
MANUALE OPERATIVO
PER LAMPADA
DA OSSERVAZIONE
USER MANUAL
FOR OBSERVATION LAMP
SOLESUD 2-LED

MO083-EN 14/02/20
EN
Rev.4
Page 1 of 17
USER MANUAL
USER MANUAL FOR OBSERVATION LAMP
SOLESUD 2-LED
Via Marconi, 1 – 20060 GESSATE (MI) ITALY
Tel. +39 02 953854209 Fax +39 02 95381167
http://www.gimaitaly.com e-mail: gima@gimaitaly.com
Introduction
Dear User, you are kindly invited to read this manual carefully before proceeding to use the Product in
order to safeguard yourself and other people from any injuries.
This appliance is a Class 1 medical device pursuant to European Directive on medical devices (MDD)
93/42/EEC (Annex IX) and 2007/47/EC.
The manufacturer declares that this Product is in compliance with Annex I (Essential requirements) of
Directive 93/42/EEC and certifies such conformity by affixing the CE marking.
This User manual is valid for the following model: SOLESUD 2-LED.
The customer service is at your disposal in case of Product details, information concerning its use,
identification of spare parts being required and for any other queries you might have concerning the
appliance, for ordering spares and for matters relating to assistance and warranty.
GIMA TECHNICAL ASSISTANCE OFFICE FOR CLIENTS
Via Marconi, 1 – 20060 GESSATE (MI) ITALY
Tel. +39 02 953854209 Fax +39 02 95381167
http://www.gimaitaly.com e-mail: gima@gimaitaly.com
The contents of this manual may be amended by GIMA, without prior notice or any further obligations,
in order to make changes and improvements. The reproduction and translation, including partial, of
any part of this manual is forbidden without the written permission of GIMA.
GIMA reserves the right to change, cancel or otherwise amend the data contained in this document at
any time and for any reason without prior notice inasmuch as GIMA is constantly seeking new
solutions which lead to product evolution. GIMA therefore reserves the right to make changes to the
supplied Product in terms of shape, fittings, technology and performances.
With regard to translations into languages other than Italian, reference shall always be made to the
Italian edition of this User manual.

MO083-EN 14/02/20
EN
Rev.4
Page 2 of 17
USER MANUAL
CONTENTS
1 General information 3
1.1 Operator qualification 3
1.2 Packaging, transport, storage and characteristics of installation premises 4
1.3 Graphic symbols used on the Product 4
1.4 Manufacturer's Declaration of Conformity 5
1.5 Warranty Certificate 6
2 Importance of personal safety 7
2.1 Intended use 7
2.2 Environmental conditions 7
2.3 Safety conditions (secondary effects) 7
2.4 Controls to be performed every time before the lamp is used 7
3 Product installation 8
3.1 Installation of wall version (S/12 MED fastening) 8
3.2 Installation of wall version (bar rail fastening) 8
3.3 Installation of 5-spoke floor version (RL) 9
3.4 First switch-on 9
3.5 Check the result of Product installation and testing before use 9
4 Description and operation 10
5 Cleaning and disinfecting 10
5.1 Cleaning the Product 10
5.2 Disinfecting 10
6 Adjustments 11
6.1 Yearly inspections by operator 11
6.2 Repairs 11
6.3 Adjustments 11
6.4 Troubleshooting 11
6.5 Routine maintenance 11
7 Technical properties 12
8 Wiring diagram 13
9 EMC Declaration 14

