
Smart One
Rev.4.0.1
User Manual
Page 1 of 35
®
PEAK FLOW AND FEV1 METER
ENGLISH (EN)
PLEASE READ ALL THE INFORMATION IN THIS USER MANUAL BEFORE USING THIS DEVICE. IF YOU D O NOT
UNDERSTAND THESE INSTRUCTIONS OR IF YOU HAVE QUESTIONS ABOUT YOUR PEAK FLOW AND FEV1 MET ER
AND ITS USE CONSULT YOUR PHYSICIAN OR OTHER LICENSED HEALTHCARE PROFESSIONAL.
IF YOU DO NOT UNDERSTAND THE INSTRUCTIONS:
USA:
Call MIR USA Tel + 1 (262) 565 - 6797 ; Fax + 1 (262) 364 - 2030 , Monday to Friday 8 AM to 5 PM (central time),
OR CONTACT US AT mirusa@spirometry.com, OR WRITE US AT MIR USA, 5462 S. Westridge Drive, New Berlin,
WI 53151 - USA
EUROPE and WORLDWIDE:
Call MIR +39 06 22754777, Monday to Friday 8 AM to 5 PM (GMT+1), OR CONTACT US AT mir@spirometry.com,
OR WRITE US AT MIR Via del Maggiolino 125, 00155 Roma, Italy.
User Manual Rev 4.0.1
Issue Date March 14th, 2023
0476

Smart One
Rev.4.0.1
User Manual
Page 2 of 35
is registered trademark of MIR S.p.A. MEDICAL INTERNATIONAL
RESEARCH
Date
Measurements
Recommendation
Physician
PEF
FEV1
Reserved for the physician or other licensed health care professional to write your good flow
rates and to provide specific interventions he/she recommends for ranges of decreased flow
rates.

Smart One
Rev.4.0.1
User Manual
Page 3 of 35
CONTENTS
1. INTENDED USE ............................................................................................................. 5
2. IMPORTANT INFORMATION CONCERNING INTENDED USE ......................................... 5
3. DETERMINING YOUR PEF BASELINE VALUE ................................................................. 6
4. WARNINGS AND PRECAUTIONS ................................................................................... 8
5. CONTRAINDICATIONS .................................................................................................. 9
6. HOW TO START USING THE MIR SMART ONE APP ..................................................... 10
7. HOW SMART ONE WORKS ......................................................................................... 11
7.1 Diary record ..................................................................................................... 12
7.2 Self–measuring of PEF and FEV1 value ............................................................ 13
7.3 Interpreting results .......................................................................................... 16
8. IMPORTANT SAFETY WARNINGS ............................................................................... 17
8.1 Data security warnings .................................................................................... 18
8.2 Warnings for use in electromagnetic environments ........................................ 19
8.3 Notes on fcc certification ................................................................................. 19
9. CARE AND CLEANING ................................................................................................. 20
9.1 Cleaning of the reusable turbine ..................................................................... 20
9.2 Cleaning of the mouthpiece............................................................................. 23
9.3 Cleaning of the device ..................................................................................... 25
9.4 Replacing batteries .......................................................................................... 26
10. ERROR MESSAGES ..................................................................................................... 27
11. TROUBLESHOOTING .................................................................................................. 27
12. Accuracy and Reliability ............................................................................................. 28
13. LABELS & SYMBOLS ................................................................................................... 30
14. TECHNICAL SPECIFICATIONS ...................................................................................... 31
15. Bluetooth Wireless Technology Information ............................................................. 32
15.1 Radio frequency (rf) communication ............................................................... 33
15.2 Radio frequency (rf) interference from other wireless devices........................ 34
16. WARRANTY TERMS .................................................................................................... 34

Smart One
Rev.4.0.1
User Manual
Page 4 of 35
Before connecting SMART ONE to a smartphone, install the MIR SMART ONE free app,
which you can download from the App Store (for iPhone and iPad) or Play Store (for Android
devices).
After removing the device from its packaging, check that there is no visible damage. If there
is, do not use the device and send it straight back for replacement.
Check if the packaging contains all the items shown below.
Keep the original packaging! If your product has a problem, use the original packaging to
ship it back to your local distributor:
MIR USA, Inc.
5462 S. Westridge Drive
New Berlin, WI 53151 - USA
Tel + 1 (262) 565 – 6797 ; Fax + 1 (262) 364 – 2030
Website: www.spirometry.com ; Email: mirusa@spirometry.com
EUROPE and WORLDWIDE:
Call MIR +39 06 22754777, Monday to Friday 8 AM to 5 PM (GMT+1), OR CONTACT US AT
mir@spirometry.com, OR WRITE US AT MIR Via del Maggiolino 125, 00155 Roma, Italy.
The manufacturer cannot be held responsible for any damage caused by users failing to
follow the instructions contained in this manual.

Smart One
Rev.4.0.1
User Manual
Page 5 of 35
1. INTENDED USE
Smart One is intended for home use by patients to monitor PEF (Peak Expiratory Flow) and
FEV1 (Forced Expiratory Volume in 1st second, VEMS). The device is intended for adult
patients, adolescents and children over five years of age.
2. IMPORTANT INFORMATION CONCERNING INTENDED USE
PEF is the maximum speed a person can blow air out of the lungs after taking as big a breath
as possible.
FEV1 is the maximum volume of air a person can exhale from the lungs in one second after
taking as big a breath as possible.
CAUTION: WHEN SMART ONE IS USED TO MONITOR LUNG CONDITIONS SUCH AS ASTHMA
YOU SHOULD BE UNDER THE CARE OF A PHYSICIAN OR OTHER LICENSED HEALTHCARE
PROFESSIONAL.
Medical studies have shown that regularly reviewing accurate measurements of PEF and
FEV1 with a physician or other licensed healthcare professional may allow individuals with
lung disease to better manage their conditions.
It is important to watch for changes from one measuring to the next, and to follow the
actions you have to take according to the action plan provided to you by your physician or
other licensed healthcare professional.
If you have breathing conditions such as asthma your physician or licensed healthcare
professional may recommend that you measure PEF/FEV1 to watch your disease and
discover if there are changes in your airflow. When you blow into the mouthpiece of the
flow meter, the device will display a number. The faster you blow, the higher the reading.
This number tells you how well air is moving through the airways in your lungs. When you
use SMART ONE regularly, you will be able to detect changes in your measurements, which
will tell you and your physician or other licensed healthcare professional what is happening
with your lungs.
These changes may require special treatment of your condition according to the action plan
given to you by your physician or licensed healthcare professional which will tell you when

