wash each part of the container from secretions under cold running water and then clean every single part
in hot water (temperature not exceeding 60°C).
Once again, carefully wash each single part using, if necessary, a non-abrasive brush to remove any de-
posits.
• Rinse with hot running water and dry all parts with a soft cloth (non-abrasive).
• Dispose of the aspiration catheter according to the provisions of local laws and regulations.
The jug and lid can be further disinfected using a common disinfectant, strictly following the instructions and
dilution values provided by the manufacturer. At the end of cleaning operations, leave to air dry in a clean
environment.
The silicone aspiration tubes and the conical tting may be carefully washed in hot water (temperature must
not exceed 60°C). After cleaning, leave the parts to dry in an open, clean environment.
When cleaning is complete, reassemble the container for liquid aspirations according to the following proce-
dure:
• Place the overow valve into its seat in the cover (under VACUUM connector)
• Insert oating valve keeping the o-ring towards the opening of the cage
• Place the o-ring into its seat around the cover
• After completing assembling operations always make sure that cover seals perfectly to avoid vacuum
leakages or liquid exit
The jar and the cover can be autoclaved by placing the parts into the autoclave and running one sterilization
stem cycle at 121°C (1 bar relative pressure – 15 min) making sure that the jar is positioned upsidedown.
Mechanical resistance of the jar is guaranteed up to 30 cycles of sterilization and cleaning at the indicated
conditions (EN ISO 10079-1). Beyond this limit the physical-mechanical characteristics of the plastic may
decrease and replacement of the part is therefore recommended.
After sterilization and cooling at environment temperature of the parts make sure that these are not damaged.
The aspiration tubes can be sterilized on autoclave using a sterilization cycle at 121°C (1 bar relative pressure – 15 min).
The conical connector can be sterilized on autoclave using a sterilization cycle at 121°C (1 bar relative pressure – 15 min).
DO NOT WASH, STERILIZE OR PUT IN AUTOCLAVE THE ANTIBACTERIAL FILTER

17
ENGLISH
PERIODICAL MAINTENANCE CHECKS
The VEGA SUCTION ASPIRATOR does not need maintenance or lubrication.
It is, however, necessary to inspect the unit before each use. With regard to training, given the information
contained in the user manual and since it is easy to understand the said device, it doesn’t appear to be nec-
essary.
Unpack the instrument and always check integrity of plastic parts and feeding cable, they might have been
damaged during previous use. Connect the cable to electrical network and turn the switch on.
Close the aspirator outlet with your nger and with suction regulator at maximum check that the vacuum
indicators reaches at least -75kPa (-0.75 bar). Rotate the knob from right to left. The vacuum indicator should
go down -25kPa (-0.25 bar).
Check that no loud noises are present. A protection fuses (F 1 x 1.6 A L 250V) reachable from exterior and
situated in the plug protects the instrument. For fuses replacing, always the type and the range.
Before changing the fuse, disconnect the plug from the power supply socket.
Fault type Cause Solution
1. The suction unit doesn’t
work
2. No aspiration Jar Cap not screwed on correctly Unscrew the cap, and re-screw it
3. No aspiration Lid seal not in its seat Unscrew the cap and insert the seal properly
4. The Vacuum power on the
patient side is either very low
or absent
5. The oat doesn’t close If the cap has been washed, ensure
6. The oat doesn’t close The oat it’s covered by dirty material Unscrewed the cap, leave the and put in on
7. Low suction Foam inside the jar Fill the jar to 1/3 full of ordinary water
8. No aspiration due to ow
leakage of mucus
Faults 1 - 2 - 3 - 4 - 5 - 6
- 7 - 8
Cable is damaged External power
source failure
• Vacuum regulator set
to minimum
• Protection lter blocked or damaged
• Connection tubes blocked, kinked
or disconnected
• Shut-off valve blocked or
damaged
• Pump motor damaged
that the oat is not partially detached
Filter blocked Replace lter
None of the procedures have
achieved the desired results
Replace the cable Check the external power
source
in its seat
• Turn the vacuum regulator clockwise and
check the value of the vacuum on the gauge
• Replace the lter
• Replace or reconnect the tubes, check the jar
connections
• Empty the jar, or disconnect the tube from the
jar and unblock the shut-off valve. The unit
twill only work in the upright position
• Refer to authorised service personnel
Fit the oat into it’s place
autoclave
Contact GIMA customer service
If the overll security system it’s activated, don’t proceede with the liquid aspiration.