MO083-EN 14/02/20
EN
Rev.4
Page 3 of 17
Installation
Ins
taller and/or quali
fied technici
an
Use
Profession
al medical personnel
Routine maintenanc
e Qualified technician with required technical
-
professi
onal skills
Spe
cial maintenance
GIMA
or author
i
zed Dealer
Assistance
GIMA
or authorized Dealer
Cleaning
Properly trained me
dica
l and param
edi
cal personnel
Comply with applicable laws on waste disposal. This product must not
ensure correct recycling.
USER MANUAL
1 General information
The EM (Electro-Medical) DEVICE to which this manual refers is a LAMP for diagnosis or observation. For
ease of description, in this manual this EM EQUIPMENT will be called “Product”.
This manual is an integral part of the Product as indicated by European Directives 93/42/EEC and
2007/47/EC. Always keep this operator’s manual close to the lamp.
GIMA disclaims all liability for any injuries to persons or damage to things caused by the installation,
maintenance or use of the Product by unqualified operators. By qualified operator is meant
whosoever has attended a course relating to the installation, maintenance and use of the product
organised by GIMA or, alternatively, whosoever has carefully read this installation manual. GIMA does
not authorize third parties to perform special maintenance jobs. Should a problem arise, contact
GIMA.
The end user is entirely responsible for Product installation activities; no costs or responsibilities
relating to the installation and/or commissioning of the Product may therefore be traced back and/or
in any case attributed to GIMA.
The ceiling or wall masonry works for Products to be installed on ceilings or walls, and the electrical
works for supplying power to the Product shall be carried out in a workmanlike manner by suitably
qualified personnel to ensure these are sturdy and safe.
By way of example only, the following professional figures are deemed as suitably qualified:
onstruction Engineer, Draughtsman, Building firm duly registered in the professional Register (for
C
the masonry works)
Electrical Engineer Electro-technical expert qualified to work as an electrician (for the electrical
works)
The Product is an EM electro-medical equipment and therefore falls within the field of application of
the IEC 62353 standard. Consequently, any operation performed on the Product must be carried out in
compliance with the IEC 62353 standard, where applicable.
1.1 Operator qualification
This paragraph describes the requirements and qualifications which the operators involved in the
various stages of Product life and use must possess.
be disposed of in standard waste disposal bins. To avoid risks for the
environment and health deriving from the dispersion of polluting
Demolition
substances in the environment, separate the various internal
component parts such as iron, aluminium, plastic and electrical
material, and dispose of these through authorized channels so as to
MO083-EN 14/02/20
EN
Rev.4
Page 4 of 17
CE marking
(month/y
ear)
Limit temperature (indicate max limit
left)
Humidity to b
e co
mplied with (indicate
bottom left)
Pressure to be complied with
(indicate
bottom left)
signal
USER MANUAL
1.2 Packaging, transport, storage and characteristics of installation premises
Boxes containing the Product together with User manual.
Transport is made by GIMA or any road-hauler as long as in compliance with the following
characteristics:
Temperature (°C): -15 / +60; Humidity: 10 / 75 %; Atmospheric pressure (hPa): 500 / 1060.
The packaged Product must be stored (warehoused) in dry premises having the following
characteristics:
Temperature (°C): -15 / +60; Humidity: 10 / 75 %; Atmospheric pressure (hPa): 500 / 1060.
The premises where the Product is started up must have the following characteristics:
Temperature (°C): +10 / +40; Humidity: 30 / 75 %; Atmospheric pressure (hPa): 700 / 1060.
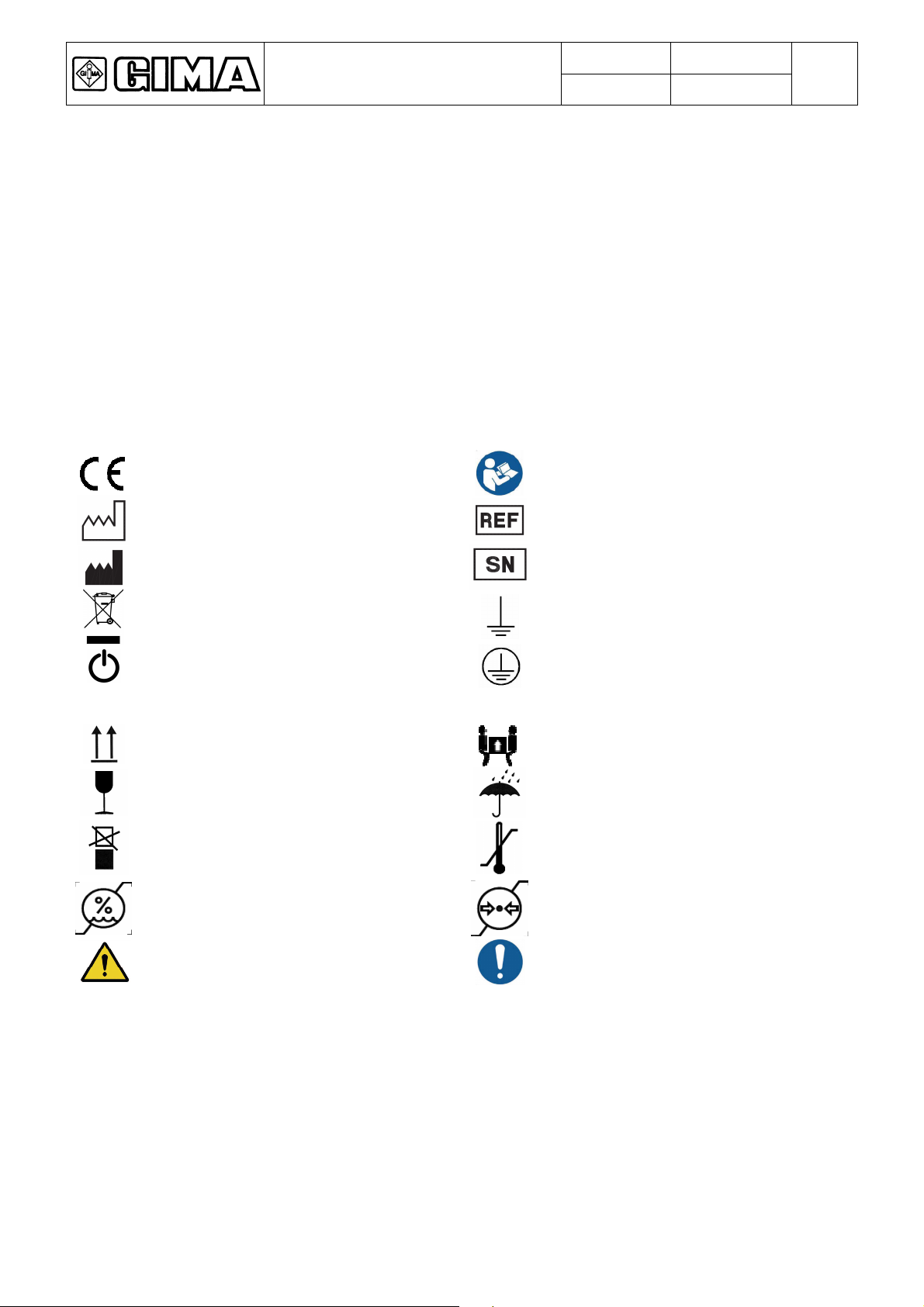
1.3 Graphic symbols used on the Product
Description of the symbols on plates, product and in manual:
Graphic symbol showing product bears
Manual reading obligation
‘I’
Symbol indicating date of manufacture
Manufacturer’s address
RECYCLING! The Product must be
recycled separately
Stand-By
ON power
Top side of packaging
Fragile packaging
Do not stack packaging
max limit at top right and min limit at
General warning signal
‘O’
Model
Serial number
Functional earth
Protection earth
OFF power
Weight of packaging
Protect from rain
at top right and min limit at bottom
max limit at top right and min limit at
General mandatory code of conduct
MO083-EN 14/02/20
EN
Rev.4
Page 5 of 17
I
EC 60
601-1 (Part
1:
General requirements for basic safety and ess
ential per
formance
)
• IEC 60601
-2-41
(Part
1:
Parti
c
ular require
ments
for the safety of surgical luminaires and
IEC 60601
-1-2 (Part 2:
General requirements for basic sa
fet
y and essential performance
-
Collateral Standard: Electrom
agnetic di
sturbances
- Requirements and tests
)
DURAT
ION:
Short term duration (Annex IX, Par.1 “D
efi
nitions”, art.1, subsection 1.1)
DESCRI
PTION
:
Non-invasive
medical d
evice (Annex IX, Par.1
“Definitions”, ar
t
.1, subsecti
on 1.2
)
A
ctive medical device
(Annex
IX,
Par.1 “Defini
tions
”,
art.1,
subsection
1.4)
CLASS I:
(Annex IX, Par.3 “Classification”
, ar
t.3, subsection 3.3, Rule 12) and
USER MANUAL
1.4 Manufacturer's Declaration of Conformity
The company: RIMSA P. LONGONI S.r.l. Via Monterosa, 18/20/22 - 20831 SEREGNO (MB) – ITALY
declares under its own responsibility that the Product (Medical device for observation and diagnosis):
made by RIMSA P.LONGONI S.r.l., complies with Annex VII of Directive 93/42/EEC dated 14/05/1993,
enforced in Italy by Legislative Decree No. 46 dated 24 February 1997 and subsequent amendments
(including Directive 2007/47/EC dated 05/09/2007, enforced in Italy by Legislative Decree No. 37 dated
25 January 2010) and with the following standards:
•
APPLY
LABEL
luminaires for diagnosis)
•
Classification with reference to article 9 and Annex IX of Directives 93/42/EEC and 2007/47/EC
(Annex IX Par.3 “Classification”, art.1, subsection 1.1 Rule 1)
• The conformity assessment is developed with reference to article 11 of Directive 93/42/EEC and
2007/47/EC.
• RIMSA Quality System complies with UNI EN ISO 9001 and UNI CEI EN ISO 13485 standards and is
certified by CSQ (CSQ certificate no. 9120.RMS1 and 9124.RMS2).
• The Medical Device to locally light up the patient’s body is marketed in NON-STERILE form.
Name: Paolo Longoni
Position: Managing Director