Smart One
Rev.4.0.1
User Manual
Page 6 of 35
and how often to use your SMART ONE meter. They also will explain how your PEF and FEV1
measurements help them monitor your lung function and how well treatments are working.
3. DETERMINING YOUR PEF BASELINE VALUE
A PEF measure with a high value usually means that your airflow is good.
The best way to determine what is a healthy PEF for you is to discuss this with your physician
or other licensed healthcare professional. In fact the importance of any changes in airflow
from one measuring to the next depends upon how much they are different from your
baseline value you should reach when you are in healthy physical condition.
Your physician or other licensed healthcare professional will use one of two possible ways
to identify your baseline value. The first method adopts the predicted value calculated
according to the results of epidemiological studies of large groups of healthy subjects of your
same age, height, sex, and origin. The second method adopts the personal best value you
can reach when you are in the healthiest physical condition.
The MIR SMART ONE app can calculate the PEF predicted value, i.e. the expected value for
healthy people, depending on age, height, sex, and origin. MIR SMART ONE app calculate
the PEF predicted value that has been endorsed by ATS (American Thoracic Society): PEF
predicted values are calculated according to Knudson, R. J., Slatin R. C., Lebowitz, M. D.,
Burrows, B., The Maximal Expiratory Flow-Volume Curve – Normal Standards, Variability,
and Effects of Age, AM REV RESPIR DIS, 1976 113;587-600.
In this case, the predicted value becomes the baseline value for your treatment plan. If your
physician or other licensed healthcare professional prefers this method, MIR SMART ONE
app provides the calculation of the predicted PEF value.
It is important to know that these predicted values are average numbers for large groups of
people. You may have a higher PEF measure than the predicted value and you may not be
healthy. Or you may have a lower PEF than the average and be healthy.

Smart One
Rev.4.0.1
User Manual
Page 7 of 35
PEF table Male (L/min)
Height (cm)
120
130
140
150
160
170
180
190
200
AGE
5
128
175
221
268
315
362
409
455
502
10
178
224
271
318
365
412
458
505
552
15
227
274
321
368
415
461
508
555
602
20
277
324
371
418
464
511
558
605
652
25
265
321
378
434
490
547
603
660
716
30
254
311
367
423
480
536
593
649
705
35
244
300
357
413
469
526
582
639
695
40
233
290
346
402
459
515
572
628
684
45
223
279
336
392
448
505
561
618
674
50
212
269
325
381
438
494
551
607
663
55
202
258
315
371
427
484
540
597
653
60
191
248
304
360
417
473
530
586
642
65
181
237
294
350
406
463
519
576
632
70
170
227
283
339
396
452
509
565
621
75
160
216
273
329
385
442
498
555
611
80
149
206
262
318
375
431
488
544
600
85
139
195
252
308
364
421
477
534
590
90
128
185
241
297
354
410
467
523
579
PEF table female (L/min)
Height (cm)
120
130
140
150
160
170
180
190
200
AGE
5
165
194
224
253
283
312
341
371
400
10
212
241
271
300
330
359
388
418
447
15
259
289
318
347
377
406
436
465
494
20
279
308
338
367
396
426
455
485
514
25
271
301
330
359
389
418
448
477
506
30
264
293
323
352
381
411
440
470
499
35
256
286
315
344
374
403
433
462
491
40
249
278
308
337
366
396
425
455
484
45
241
271
300
329
359
388
418
447
476
50
234
263
293
322
351
381
410
440
469
55
226
256
285
314
344
373
403
432
461
60
219
248
278
307
336
366
395
425
454
65
211
241
270
299
329
358
388
417
446
70
204
233
263
292
321
351
380
410
439
75
196
226
255
284
314
343
373
402
431
80
189
218
248
277
306
336
365
395
424
85
181
211
240
269
299
328
358
387
416