If the overll security system doesn’t work there are two cases:
1° case – If the overll security system doesn’t work the aspiration will be stopped by the bacteriological lter
who avoid the liquid penetration inside the device.
2° case – If both the security system doesn’t work, there is the possibility that liquid comes inside the device,
in this case return the device to GIMA technical service.
Gima S.p.A. will provide upon request electric diagrams, components list, descriptions, setting instructions and any other information that can help the technical assistance staff for product repair.
BEFORE EVERY CHECKING OPERATION, IN CASE OF ANOMALIES OR BAD FUNCTIONING,
PLEASE CONTACT GIMA TECHNICAL SERVICE. GIMA DOES NOT GIVE GUARANTEE IF INSTRUMENT, AFTER THE TECHNICAL SERVICE CHECKING, APPEARS TO BE TAMPERED.

ENGLISH
18
INSTRUCTION FOR USE
• The device must be checked before each use in order to detect malfunctions and / or damage caused by
transport and / or storage.
• The working position must be such as to allow one to reach the control panel and to have a good view of
the empty indicator, the jar and the antibacterial lter.
• It is recommended not to keep the device in your hands and / or to avoid prolonged contact with the body
of apparatus.
WARNING: For proper use, place the aspirator on a at, stable surface in order to have the full volume
of use of the jar and better efciency of the overow device.
• Connect one end of the short silicon tube, with antibacterial lter, to the suction connector on the lid of the
jar.
• The other tube, connected to the lter on one end, must be attached to the connector on the ask cover to
which the oat is secured inside. (overow device). The overow device starts working (the oat closes the
connector on the cover) when the maximum volume of liquid is reached, so no liquid can enter the machine
(90% of the ask’s total volume), thus ensuring that the liquid cannot penetrate inside the machine. The
device must be used on a at work top.
Filter assembling
Make sure the lter is assembled with the arrows on the side of the patient.
WARNING: The inside of the medical device must be regularly checked for the presence of liquids
or other visible contamination (secretions). In the presence of liquids or other visible contamination,
immediately replace the medical device due to the risk of an insufcient vacuum ow rate.
These products have been designed, tested and manufactured exclusively for single patient use and
for a period no longer than 24 hours.
• Connect the long silicone tube to the “PATIENT” jar outlet
• Connect the other end of the long silicon tube to the probe plastic connector, then connect the suction
probe to it.
• Connect the power cord to the device then connect the plug to the electrical mains supply.
• Push switch on position I to start suction.
• Unscrew the lid of the jar and ll the jar 1/3 full or ordinary water (this assists the unit to reach peak vacuum
performance and makes clean-up easier) then re-screw the lid on the jar correctly.
• During operation the jar has to be in vertical position to avoid overow valve to cut off aspiration. Should
this happen, switch off the device and disconnect the tube from the jar cover (from “VACUUM” outlet).
• Once nished push switch on O position and unplug.
• Remove the accessories and clean.
• At the end of each use, place the device in its box away from dust.
WARNING: The power supply cable plug is the element of separation from the electrical mains system: even
if the units equipped with a special on / off switch button, the power supply plug must be kept accessible once
the device is in use so as to allow a further method of disconnection from the mains supply system.