MO083-EN 14/02/20
EN
Rev.4
Page 6 of 17
USER MANUAL
1.5 Warranty Certificate
1. The Product is covered by an 18-month warranty, including electrical parts
2. The warranty begins on the date of product shipment from the GIMA warehouse to the buyer.
3. In case of disputes, the date indicated on the “transport document” attached to the goods shall
be deemed valid.
4. The warranty only covers the sending of Product spare parts to the buyer or, in the event of GIMA
considering the replacement of spare parts not feasible, the replacement of the entire product,
after fabrication faults have been properly ascertained at the undisputable judgement of GIMA.
The warranty does not therefore cover any other costs or expenses (including, by way of example
but without limitation, labour costs, packaging costs and transport costs, etc.).
5. The guarantee does not include the components subject to normal wear, such as halogen bulbs,
LEDs, fuses, relays, ball bearings, etc.)
6. The warranty does not cover:
- malfunctions due to failure to comply with user manuals;
- malfunctions due to installation and/or maintenance errors;
- malfunctions or faults caused by carelessness, negligence, incorrect use or other causes not
attributable to GIMA;
- malfunctions or faults due to the fact that the electrical system of the premises where the
device is installed is not in compliance with CEI 64-8 standards (standards for electrical
systems in premises used for medical purposes) and similar standards.
7. GIMA shall repay direct damages suffered by the buyer and which are documented as
attributable to its product, caused within the warranty period, for an amount not above 40% of the
net value of the product as indicated on the buyer’s invoice. GIMA’s liability is expressly ruled out
for indirect damages or consequential damages (including cases of the lamp not being used)
deriving from the supply.
8. This warranty certificate replaces legal warranties for faults and non-conformities and rules out
any other possible liability of GIMA originating from the supplied products.
9. The payment of any damages to persons or things due to product malfunction or faults shall be
limited to the maximum amount of GIMA’s insurance coverage for civil liability.
10. The warranty shall be automatically invalidated in the event of:
- the Product having been tampered with or modified by the buyer or third parties;
- the Product having been repaired by the buyer or third parties, without following the
instructions in user manuals;
- the Product serial number having been cancelled, defaced or removed;
- the buyer not being up to date with payments.
11. For jobs to be done under warranty, the buyer shall contact GIMA only.
12. The component parts replaced under warranty must only be returned to GIMA, if so requested by
GIMA, carriage free and suitably packed.
13. In case of failure to return a part requested by GIMA, the cost of the component part will be
charged.
14. GIMA cannot accept returns from end users or in any case from parties other than the buyer.
15. Products returned to GIMA must be complete with documentation authorising such return and
another document describing the malfunction.
16. For everything not indicated on this warranty certificate, reference shall be made to the laws of
Italy.
17. For all disputes deriving from or related to the orders to which this warranty certificate applies and
which cannot be amicably settled between the parties, the only competent law court shall be that
of Milan.