Smart One
Rev.4.0.1
User Manual
Page 8 of 35
90
174
203
233
262
291
321
350
380
409
CAUTION: NO MATTER WHICH METHOD YOUR PHYSICIAN OR OTHER LICENSED
HEALTHCARE PROFESSIONAL PREFERS TO USE, IT IS IMPORTANT THAT YOU CLEARLY
UNDERSTAND THE MEANING OF YOUR BASELINE VALUE AND HOW IT RELATES TO YOUR
TREATMENT PLAN. IF YOU HAVE TROUBLE DETERMINING YOUR BASELINE VALUE, ASK
YOUR PHYSICIAN OR OTHER LICENSED HEALTHCARE PROFESSIONAL FOR ASSISTANCE.
4. WARNINGS AND PRECAUTIONS
PLEASE READ ALL THE INFORMATION IN THIS USER MANUAL BEFORE USING THIS
DEVICE. IF YOU DO NOT UNDERSTAND THESE INSTRUCTIONS OR IF YOU HAVE QUESTIONS
ABOUT YOUR PEF AND FEV1 METER AND ITS USE CONSULT YOUR PHYSICIAN OR OTHER
LICENSED HEALTHCARE PROFESSIONAL.
WHEN SMART ONE IS USED TO MONITOR LUNG CONDITIONS SUCH AS ASTHMA YOU
SHOULD BE UNDER THE CARE OF A PHYSICIAN OR OTHER LICENSED HEALTHCARE
PROFESSIONAL.
A HEALTHCARE PROFESSIONAL’S ADVICE IS REQUIRED TO INTERPRET THE MEANING
AND IMPORTANCE OF THE MEASUREMENTS REPORTED BY SMART ONE AND HOW TO
DECIDE ON AN APPROPRIATE ACTION PLAN
DIAGNOSIS AND APPROPRIATE TREATMENTS ARE TO BE GIVEN ONLY BY A PHYSICIAN
OR OTHER LICENSED HEALTHCARE PROFESSIONAL. THE ACTION PLAN WILL TELL YOU
WHAT ACTION TO TAKE WHEN THERE ARE CHANGES IN YOUR MEASURES.
SELF–MEASUREMENT MEANS CONTROL, NOT DIAGNOSIS OR TREATMENT. IN ANY
EVENT PLEASE BE SURE TO DISCUSS YOUR MEASURED VALUES WITH YOUR PHYSICIAN OR
OTHER LICENSED HEALTHCARE PROFESSIONAL. THEY WILL ALSO EXPLAIN WHICH VALUES
ARE NORMAL FOR YOU.
NO MATTER WHAT YOUR MEASURES ARE, IF YOU HAVE SIGNS AND SYMPTOMS SUCH
AS CHEST TIGHTNESS, SHORTNESS OF BREATH, COUGHING OR WHEEZING YOU SHOULD
CONTACT YOUR PHYSICIAN OR LICENSED HEALTH CARE PROFESSIONAL.
FOLLOW INSTRUCTIONS CAREFULLY IN ORDER TO GET AN ACCURATE READING OF
YOUR MEASURES. IF YOU ARE UNABLE TO OBTAIN A READING, CONTACT YOUR
HEALTHCARE PROFESSIONAL.
Smart One
Rev.4.0.1
User Manual
Page 9 of 35
ASK YOUR PHYSICIAN OR OTHER LICENSED HEALTH CARE PROFESSIONAL TO WATCH
YOU USE YOUR SMART ONE BEFORE YOU RELY ON ANY MEASUREMENTS.
MODIFYING YOUR ACTION PLAN OR MODIFYING YOUR BASELINE VALUE FOR YOUR
PEF MEASUREMENT SHOULD ONLY BE DONE WITH DIRECTION FROM YOUR PHYSICIAN OR
OTHER LICENSED HEALTHCARE PROFESSIONAL. DISCUSS WITH YOUR PHYSICIAN BEFORE
PROCEEDING.
YOU SHOULD NEVER ALTER THE DOSAGE OF ANY MEDICATION WITHOUT TALKING TO
YOUR PHYSICIAN.
THE DEVICE SHOULD NOT BE USED BY MORE THAN ONE PERSON. IF MORE THAN ONE
PERSON WISHES TO USE THE DEVICE, ONE USER’S MEASUREMENTS MUST NOT BE
ATTRIBUTED TO ANOTHER AND BOTH TURBINE AND MOUTHPIECE MUST BE CLEANED
THROUGHLY AFTER EACH USE UNLESS MULTIPLE TURBINE AND MOUTHPIECE ARE
AVAILABLE.
IF ANOTHER PERSON INTENDS TO USE THE DEVICE PERMANENTLY, THE PREVIOUS
DATA STORED BY THE MIR SMART ONE APP MUST BE REMOVED AND THE NEW PEF
BASELINE VALUE MUST BE DEFINED ACCORDING TO A PHYSICIAN OR OTHER LICENSED
HEALTHCARE PROFESSIONAL.
ANY SERIOUS INCIDENTS OCCURRING WITH THE DEVICE MUST BE REPORTED TO THE
MANUFACTURER AND THE COMPETENT AUTHORITY OF THE MEMBER STATE WHERE THE
USER AND/OR PATIENT IS ESTABLISHED, IN ACCORDANCE WITH REGULATION (EU)
2017/745.
5. CONTRAINDICATIONS
The ATS/ERS guideline updated 2019 sets out the relative contraindications of spirometry as
follows: Acute myocardial infarction within 1 week; Systemic hypotension or severe
hypertension; Significant atrial/ventricular arrhythmia; Uncompensated heart failure;
Uncontrolled pulmonary hypertension; Acute pulmonary heart; Clinically unstable
pulmonary embolism; History of syncope related to forced exhalation/cough. Cerebral
aneurysm; Brain surgery within 4 weeks; Recent concussion with persistent symptoms; Eye
surgery within 1 week.

Smart One
Rev.4.0.1
User Manual
Page 10 of 35
Due to increased sinus and middle ear pressure: Sinus or middle ear surgery or infection
within 1 week. Presence of pneumothorax; Thoracic surgery within 4 weeks; Abdominal
surgery within 4 weeks; Pregnancy beyond term. Active or suspected transmissible
respiratory or systemic infection, including tuberculosis; Physical conditions predisposing to
transmission of infection, such as haemoptysis, significant discharge or oral lesions or oral
bleeding.
If at least one of these conditions affects you, consult your doctor or a health care
professional before using the device.
6. HOW TO START USING THE MIR SMART ONE APP
Follow the instructions in the Maintenance section for correct battery insertion.
Before connecting SMART ONE to a smartphone, install the MIR SMART ONE free app,
which you can download from Apple Store (for iPhone and iPad) or from Play Store (for
Android devices).
Launch the MIR SMART ONE APP and carry out the following steps.
These are one–off operations that do not need to be repeated next time you enter the app.
a) Authorize data exchange with the Health app, which is already installed on your
smartphone. The user can decide whether or not to allow
• the following data to be written to the Health app: height, weight, PEF and FEV1
• the following data to be read from the Health app: height, weight, date of birth, gender.
You can allow or deny authorization for each parameter.
b) Enter your personal details: date of birth, origin, weight, height, sex.
The MIR SMART ONE APP will use these data to calculate PEF baseline value, and will use it
to assign a colored marker to your test (green, yellow, red). Please refer to the section
DETERMINING YOUR PEF BASELINE VALUE for a clear understanding of the baseline value.
If you don’t enter your data, a warning message will be issued.
Connection between SMART ONE and your smartphone is automatic. To check whether
there is a connection, read the messages from the app.