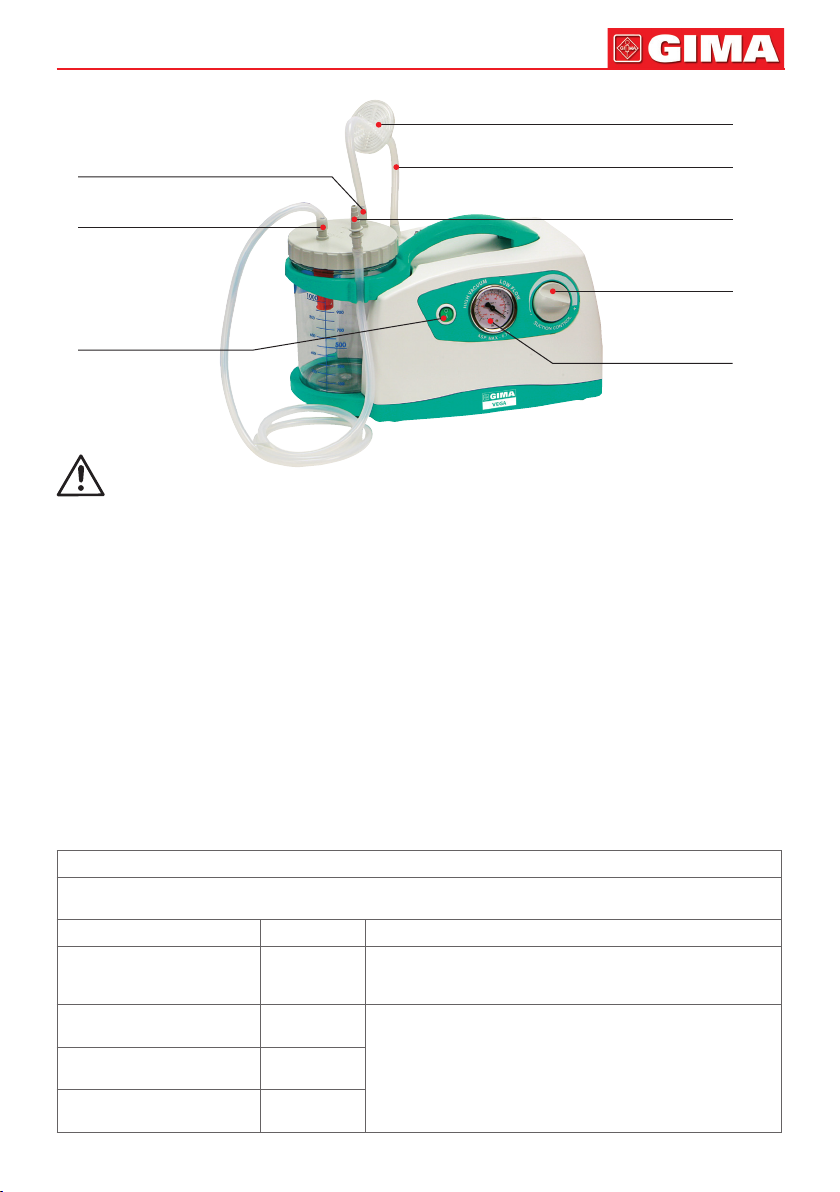
19
ENGLISH
Antibacterial Filter
PATIENT Port
VACUUM Port
ON/OFF Switch
Autoclavable Silicone Tube
Conical Fittings
Vacuum
Regulator Knob
Vacuum Indicator
(kPa /Bar)
NEVER USE THE DEVICE WITHOUT JAR AND / OR PROTECTION FILTER
MAKE SURE THAT CHILDREN AND/OR MENTALLY ILL PEOPLE DO NOT USE THE DEVICE WITHOUT
ADULT SURVEILLANCE
RISK OF ELECTROMAGNETIC INTERFERENCE AND POSSIBLE REMEDIES
This section contains information regarding the conformity of the compliance with the EN 60601-1-2 Standard
(2014).