MO083-EN 14/02/20
EN
Rev.4
Page 7 of 17
USER MANUAL
2 Importance of personal safety
2.1 Intended use
The Product has been designed to light up the area of the patient undergoing observation and
diagnosis and is intended for use in doctors’ surgeries.
The Product correctly lights up the operating field from a minimum distance of 40 cm and a
maximum distance of about 70 cm, from the point of light emission.
The Product, in conformity with the IEC 60601-2-41 standard, is defined as a lamp for diagnostics:
- a lamp for diagnostics is a lamp used to locally light up the body of a patient, in order to make
diagnosis or treatment easier. These can be interrupted without any danger for the patient in case
of the light going off. (The Product is not intended for use in operating theatres).
2.2 Environmental conditions
- The Product is not suitable for use in explosion-risk areas.
- The Product is not suitable for use wherever there are flammable mixes of anaesthetics with air,
oxygen or N2O (laughing gas).
- The Product is not suitable for use in environments rich in oxygen and use is not intended in the
presence of flammable agents.
- During operation, the ambient temperature must be between 10°C and 40°C.
- Relative humidity must be between 30% and 75%.
- Atmospheric pressure must be between 700 and 1060hPa.
2.3 Safety conditions (secondary effects)
- Do not direct the light source into the patient’s and/or operator’s eyes.
- Obligation to adequately protect the patient’s eyes.
Failure to follow such precautions could cause glare and potential damage to the retina.
- Never place and/or hang anything on the Product.
Unless this precaution is taken, positioning will not be reliable and the danger exists of such
objects falling in the operating area.
- Never hang on the Product with the body weight of a person.
Failure to follow such precaution could damage the Product structure.
- Never cover the head of the Product during operation.
Failure to comply could prevent heat exchange with the environment and the Product could
overheat.
- Avoid knocking the rocker arms and Product head.
A violent knock could damage the Product and pieces of paint could chip off and fall onto the
operating field in the patient area.
- To avoid any significant risk of reciprocal interference due to the presence of the Product during
specific exams or treatments, see section 9 of the manual.
2.4 Controls to be performed every time before the lamp is used
To make sure the Product is safe and provides a correct diagnosis, every time before use, the operator
must check:
- The lamp has been correctly disinfected;
- The emitted light is stable and of adequate intensity;
- The flexible arm remains in the selected position, without falling.