Smart One
Rev.4.0.1
User Manual
Page 11 of 35
7. HOW SMART ONE WORKS
SMART ONE is an electronic device for home use that accurately measures your PEF (Peak
Expiratory Flow) and FEV1 (Forced Expiratory Volume in 1 sec).
PEF is the maximum speed a person can blow air out of the lungs after taking as big a breath
as possible while FEV1 is the maximum volume of air a person can exhale from the lungs in
one second after taking as big a breath as possible.
WHAT IS THE SCIENTIFIC BASIS FOR PEF AND FEV1 HOME MEASUREMENT?
The first portable mechanical meter to measure PEF was introduced in 1959 by B. Wright.
The widespread use of this device to monitor children greater than five years of age and
adults has made it a popular means of tracking the degree of respiratory conditions in
patients with asthma and other pulmonary conditions.
Inexpensive, small, portable, and easy to use electronic meters for evaluating respiratory
conditions are now widely available. They offer several advantages including the ability to
record PEF and FEV1, and to store and transfer data to a physician or other licensed
healthcare professional.
SMART ONE provides a warning message if a test is not correctly performed, for example if
instead of blowing out as hard and fast as you can, you exhale too slowly. This is another
clear advantage compared to a mechanical peak flow meter that does not provide any
message.
PEF and FEV1 are measured during the same exhalation. When the test is correctly
performed, PEF is measured 0.10–0.15 seconds after the blow start, while FEV1 is measured
exactly 1.0 second after the blow start.
According to best current evidence from several scientific studies, research paper topics and
clinical expertise, both PEF and FEV1 have proven to be good indicators of airflow function
in health and illness that can indicate how well you are breathing, and can help you
determine if there are changes in your airflow. Regular measurements of PEF and FEV1
provide evidence of disease progression.
The GUIDE FOR ASTHMA MANAGEMENT AND PREVENTION published in 2016 by GINA
(Global Strategy for Asthma Management and Prevention) states:
Effective asthma self–management education requires:

Smart One
Rev.4.0.1
User Manual
Page 12 of 35
• Self–monitoring of symptoms and/or lung function
• Written asthma action plan
• Regular medical review
The above indicates that when engaged in self–management of asthma, your lung
conditions can be effectively monitored according with the written action plan prepared
by a physician or a licensed healthcare professional.
CAUTION: A PHYSICIAN’S OR OTHER HEALTHCARE PROFESSIONAL’S ADVICE IS REQUIRED TO
INTERPRET THE MEANING AND IMPORTANCE OF THE MEASUREMENTS REPORTED BY
SMART ONE AND HOW TO DECIDE ON AN APPROPRIATE ACTION PLAN
SMART ONE connects to a smartphone via Bluetooth SMART technology. Connection is
automatic once the MIR SMART ONE APP has been installed on your smartphone.
Each measure of PEF and FEV1 is transferred from the device to the smartphone to be
displayed. Please use the PEF colored indicator (green, yellow or red) following your
physician’s or other licensed health care professional’s advice. They will help you determine
how to perform the test accurately and recommend actions when decreased values are
measured.
CAUTION: WHEN SMART ONE IS USED TO MONITOR LUNG CONDITIONS SUCH AS ASTHMA
YOU SHOULD BE UNDER THE CARE OF A PHYSICIAN OR OTHER LICENSED HEALTHCARE
PROFESSIONAL.
A higher value of PEF and FEV1 usually means air is moving easily through the lungs. When
asthma attacks occur air cannot move easily through the lungs and lower values will be
measured. It is generally recommended to take measurements as directed by the licensed
healthcare professionals.
SMART ONE should also be used when you are feeling symptoms of breathing problems in
order to help you and your physician or other licensed healthcare professional determine
how serious the breathing problem is and how well your treatment is working. Discuss with
your physician or other licensed healthcare professional when and how often to use your
SMART ONE meter.
7.1 Diary record
The MIR SMART ONE APP keeps a record of your highest reading of PEF and FEV1 for both
morning and evening session, complete with date and time. Dots between consecutively

Smart One
Rev.4.0.1
User Manual
Page 13 of 35
recorded readings are connected in order to form a trend graph. This on–going record is an
important part of your asthma action plan.
The MIR SMART ONE APP can transfer the measured data to your physician or other
licensed healthcare professional. When used properly, SMART ONE will help you and your
physician or licensed healthcare professional monitor your asthma or other lung disease to
provide the best treatment.
Reviewing the measured data can help you and your physician or other licensed healthcare
professional check closely on your respiratory disease to provide the best treatment for you.
Because your smartphone has an automatic memory of hundreds of readings, you can take
the device with you the next time you visit your physician or other licensed healthcare
professional for a review of many readings.
7.2 Self–measuring of PEF and FEV1 value
PLEASE READ ALL THE INFORMATION IN THIS USER MANUAL BEFORE USING THIS DEVICE. IF YOU
DO NOT UNDERSTAND THESE INSTRUCTIONS OR IF YOU HAVE QUESTIONS ABOUT YOUR PEAK
FLOW AND FEV1 METER AND ITS USE CONSULT YOUR PHYSICIAN OR OTHER LICENSED
HEALTHCARE PROFESSIONAL.
IF YOU DO NOT UNDERSTAND THE INSTRUCTIONS :
USA:
Call MIR USA 1-844-4MIRUSA (1-844-464-7872), Monday to Friday 8 AM to 5 PM (central time),
OR CONTACT US AT mirusa@spirometry.com, OR WRITE US AT MIR USA, Inc. 5462 S. Westridge
Drive New Berlin, WI 53151 – USA.
EUROPE and WORLDWIDE:Call MIR +39 06 22754777, Monday to Friday 8 AM to 5 PM (GMT+1),
OR CONTACT US AT mir@spirometry.com, OR WRITE US AT MIR Via del Maggiolino 125, 00155
Roma, Italy.
ASK YOUR PHYSICIAN OR OTHER LICENSED HEALTH CARE PROFESSIONAL TO WATCH YOU USE
THE PEAK FLOW METER. THIS WILL HELP ASSURE YOU ARE USING IT CORRECTLY.
NO MATTER WHAT YOUR PEAK FLOW MEASURES ARE, IF YOU HAVE SIGNS AND
SYMPTOMS SUCH AS CHEST TIGHTNESS, SHORTNESS OF BREATH, COUGHING OR