The VEGA surgical aspirator is an electro-medical device that requires particular precautions regarding elec-
tro-magnetic compatibility and which must be installed and commissioned according to the electro-magnetic
compatibility information supplied. Portable and mobile radio communication devices (mobile phones, transceivers, etc.) may interfere with the medical device and should not be used in close proximity with, adjacent
to or on top of the medical device. If such use is necessary and unavoidable, special precautions should be
taken so that the electro-medical device functions properly in its intended operating conguration (for example, constantly and visually checking for the absence of anomalies or malfunctions). The use of accessories,
transducers and cables differing from those specied, with the exception of transducers and cables sold by
the appliance and system manufacturer as spare parts, can lead to an increase in emissions or in a decrease
of the immunity of the device or system. The following tables supply information regarding the EMC (Electromagnetic Compatibility) characteristics of the electro-medical device.
Guidance and manufacturer’s declaration – electromagnetic Emissions
The VEGA SUCTION UNIT is intended for use in the electromagnetic environment specied below. The customers or the
user of the VEGA SUCTION UNIT should make sure that it’s used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
Irradiated / Conducted
emissions CISPR11
Irradiated / Conducted
emissions CISPR11
Harmonic emissions
EN 61000-3-2
Voltage uctuations / icker
emissions EN 61000-3-3
Group 1 The VEGA SUCTION UNIT only used RF energy only for its internal
Class [B] The VEGA SUCTION UNIT can be used in all environments, in-
Class [A]
Complies
functioning. Therefore, its RF emissions are very low and are not
cause interference in proximity of any Electronic appliances.
cluding domestic and those connected directly to the public mains
distribution that supplies power to environments used for domestic
scopes.

ENGLISH
Guidance and manufacturer’s declaration – Immunity Emissions
The VEGA SUCTION UNIT is intended for use in the electromagnetic environment specied below. The customers or the
user of the VEGA SUCTION UNIT should make sure that it’s used in such an environment.
Immunity Test Level indicated by the
Compliance Level Electromagnetic environment - guidance
EN 60601-1-2
Electrostatic
discharge (ESD)
EN 61000-4-2
+/-8kV on contact
+/-15kV in air
The device doesn’t
change its state
Floors should be wood, concrete or ceramic
tile. If oors are covered with synthetic material, the relative humidity should be at least
30%.
Electrical fast
transient / burst
EN 61000-4-4
± 2kV power supply
lines
± 1kV for input /
The device doesn’t
change its state
Mains power quality should be that of a typical
commercial environment or hospital.
output lines
Surge
EN 61000-4-5
Loss of voltage, brief
voltage interruptions
and variations
EN 61000-4-11
± 1kV differential mode
+/-2 kV ordinary mode
<5% UT (>95% dip UT)
for 0,5 cycle
40% UT (60% dip UT)
for 5 cycle
70% UT (30% dip UT)
The device doesn’t
change its state
Mains power quality should be that of a typical
commercial environment or hospital.
- - Mains power quality should be that of a typi-
cal commercial environment or hospital If the
user of the VEGA SUCTION UNIT request
that the appliance operates continuously, the
use of a continuity unit is recommended.
for 25 cycle
<5% UT (>95% dip UT)
for 5 sec
Magnetic eld with
network frequency
(50/60 HZ)
30A/m The device doesn’t
change its state
The power frequency magnetic eld should
be measured in the intended installation lo-
cation to make sure that it’s sufciently low.
EN 61000-4-8
Note UT is the value of the power supply voltage
20
Guidance and manufacturer’s declaration – Immunity Emissions
The VEGA SUCTION UNIT is intended for use in the electromagnetic environment specied below.
The customers or the user of the VEGA SUCTION UNIT should make sure that it’s used in such an environment.