MO083-EN 14/02/20
EN
Rev.4
Page 8 of 17
Before proceeding to install the Pr
oduct, first of all check t
he presence
of all the
Keep the
original packaging in case the Prod
uct has
to be re
-
dispatched
.
USER MANUAL
3 Product installation
packaging and that this is in good condition and has not been damaged during transport.
Claims will only be taken into consideration if the seller or carrier has been immediately
notified. All claims must be made in writing. Goods always travel under the responsibility
and at the risk of the buyer.
The product is supplied with different support systems, to be selected as required:
- ‘S/11’ wing-nut vice for fastening to table;
- ‘S/12 MED’ wall-fastening clamp;
- ‘Z400072’ rail bar clamp, ‘Z400075’ rail bar supplied with 1 metre bar length, 3 spacers, 3 wall
anchors and 3 screws for fastening the anchors to the bar;
- ‘RL’ floor lamp consisting of upright and 5 wheels with pedal-operated lock system.
To avoid the risk of electric shocks, this appliance
supplies with earth connection.
must only be connected to mains
3.1 Installation of wall version (S/12 MED fastening)
• Fasten the clamp S/12 MED to the wall with 3 expansion screws. GIMA does not
supply screws.
• The wall must be a supporting wall and be made of solid brick. Installation on
walls of perforated bricks and plasterboard is only allowed with the fitting of a
plate on the opposite side of the wall (sandwich closing). GIMA suggests using M5
screws.
• Fit the lamp in the hole located in the upper part of the clamp S/12 MED.
• Screw up the threaded knob, making sure this fit into the mill hole of the lamp pin in such a way as
to prevent it accidentally coming out.
• Insert the pin situated in the end of the power cable in the power socket.
3.2
• Fasten the bar rail according to attached instructions MO002i.
• Fit the clamp on the bar and tighten the lower knob.
• Fit the lamp in the hole located on the clamp.
• Screw up the threaded knob, making sure this fit into the mill hole of the
• Insert the pin situated in the end of the power cable in the power socket.
Installation of wall version (bar rail fastening)
lamp pin in such a way as to prevent it accidentally coming out.