Smart One
Rev.4.0.1
User Manual
Page 14 of 35
WHEEZING YOU SHOULD FOLLOW YOUR LICENSED HEALTH CARE PROFESSIONAL'S
ADVICE FOR CONTACTING HIM OR HER.
IF YOU ARE UNABLE TO OBTAIN A READING YOU SHOULD CONTACT YOUR PHYSICIAN
IMMEDIATELY.
SMART ONE must be cleaned as shown in CARE AND CEANING section before your initial
trial and periodically thereafter.
To carry out a measurement:
• Run the MIR SMART ONE APP on your smartphone
• Press the START icon
• Wait for Bluetooth connection
1
Push the turbine into the slot until it stops
2
Turn the turbine clockwise until it
stops

Smart One
Rev.4.0.1
User Manual
Page 15 of 35
3
Insert mouthpiece at least 0.5 cm into the
turbine socket.
4
Your SMART ONE is now ready.
Hold SMART ONE with your hand
as if it were a cell phone and make
sure not to obstruct the turbine
with your hand.
5
Insert the mouthpiece end into your mouth beyond your teeth, and close lips around
the mouthpiece. Make sure your lips form a tight seal around the mouthpiece.
To prevent turbulence that might otherwise affect the results do not put your tongue
in the mouthpiece. Do not bend your neck.

Smart One
Rev.4.0.1
User Manual
Page 16 of 35
6
It is best to do the test standing or sitting
upright (makes no difference to test
results).
7
• Take a slow deep breath as deep as
you can.
• Blow out as hard and fast as you can
until you can read the results on the
screen of your smartphone.
• This is your PEF and FEV1 measure.
NOTE: Avoid long slow exhalation
Since each test session should
consist of three trials, repeat steps
4–7 twice more.
SMART ONE will save the highest
value.
Warning: Supervision of an adult is required for testing elderly subjects, children and
differently-able persons
7.3 Interpreting results
Three tests are performed per each session, after
which the MIR SMART ONE APP automatically
selects and save the highest PEF value and
compares it with the baseline value. The app
shows a graphic marker (green, yellow or red),
which is then displayed as a colored circle around
the PEF test result.
The meaning of the graphic marker is displayed in
the following table.
COLOR
RESULT
MEANING
ACTION
Green
Above 80% of the baseline
OK
Your breathing condition appears
under control. Your medication is

Smart One
Rev.4.0.1
User Manual
Page 17 of 35
working. Go ahead with your normal
activities.
Yellow
Above 50% and below or
equal to 80% of the
baseline
Warning
Use caution in your activity. Refer to
your action plan made by your
physician or other licensed
healthcare professional for action to
be taken.
Red
Below or equal to 50% of
the baseline
Danger
Medical alert. You should get
immediate medical attention. Act as
discussed with your physician or
other licensed healthcare
professional.
CAUTION: ASK YOUR PHYSICIAN OR OTHER LICENSED HEALTHCARE PROFESSIONAL TO
WATCH YOU USE YOUR SMART ONE BEFORE YOU RELY ON ANY MEASUREMENTS.
CAUTION: WHEN SMART ONE IS USED TO MONITOR LUNG CONDITIONS SUCH AS ASTHMA
YOU SHOULD BE UNDER THE CARE OF A PHYSICIAN OR OTHER LICENSED HEALTHCARE
PROFESSIONAL.
CAUTION: THE ACTION PLAN GIVEN TO YOU BY YOUR PHYSICIAN OR OTHER LICENSED
HEALTH CARE PROFESSIONAL WILL TELL YOU WHAT ACTION TO TAKE WHEN THERE ARE
CHANGES IN YOUR MEASURES.
CAUTION: NO MATTER WHAT YOUR MEASURES ARE, IF YOU HAVE SIGNS AND SYMPTOMS
SUCH AS CHEST TIGHTNESS, SHORTNESS OF BREATH, COUGHING OR WHEEZING YOU
SHOULD CONTACT YOUR PHYSICIAN OR LICENSED HEALTH CARE PROFESSIONAL.
8. IMPORTANT SAFETY WARNINGS
Warning: indicates a potentially hazardous situation, which, if not prevented, could
result in minor or moderate injury to the user or patient or damage the device.
Supervision of an adult is required for testing elderly subjects, children and
differently abled persons
The manufacturer cannot be held responsible for damage caused by the failure of
the user to follow these instructions correctly.

Smart One
Rev.4.0.1
User Manual
Page 18 of 35
Only original accessories as specified by the manufacturer must be used with the
device.
Periodically check that no impurities or foreign bodies, such as skin, hairs have
deposited inside the turbine. This may cause errors in measurement or compromise
the correct functioning of the device.
Do not drop the device or treat it roughly in any way. Avoid strong vibration. Do not
expose the device to extreme temperature, humidity, dust, sand, or chemical
substances, or direct air currents (e.g. wind), sources of heat or cold, direct sunrays
or other sources of light or energy.
Use and store the device in compliance with the environmental conditions specified
in the Technical Specifications. If the device is subjected to environmental
conditions other than those specified, it may malfunction and/or display incorrect
results.
The maintenance operations set out in the User Manual must be carried out with
the utmost care. Failure to follow the instructions may lead to measurement errors
or misinterpretation of the measured values.
Do not modify the device without authorization from the manufacturer. All
modifications, adjustments, repairs, reconfigurations must be performed by the
manufacturer or by authorized personnel. In case of problems, do not try to repair
the device yourself.
8.1 Data security warnings
Your smartphone stores your personal data.
Potential threats such as the following:
• Malware installation
• Physical access to the smartphone
• Interception of communications
• Physical damage to the smartphone
• Theft of the smartphone
could have an impact on the integrity or confidentiality of such data, such as:
• Accessing data in memory by unauthorized persons
• Loss of data in memory
• Inability to use smartphone for communications
Smart One
Rev.4.0.1
User Manual
Page 19 of 35
• The integrity check of the data is made automatically and in case of transmission error
it will create a corruption of the data and the file will be illegible
The following actions help reduce the risk of such events:
• Do not open or install files from suspicious sources
• Use antivirus software
• Back up your data periodically
• Do not leave your smartphone unattended
• Use a password to access the data
• Verify the correct Email address where to send the test results
• When data are transmitted call the physician to ask for confirmation of receipt
8.2 Warnings for use in electromagnetic environments
Due to the increasing number of electronic devices (computers, cordless phones, cell
phones, etc.) medical devices may be susceptible to electromagnetic interference from
other equipment. Such electromagnetic interference could cause the medical device to
malfunction, as well as a measurement accuracy lower than the one stated in paragraph 11,
and create a potentially unsafe situation.
SMART ONE complies with EN 60601–1–2:2015 on electromagnetic compatibility (EMC for
medical devices) for both immunity and emissions.
For the device to function properly, however, the following precautions must be taken:
• Make sure that the SMART ONE and the smartphone on which the MIR SMART ONE
APP is installed are no more than 2 metres apart.
• Do not use SMART ONE near other devices (computers, cordless phones, cell phones,
etc.) that generate strong electromagnetic fields. Keep such equipment at a minimum
distance of 30 cm. If a use under lower distances is necessary, SMART ONE and the
other devices should be kept under observation to verify their normal function.
8.3 Notes on fcc certification
SMART ONE complies with Part 15 of the FCC Rules. Operation is subject to the following
conditions:
(1) this device may not cause harmful interference