Immunity Test Level indicated by the
EN 60601-1-2
Conducted
Immunity
EN 61000-4-6
3Vrms 150kHz
to 80Mhz
(for non life-supporting
devices)
Compliance
Level
V1 = 3 V rms
Electromagnetic environment - guidance
The portable and mobile RF communication devices,
including cables, must not be used closer to the VEGA
SUCTION UNIT device, than the separation distance
calculated by the equation applicable to the transmitter
frequency.
Recommended separation distance
Radiated
Immunity
EN 61000-4-3
10V/m 80MHz
to 2.7GHz
(for non life-supporting
devices)
E1 = 10 V / m
d=
d=
d=
3,5
V
12
E
23
E
√P
1
from 80 MHz to 800MHz
√P
1
1
from 800 MHz to 2.7 GHz
√P

21
ENGLISH
Where P is the maximum nominal output voltage of the
transmitter in Watt (W) depending on the manufacturer of the transmitter and the recommended separation
distance in metres (m). The intensity of the eld from
the xed RF transmitters, as determined by an elec-
tro-magnetic study of the sitea), could be lower than the
level of conformity of each frequency interval b).
It is possible to check for interference in
proximity to devices identied by the following symbol:
Note 1: At 80 MHz and 800 MHz the interval with the highest frequency is applied
Note 2: These guide lines may not be applicable in all situations. The electro-magnetic propagation is inuenced by the
absorption and by reection from buildings, objects and people.
a) The eld intensity for xed transmitters such as the base stations for radiotelephones (mobile and cordless) and terrestrial mobile radio, amateur radio devices, radio AM and FM transmitters and TV transmitters can not be theoretically and
accurately foreseen. To establish an electro-magnetic environment generated by xed RF transmitters, an electro-magnetic study of the site should be considered. If the eld intensity measured in the place where the device will be used surpasses the above mentioned applicable level of conformity, the normal functioning of the device should be monitored. If abnormal performance arises, additional measures such as changing the device’s direction or positioning may be necessary.
b) The eld intensity on an interval frequency of 150 kHz to 80 MHz should be less than 3 V/m.
Recommended separation distance between portable and mobile radio-communication devices and the monitor
The VEGA SUCTION UNIT surgical aspirator is intended to operate in an electro-magnetic environment where RF irradiated interferences are under control. The client or operator of the VEGA SUCTION UNIT device can help prevent elec-
tro-magnetic interference by keeping a minimum distance between the portable and mobile RF communication devices
(transmitters) and the VEGA SUCTION UNIT device, as recommended below, in relation to the radio-communication
maximum output power.
Maximum nominal
output power of the
Transmitter W
Separation distance from the frequency transmitter (m)
150KHz to 80MHz 80MHz to 800MHz 800MHz to 2,7GHz
d=
3,5
V
√P
1
d=
12
√P
1
E
d=
23
E
√P
1
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters with a maximum nominal output power not shown above, the recommended separation distance in metres
(m) can be calculated using the equation applicable to the transmitter frequency, where P is the maximum nominal output
power of the transmitter in Watt (W) depending on the transmitter’s manufacturer.
Note 1: At 80 MHz and 800 MHz the interval with the highest frequency is applied
Note 2: These guide lines may not be applicable in all situations. The electro-magnetic propagation is inuenced by the
absorption and by the reection from buildings, objects and people

SYMBOLS
ENGLISH
22
Caution: read instructions (warnings) carefully
Keep in a cool, dry place Keep away from sunlight
Manufacturer Date of manufacture
Product code Lot number
Medical Device complies
with Directive 93/42/EEC
WEEE disposal Class II applied
Serial number Temperature limit
Fuse
IP21
Covering Protection rate
ON
OFF
Disposal: The product must not be disposed of along with other domestic waste. The users must
dispose of this equipment by bringing it to a specic recycling point for electric and electronic equipment.
~
Follow instructions for use
Type BF applied part
Atmospheric Pressure limit
Alternating current
Mains frequency
Hz
Humidity limit
GIMA WARRANTY TERMS
The Gima 12-month standard B2B warranty applies.
 Loading...
Loading...