MO083-EN 14/02/20
EN
Rev.4
Page 9 of 17
USER MANUAL
3.3 Installation of 5-spoke floor version (RL)
• To fit the stand keep the iron rod (1) in vertical position with side up of the lamp so
the internal conical nut goes down (2).
• Put the iron steel on the mobile (3) and screw down bolt (4) with washer and plate
(5) pulling down while screwing.
• Then fit the lamp in the hole located in the top part of the stand rod.
• Screw up the threaded knob, making sure this fits into the mill hole of the
lamp pin in such a way as to prevent it accidentally coming out.
• Insert the pin situated in the end of the power cable in the power socket.
In the floor version, operate all 5 wheel brakes dur
ensure stability.
3.4 First switch-on
At this point, the Product can be switched on to make sure it works properly.
1. Press the green switch on the base;
2. Make sure all LEDs and functions are working properly.
3.5 Check the result of Product installation and testing before use
The following instructions are to be deemed mandatory during the installation inspection phase, as
they prove that all the various jobs referred to have been correctly done. Hence each single step must
be ticked.
1. Make sure the wall is suitable for Product installation.
2. Make sure the stand pin has been correctly fitted in its fastening point.
3. Make sure movement mechanisms are working properly. Check mechanical operation by means
of direction and rotation movements.
4. After switch-on, the Product must emit light from the reflector.
Installer’s stamp and signature:
ing operation to

MO083-EN 14/02/20
EN
Rev.4
Page 10 of 17
Prod
uct when it
is col
d.
Failure to comply with the instruc
tions could caus
e the
p
aint
to come off
with possi
ble
glass
.
the Product
when
it is col
d.
USER MANUAL
4 Description and operation
The Product locally lights up the patient’s body thanks to 3 LEDs focalized by means of specific lenses.
Positioning is easy thanks to the articulated arm and is done manually.
The Product does not have a keyboard to operate. To switch on the Product, press the ‘I’ (ON) green
light switch on the Product base. The intensity of the light cannot be adjusted. It can only be directed
at the desired point thanks to the flexible arm, gripping it from the reflector. After use, press the ‘O’
(OFF) switch. To disconnect from the mains, remove the plug.
Do not position the device so it is hard to reach and remove the power plug in case of an
emergency.
5 C
leaning and disinfecting
5.1 Cleaning the Product
Before going ahead with cleaning operations switch off the Product by detaching the
plug, make sure it cannot be switched back on and leave it to cool down. Only clean the
Protect the Product from water spray and detergents and do not clean it with liquids. Clean with
suitable detergents with low alkaline content and chlorine free.
Do not use abrasive products, petrol, paint thinners, alkaline detergents, acids, containing alcohol or
aldehydes; dose the cleaning agents so no liquids penetrate inside the lamp elements and into the
support arm system.
Clean the Product with a damp, but not wet, cloth.
accidental dropping of such paint into the patient area, the early ageing of the plastic
parts with consequent weakening and the possibility of breakages, and the tarnishing of
The product is best cleaned at least once a day when used. To clean the lamp, the support need not
be removed.
5.2 Disinfecting
Before going ahead with disinfecting operations switch off the Product by detaching the
plug, make sure it cannot be switched back on and leave it to cool down. Only disinfect
Protect the Product from water spray and detergents and do not clean it with liquids.
Disinfectants can contain substances which are harmful for the health - only use disinfectants in
accordance with the rules on hygiene established by the hospital; the Product operator must comply
with the rules established by the national commission for hygiene and disinfection.
To prevent damaging parts in stainless steel or aluminium, only use disinfectants which are chlorine
and halogen free; to prevent the plastic parts becoming fragile, use only disinfectants with low
alcohol content; dose the disinfectants so no liquids penetrate inside the lamp elements and into the
support arm system.
Clean the Product with a damp, but not wet, cloth.