Smart One
Rev.4.0.1
User Manual
Page 20 of 35
(2) this device must accept any interference received, including interference that may cause
undesired operation.
Any modifications not expressly approved by this Company could compromise use of the
device by the user.
N.B.: This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference when the equipment is operated in a residential
installation. This equipment generates, uses, and can radiate radio frequency energy and, if
not installed and used in accordance with the instructions, may cause harmful interference
to radio communications. However there is no guarantee that interference will not occur. If
this device does cause interference to radio or television reception, which can be
determined by turning the device off and on, the user is encouraged to correct the
interference by taking one of the following measures:
• Reorient or relocate the antenna
• Increase the distance between the equipment and receiver
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected
• Consult the dealer or an experienced radio/television technician for help.
9. CARE AND CLEANING
SMART ONE is a device that requires little maintenance. The following operations are to be
performed regularly:
• Cleaning of the reusable turbine
• Cleaning of the mouthpiece
• Cleaning of the device
• Replacing batteries
9.1 Cleaning of the reusable turbine
To avoid irreparable damage to the turbine, do not use any alcoholic or oily cleaning
solutions, and do not immerse in hot water or solutions. Do not try to sterilize the
turbine in boiling water. Never try to clean the turbine under a direct jet of water or

Smart One
Rev.4.0.1
User Manual
Page 21 of 35
other liquids. If there are no liquid detergents, the turbine must at least be washed in
clean water.
Correct operation of the turbine is guaranteed only if it is "clean" and free of foreign objects
that affect its movement. The presence of dust or foreign bodies (such as hairs, sputum etc.)
could slow or block the moving parts of the turbine and make the result less accurate, or
damage the turbine itself. After each use, check the cleanliness of the turbine.
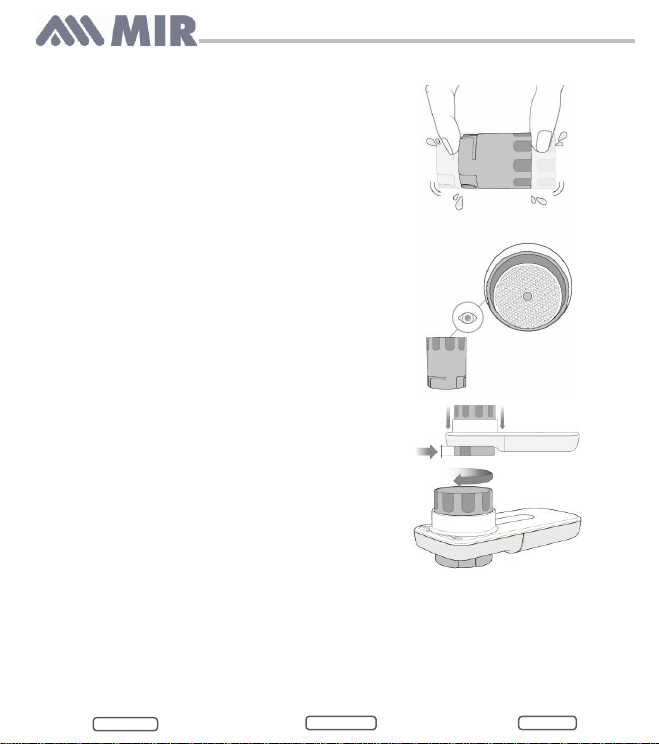
To clean the turbine, follow the steps below:
1) Remove the turbine from its housing by
turning anti-clockwise and apply light
pressure with your fingers from the bottom
of the turbine to lift it out of its housing.

Smart One
Rev.4.0.1
User Manual
Page 22 of 35
2) Mix ¾ cup of Clorox™ bleach (7.5%) in one
quart of water. Place orange turbine in
solution.
3) Shake the turbine to remove all impurities
for at least 1 minute.
4) Let the turbine soak for 15 minutes.
5) Clean the turbine by immersing it in clean
(not hot) water for at least 1 minute.
Smart One
Rev.4.0.1
User Manual
Page 23 of 35
6) Remove excess water from the turbine by
shaking it and let it dry by placing it
vertically on a dry surface
7) Check that it is clean and free of any foreign
bodies.
8) After cleaning, insert the turbine into the
socket in the direction indicated by the
closed padlock symbol screen-printed on
the SMART ONE device. To insert the
turbine correctly, push it down and turn it
clockwise until it stops, to ensure that it is
fully inserted into the plastic housing.
9.2 Cleaning of the mouthpiece
Be sure to clean the mouthpiece after each use, as outlined in the instructions below.

Smart One
Rev.4.0.1
User Manual
Page 24 of 35
1) To clean the nozzle, simply remove it from
the turbine.
2) Immerse the mouthpiece in warm water
3) Shake the mouthpiece for 2-3 minutes.

Smart One
Rev.4.0.1
User Manual
Page 25 of 35
4) Rinse it in clean water.
5) Shake it gently to remove any excess water.
6) Let it dry on a cloth.
Afterwards, insert the mouthpiece into the
turbine with gentle pressure.
9.3 Cleaning of the device
Clean the device once a day. To clean, wipe the device’s surfaces with a soft damp cloth. Dry
with a soft cloth, or allow to air dry. Ensure that all surfaces are completely dry.
Never put the device into water or other fluids.

Smart One
Rev.4.0.1
User Manual
Page 26 of 35
9.4 Replacing batteries
The device continuously monitors the battery level. A message on the smartphone display
alerts the user when the device battery is low. On fully charged batteries, the device is
expected to work for 5 years or 1000 tests whichever comes first.
Used SMART ONE batteries should only be disposed of in special containers or
preferably returned to the dealer of the device or to a special collection centre. In any
case, all applicable local regulations must be complied with.
Remove the battery cover on the back
of the SMART ONE
2
Remove the two batteries and replace
them with two new ones, following the
polarity as indicated by the symbols in
the compartment
3
Reattach the battery cover

Smart One
Rev.4.0.1
User Manual
Page 27 of 35
10. ERROR MESSAGES
If you encounter any problems when using the SMART ONE, a message will appear on the
smartphone display to warn of the malfunction.
MESSAGE
POSSIBLE CAUSE
REMEDY
Bluetooth
Bluetooth is off
To perform measurements with the
device, you must activate Bluetooth
on the smartphone. Exit the app and
activate Bluetooth from the
smartphone settings menu
Battery low
When the SMART ONE
batteries are below 15%
Replace the SMART ONE batteries
It seems that you
have not configured
an Email account
The user wants to share
the results of the tests, but
has not configured an
Email account on the
smartphone
Set up an Email account from the
smartphone settings menu
11. TROUBLESHOOTING
If you receive an unusually low reading, it could mean that your SMART ONE meter is
broken, or it could mean that the reading is accurate and your asthma is getting worse.
Check to make sure that the meter is not broken. You must follow directions exactly as
instructed to obtain accurate results. If your meter is not broken, follow the instructions in
your action plan for low readings and contact your physician or other licensed healthcare
professional.
If you have any questions regarding the use of this device, please ask your physician or other
licensed healthcare professional, or contact MIR USA, Inc.
Toll free number: 844–464–7872.
USA:
Call MIR USA 1-844-4MIRUSA (1-844-464-7872), Monday to Friday 8 AM to 5 PM (central
time), OR CONTACT US AT mirusa@spirometry.com, OR WRITE US AT MIR USA, Inc. 5462 S.
Westridge Drive New Berlin, WI 53151 – USA.
Smart One
Rev.4.0.1
User Manual
Page 28 of 35
EUROPE and WORLDWIDE:
Call MIR +39 06 22754777, Monday to Friday 8 AM to 5 PM (GMT+1), OR CONTACT US AT
mir@spirometry.com, OR WRITE US AT MIR Via del Maggiolino 125, 00155 Roma, Italy.
If problems occur when using the device, the following points should be checked.
MALFUNCTION
POSSIBLE CAUSE
REMEDY
SMART ONE can’t
connect with the
smartphone
The Bluetooth connection
is not working properly
Look for SMART ONE on the list of
recognized devices. For correct use,
the smartphone needs Bluetooth
version 4.0 or higher
The test results are
unreliable
The turbine may be dirty
Clean the turbine as described in the
Maintenance section. If necessary,
replace the turbine with a new one. If
necessary, contact the manufacturer
The test was performed
wrongly
Repeat the test, following the
directions on the smartphone screen.
Avoid sudden movements when you
finish exhaling. Discuss the value with
your physician
The turbine has not been
inserted properly
Insert the turbine from the front of the
device by pushing it all the way down
and turning it clockwise
12. ACCURACY AND RELIABILITY
This device meets the requirements of the following standard:
• ATS Standardization of Spirometry 2005, 2019 update
• ISO 23747: 2015
• ISO 26782: 2009
Volume max 10 L
Volume accuracy 2.5% or 0.05 L whichever is greater
Peak Flow max 960 L/min (16 L/s )
Smart One
Rev.4.0.1
User Manual
Page 29 of 35
Peak Flow accuracy 10% or 20 L/min (0.33 L/s ) whichever is greater

Smart One
Rev.4.0.1
User Manual
Page 30 of 35
13. LABELS & SYMBOLS
Symbols are described in the table below
SYMBOL
DESCRIPTION
Model:
Product Name
SN
Device serial number
Manufacturer’s name and address
0476
This product is a certified Class IIa medical device, and complies with the
requirements of Regulation (EU) 2017/745 for medical devices.
In accordance with IEC 60601–1 the product and its applied parts are type BF and
thus protected against the risks of electrical leakage.
This symbol is required by European directive 2012/19/EEC on waste electrical and
electronic equipment (WEEE). At the end of its useful life this device must not be
disposed of as normal domestic waste. Instead it must be delivered to a WEEE
authorised collection centre. As an alternative, the device may be returned without
charge to the dealer or distributor, when it is replaced by another equivalent
device.
Due to the construction materials used for the device, disposal as normal waste
could cause harm to the environment and/or health. Failure to observe these
regulations can lead to prosecution.
IP22
Indicates the degree of resistance to liquids. The device is protected against falling
drops of water with a maximum inclination of 15° from vertical.
The symbol is used for products including RF transmitters.
FCC ID
Identification showing traceability to FCC compliance
Instruction for use symbol. Read this manual carefully before using the medical
device
Manufacturing date