MO083-EN 14/02/20
EN
Rev.4
Page 11 of 17
USER MANUAL
The Product is best disinfected every time before use. To clean the lamp, the support need not be
removed.
6 Adjustments
6.1 Yearly inspections by operator
Keep to the yearly inspection schedules and inspect the product according to IEC 62353 standard.
6.2 R
The Product must only be opened and repaired by the manufacturer. Contact customer service as
indicated on page 1 in case of need.
Each Product, over time, is subject to a certain amount of wear. Product safety and
operation must therefore be checked during inspection and maintenance intervals.
epairs
Making any changes to this appliance is forbidden.
6.3 Adjustments
The Product is sold balanced and does not require further adjustment. In the event of the Product
becoming stiff or loose over time, contact GIMA customer service.
6.4 T
roubleshooting
No.
1 The Product fails to work Contact the after-sales service
2 The Product does not remain in position Contact the after-sales service
3 The light flickers Contact the after-sales service
4 The light beam is not focalised Contact the after-sales service
Problem Solution
6.5 Routine maintenance
No.
Internal Action
1 Once a year
2 Once a year
3 Once a year
Perform complete movements of Product arm and make sure movement
is smooth. If the Product fails to maintain its position or its movements
are hard, contact the after-sales service.
Make sure the retention screws of connections are tightened properly.
If these are not properly fastened, adequately tighten.
Check the condition of the Product paint. Make sure there are no paint
pieces that could fall in the patient area. If any paint pieces deemed
hazardous are found, contact the after-sales service.

MO083-EN 14/02/20
EN
Rev.4
Page 12 of 17
T
echnical properties
SOLESUD 2
-
LED Illumination Ec a
t 50cm distance
± 10
% [Lux]
60,
000 C
olour temperature (±5%) [K]
4,000
Colour
rendering index R
[-] 94 Max irradiance [
W/m
] 214 Max radiation in UV [W/m
] 0.016
Power
connection
details
Primary alternate voltage [V ac
] 100-240 Frequency [Hz]
50/60 Absorbed power [VA]
18 Light s
ource
N°3 LED
Duration of LED diode li
ght source [hr]
operating frequency)
General data
Colou
r RAL 9003
Directive
93/42
/EEC
(i
ncl. 20
07/47/EC)
Standard
s EN 60601
-
1 and EN 60601
-2-41 Clas
sification of Medical Device
Class I
Distribution of minimum and adequate lighting (luminous flux emit
ted by the ME
3000K
-
6700K
and 85
-
100, respect
ively)
.
Limitation of energy in the operating field (UV
-
irradia
nce for wavelengths belo
w
does no
t exceed 10
00 W/m2
at a distance of 500 mm
).
IP Classificat
ion IP20
Operating conditions
Continuous operation
Mains power voltage insulatio
n means
Integrated powe
r plug
Fuses incorporated
T1AH 250V,
5x20
Dimensions
Diameter of l
amp body [cm]
9
Lens diameter
[cm]
Light emission surface [cm
] 22
Overall dimensions [
cm] 100
x35
x15
Lamp
weight [k
g] 3 Markings
In c
onf
ormity with Directive 93/42/EEC (and
2007/47/EC)
Al
l technical light measurements are to be de
emed with a tolerance
of ±6% for m
etrol
ogical and
manufacturing reasons
.
USER MANUAL
7 Technical properties
a
2
2
(this figure can vary according to power peaks and
equipment does not vary by more than 20% during use and the colour
Essential
performance
temperature and the colour rendering index are stable and are within the range
400 nm does not exceed 10 W/m2 and the total irradiance Ee in the lighted area
60,000
2
3.2

MO083-EN 14/02/20
EN
Rev.4
Page 13 of 17
8 Wiring diagram
USER MANUAL

MO083-EN 14/02/20
EN
Rev.4
Page 14 of 17
Immuni
t
y test
Conformity
Electromagnetic environment
- directives
The Produc
t uses RF energy only for its internal
func
t
ion.
equip
men
t.
T
he Product is suitabl
e for use in al
l
establishments
location.
NOTE
: The
E
MISSIO
NS characteristics of this equipment mak
e it suitable
f
or use in indu
st
rial areas
re-orien
ting
the equi
pment
.
USER MANUAL
9 EMC Declaration
The Product has been tested according to EN60601-1-2 standard to ensure correct electromagnetic
compatibility.
Portable and mobile RF-communications equipment can affect the Product. The Product should not
be used adjacent with other equipment and that if adjacent use is necessary the Product should be
observed to verify normal operation.
The Product is intended for use in the electromagnetic environment specified below. The customer or
the user of the Product should assure that is used in such an environment.
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations
/flicker emissions
IEC 61000-3-3
and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR 11 class B is
normally required) this equipment might not offer adequate protection to radio-frequency
communication services. The user might need to take mitigation measures, such as relocating or
Group 1
Class A
Class A
Conforming
Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic
other than domestic, and may be used in domestic
establishments and those directly connected to the
public low-voltage power supply network that supplies
buildings used for domestic purposes, provided the
following warning is heeded:
WARNING: This equipment/system is intended for use
by healthcare professionals only. This
equipment/system may cause radio interference or
may disrupt the operation of nearby equipment. It may
be necessary to take mitigation measures, such as reorienting or relocating the Product or shielding the

MO083-EN 14/02/20
EN
Rev.4
Page 15 of 17
Test l
evel t
o
IEC 60601
-1-2
Electromagnetic env
ironment
-
d
irectives
F
loor
s sho
uld be w
ood
, concrete or
should be
a
t leas
t 30%
+/-
2 kV
lines
+/- 2
kV
lines
Mai
ns po
w
er quality should be that
+/-
1 kV
common mode
+/- 1 kV
common m
ode
Mains power quality should be that
<5% U
For 5 sec
<5%
UT
For 5 sec
M
ains
power qu
ali
ty should be that
Pow
er frequency
Power frequency m
ag
netic fields
environmen
t.
USER MANUAL
Immunity test
Electrostatic
discharge
(ESD)
IEC 61000-4-2
Electrical fast
transient / burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips,
short interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
+/- 8 kV contact
+/- 15 kV air
for power supply
unit
+/- 1 kV
for input/output
differential mode
+/- 2 kV
T
(>95% dip in UT)
For 0,5 cycle
40% of U
(60% dip in UT)
For 5 cycles
70% of U
(30% dip in UT)
For 25 cycles
<5% U
(>95% dip in UT)
T
T
T
Conformity level
+/- 8 kV contact
+/- 15 kV air
for power supply
lines
+/- 1 kV
for input/output
differential mode
+/- 2 kV
(>95% dip in UT)
For 0,5 cycle
40% of U
(60% dip in UT)
For 5 cycles
70% of U
(30% dip in UT)
For 25 cycles
<5% U
(>95% dip in UT)
T
T
T
ceramic tile.
If floors are covered with synthetic
material, the relative humidity
of a typical commercial or
residential environment.
of a typical commercial or hospital
environment.
of a typical commercial or hospital
environment. If the user of the
Product requires continued
operation during power mains
interruptions, it is recommended
that the Product be powered from
an uninterruptible power supply or
battery.
(50/60Hz)
magnetic field
IEC 61000-4-8
NOTE: UT is is the a.c mains voltage prior to application of the test level.
30 A/m 30 A/m
should be at levels characteristic
of a typical location in a typical
commercial or hospital

MO083-EN 14/02/20
EN
Rev.4
Page 16 of 17
Test level to
IEC 60601
-1-2
Co
nformity
level
Ele
ctrom
agn
etic environment
-
directives
Portable and mobile RF
NOTE 1
:
At 80 MHz and
800 MHz, the hi
gher frequency range applies.
a
bsorp
tion and
reflection from structures, obj
ects an
d p
eople.
Immunity test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
USER MANUAL
3 Veff
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5GHz
3 Veff
3 V/m
communications equipment should
be used no closer to any part of the
Product, included cables, than the
recommended separation distance
calculated from the equation
applicable to the frequency of the
transmitter.
Recommended separation distance
d = 1.2√P 150 KHz to 80 MHz
d = 1.2√P 80 MHz to 800 MHz
d = 2.3√P 80 MHz to 2.5 GHz
where P is the maximum output
power rating of the transmitter in
watts (W) according to the
transmitter manufacture and d is the
recommended separation distance in
meters (m).
Field strengths from fixed transmitters,
as determined by an electromagnetic
site survey, should be less than the
compliance leave in each frequency
range.
Interference may occur in the vicinity
of equipment marked with the
following symbol.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by

MO083-EN 14/02/20
EN
Rev.4
Page 17 of 17
equipm
ent and the Produc
t
The Product
is intended for use in an electromagnetic environment in
whic
h radiat
ed RF
the maximu
m output p
ower of the communications equ
ipm
ent.
W
d = 1.
2√P
d = 1
.2√P
d = 2
.3√P
For transmitters rated at a maximum output power not listed above, the recommended separation
USER MANUAL
Recommended separation distance between portable and mobile RF communications
disturbances are controlled. The customer or the user of the Product can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the Product as recommended below, according to
Rated maximum output
power of transmitter
0.01 0.12 0.12 0.24
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz



 Loading...
Loading...