Smart One
Rev.4.0.1
User Manual
Page 31 of 35
SYMBOL
DESCRIPTION
Temperature limits: indicates the temperature limits to which the medical device
can be safely exposed
Humidity limitation: indicates the range of humidity to which the medical device
can be safely exposed
The symbol indicates that the product is a medical device
The symbol indicates the Unique Device Identification
SMART ONE complies with the Essential Requirements of Directive 93/42/EEC on Medical
Devices. This statement is made on the basis of CE Certificate no. MED 9826 issued by Kiwa
Cermet, Notified Body no. 0476.
14. TECHNICAL SPECIFICATIONS
Peak Expiratory Flow
PEF (L/min)
Forced Expiratory Volume in 1 second
FEV1 (L)
Measurement system
Bi–directional turbine (rotating blade)
Measurement principle
Infrared interruption
Peak Flow max
PEF 960 L/min (16 L/s)
Volume max
FEV1 10 L
Volume accuracy (ATS 2019)
2.5% or 0.05 L whichever is greater
Peak Flow accuracy
10% or 20 L/min ( 0.33 L/s ) whichever is
greater
Dynamic resistance at 12 L/s
<0.5 cm H2O/L/s
Communication interface
Bluetooth SMART (4.0 or higher)
Power supply
2 x 1.5V AAA alkaline batteries
Size
Main body 109x49x21 mm
Weight
60.7 g (including batteries)
Type of electrical protection
Internally powered
Electrical protection level
BF
IP protection level
IP22
Regulations applicable
ATS/ERS Guidelines: 2005, 2019 update

Smart One
Rev.4.0.1
User Manual
Page 32 of 35
ISO 26782: 2009
ISO 23747: 2015
ISO 14971: 2019
ISO 10993-1: 2018
2011/65/UE Directive
EN ISO 15223-1:2021
IEC 60601-1:2005 + A1: 2012
EN 60601-1-2: 2015
EN IEC 60601-1-6: 2010+Amd2013
EN 60601-1-11: 2015
IEC 62304:2006/A1:2015
Directive 2014-53-EU-RED
Conditions of use
Device for continuous use
Storage conditions
Temperature: MIN –25°C, MAX +70°C
Humidity: MIN 10% RH; MAX 93%RH
Transport conditions
Temperature: MIN –25°C, MAX +70°C
Humidity: MIN 10% RH; MAX 93%RH
Operating conditions
Temperature: MIN +5°C, MAX +40°C
Humidity: MIN 15% RH; MAX 93%RH
SMART ONE complies with the Basic Requirements of Regulation (EU) 2017/745 for medical
devices.
15. BLUETOOTH WIRELESS TECHNOLOGY INFORMATION
Bluetooth Compliance:
Bluetooth 5-Ready
Operating Frequency:
2.4 to 2.4835 GHz
Max Output Power:
TX: 0 dBm; 1 mW
Operating Range:
10 meter radius (line of sight)
Network Topology:
Star – bus
Operation:
Server
Antenna Type:
Antenna integrated in the module
Modulation Technology:
FHSS
Modulation Type:
GFSK
Data Rate:
1 Mbit/second
Data Latency:
7 – 40 ms

Smart One
Rev.4.0.1
User Manual
Page 33 of 35
Data Integrity:
Adaptive frequency hopping, Lazy Acknowledgement, 24-bit
CRC, 32-bit Message Integrity Check Data
Format:
Sends data packets once per 60 ms. Includes 3 control bytes
that allows the host to detect if packets are missing and the
device to retransmit.
Quality of Service:
This device uses Bluetooth Smart technology for wireless
communications, which allows for reliable communications in
electrically noisy environments and transmits packets once
per 60 ms.
It includes 3 control bytes that allows the host to detect if
packets are missing and the device to re-transmit. If the
connection is lost, the App changes the connected status
from connected to disconnected and become available for a
connection immediately.
Bluetooth Profiles
Supported:
GATT-based profile
Authentication and
Encryption:
Supported
Encryption Key Size:
128-bit AES with Counter Mode CBC-MAC and application
layer user defined
The Bluetooth® word mark and logo are registered trademarks owned by Bluetooth SIG, Inc.
15.1 Radio frequency (rf) communication
This device complies with the United States Federal Communications Commission (FCC) and
international standards for electromagnetic compatibility. The following information is
provided in accordance with Federal Communications Commission (FCC) regulations.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) This device may not cause harmful interference, and (2) this device must
accept any interference received, including interference that may cause undesirable
operation. This device does not interfere with any radio frequency signals transmitted from
outside sources. These FCC standards are designed to provide reasonable protection against
excessive radio frequency interference and prevent undesirable operation of the device
from unwanted electromagnetic interference.

Smart One
Rev.4.0.1
User Manual
Page 34 of 35
15.2 Radio frequency (rf) interference from other wireless devices
Common consumer electronic devices that transmit in the same frequency bands used by
the Smartone may prevent the uploader or mobile device from receiving data.
This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment
generates, uses and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception, which
can be determined by turning the equipment off and on, the user is encouraged to try to
correct the interference by increasing the separation between the equipment and receiver.
16. WARRANTY TERMS
SMART ONE is guaranteed for a period of 12 months in the case of professional use
(physician, hospital, etc.) or 24 months for other use. The warranty period is effective from
the date of purchase, which must be proven by an invoice or sales receipt. The device must
be checked at the time of purchase, or upon delivery, and any claims must be made
immediately in writing to the manufacturer.
This warranty covers the repair or the replacement (at the discretion of the manufacturer)
of the product or of the defective parts without charge for the parts or for the labour. All
batteries and other consumable parts, including the turbine flow meter, are specifically
excluded from the terms of this guarantee.
The product warranty shall not apply, at the discretion of the manufacturer, in the following
cases:
• Improper handling, improper installation, improper operation of the device, or if the
installation does not comply with local technical or safety regulations
• Use of the product for purposes other than those provided or failure to follow
instructions
• Repair, adaptation, modification or tampering by third party
• Damage caused by lack of or incorrect maintenance
• Damage caused by abnormal physical or electrical stress, or by leaking batteries
• Serial number altered, deleted, removed or rendered illegible

Smart One
Rev.4.0.1
User Manual
Page 35 of 35
The repair or replacement described in this warranty is provided for goods returned at the
customers' expense to certified service centres authorized by manufacturer. For details of
these centres please contact either your local supplier or the manufacturer. Any unautorized
opening of the device invalidates all guarantee claims.
Customer shall be responsible for all transport, customs and delivery charges regarding the
goods. Each product, or accessory, sent in for repair must be accompanied by a clear and
detailed explanation of the fault. Forwarding to the manufacturer requires the written
permission of the manufacturer himself.
The manufacturer – MIR MEDICAL INTERNATIONAL RESEARCH S.p.A. – reserves the right
to replace the product or make any changes deemed necessary.
 Loading...
Loading...