For In Vitro Diagnostic Use Only
【Packing Specifications】
Type A
SARS-CoV-2 Antigen Rapid Test Kits for Self-testing
No. Catalogue number Spec.
1
CG3601 1 test / kit 1 CG4401 1 test / kit
2
CG3602 2 tests / kit 2 CG4402 2 tests / kit
3
CG3605 5 tests / kit 3 CG4405 5 tests / kit
4
CG3610 10 tests / kit 4 CG4410 10 tests / kit
5
CG3625 25 tests / kit 5 CG4425 25 tests / kit
6
CG3650 50 tests / kit 6 CG4450 50 tests / kit
(Colloidal Gold Immunochromatography)
No. Catalogue number Spec.
Type B
0197
EN
【Product name】
SARS-CoV-2 Antigen Rapid Test Kits for Self-testing (Colloidal Gold Immunochromatography)
【Intended Use】
This product is a rapid, lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 nucleocapsid
antigens from anterior nasal swabs that are self-collected by an individual aged 18 years or older or are collected by an
adult from an individual younger than 18 years old. This test is intended for use in individuals with symptoms or other
epidemiological reasons to suspect a COVID-19 infection.
This product is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
Results are for the identification of the SARS-CoV-2 nucleocapsid protein antigen. The antigen is generally detectable
in anterior nasal swab specimens during the acute phase of infection. Positive results indicate the presence of viral
antigens, but the clinical correlation with past medical history and other diagnostic information is necessary to
determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The
agent detected may not be the definite cause of disease. Negative results should be treated as presumptive and
confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not
rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions
including infection control decisions. Negative results should be considered in the context of a patient’s recent
exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
Individuals who test negative and continue to experience COVID-like symptoms should seek follow up care from their
healthcare provider.
【Introduction 】
Coronavirus, as the broad family of viruses, is a single strand plus RNA virus with an envelope. The virus is known to
cause major diseases such as cold, Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory
Syndrome (SARS). The core protein of SARS-CoV-2 is N protein (Nucleocapsid), which is a protein component
inside the virus. It is relatively conservative among β-coronaviruses and is commonly used as a diagnostic tool for
coronavirus. As the key receptor for SARS-CoV-2 to enter cells, ACE2 is significant for the study of viral infection
mechanisms.
【Principle 】
The current test kit is based on specific antibody-antigen reaction and immunoassay technique. The test strip consists
of gold marked pad (coated with gold-marked SARS-CoV-2 N protein mouse anti human monoclonal antibody),
sample pad, NC membrane (paired SARS-CoV-2 N protein mouse anti human monoclonal antibody coated on the test
line (T) and goat anti-mouse IgG polyclonal antibody coated on the quality control line (C)) and absorbing paper.
During the test, the N protein in the specimen binds to the gold-marked SARS-CoV-2 N protein mouse anti human
monoclonal antibody pre-coated on the gold marked pad, and the conjugate moves upward under the capillary effect,
and then is trapped by N protein mouse anti human monoclonal antibody conjugate fixed in the Test Line (T). The
higher the N protein content in the specimen, the more conjugates are trapped, and the darker the color of the Test
Line (T). If there is no SARS-CoV-2 in the specimen or the virus content is below the detection limit, no color appears
in the Test Line (T). A purple-red band will appear in the Control Line (C) regardless of whether there is a virus in the
specimen. The purple-red band that appears in the Control Line (C) is the criteria for determining whether there is
enough specimen and whether the chromatography process is normal.
【Main Components】
The product includes test cards, instruction for use, operation card, disposable sterile swabs and sample treatment
solution. Each reagent kit contains 1 novel coronavirus (SARS-CoV-2) antigen test card and 1 bag of desiccant.
Disposable sterile swab information:
Nasal Swab could be provided based on customer’s requirement.
Name
Disposable sterile swab information Nasal swab
0123 MDD 93/42/EEC
Manufacturer 1: Zhejiang Gongdong Medical Technology Co., Ltd.Beicheng Industrial Area 318020 Huangyan China
0197 MDD 93/42/EEC
Manufacturer 2: Jiangsu Changfeng Medical Industry Co., Ltd. Touqiao Town,Guangling District Yangzhou 225109
Jiangsu China
0197 MDD 93/42/EEC
Manufacturer 3: Shenzhen KangDaAn Biological Technology Co., Ltd.Liuxiandong industrial zone,Xili street
Nanshan district,Shenzhen 518055 Guangdong China
0413 MDD 93/42/EEC
Manufacturer 4: Medico Technology Co., Ltd.Zhangbei Industrial Park, Longcheng Street, Longgang district,
Shenzhen, 518100 Guangdong, China
0197 MDD 93/42/EEC
Manufacturer 5: Goodwood Medical Care Ltd. 1-2 Floor, 3-919, Yongzheng Street, Jinzhou District, Dalian 116100
Liaoning, China
1 test / kit 1 test / kit 1 test
2 tests / kit 2tests / kit 2tests
5 tests / kit 5 tests / kit 5 tests
10 tests / kit 10 tests / kit 10 tests
25 tests / kit 25 tests / kit 25 tests
50 tests / kit 50 tests / kit 50 tests
Spec.
Spec.
Test card Instruction manual Operation card Sample treatment solution Swabs
1
1 1
1
1
1
1
1
1
1
1
1
Application
300μl×1 1 piece
300 μl×5 5 pieces
300 μl×10 10 pieces
300 μl×25 25 pieces
300 μl×50 50 pieces
300μl×2 2 pieces
Test card consists of paper shell, test strip, sample well and adhesive tape. The test strip, sample well and adhesive
tape are attached on the paper shell.
The test strip consists of gold marked pad (coated with gold-marked SARS-CoV-2 N protein mouse anti human
monoclonal antibody), sample pad, NC membrane (paired SARS-CoV-2 N protein mouse anti human monoclonal
antibody coated on the test line (T) and goat anti-mouse IgG polyclonal antibody coated on the quality control line (C))
and absorbing paper.
The main compositions of sample treatment solution include tris, tritonX-100, sodium caseinate.
【General description】
The SARS-CoV-2 Antigen Rapid Test Kits for Self-testing (Colloidal Gold Immunochromatography) contains 3 core
elements for operation:
Test card: Test card which is book-shaped hinged test cardboard containing the test strip (for single use)
Sample Treatment Solution: Bottle containing sample treatment solution (for single use)
Nasal Swabs: Sterile swab (for single use)
1 Test Card
1 Sample Treatment Solution(buffer)
1 Swab
【Material required but not provided 】
Clock or timer or stopwatch, Waste container
【Storage Conditions and Validity Period】
1. The test kit should be stored in a dry and dark place with temperature of 4-30℃, valid for 18 months.
2. The validity period of the test card is 1 hour after opening its inner package and it is suggested that the storage
temperature should be 4 ~ 30 ℃ and the humidity should not exceed 70%.
3. The sample treatment solution should be used immediately after opening. See package label for date of
manufacture and expiration.
【Specimen Requirements 】
This test kit is suitable for testing human anterior nasal swab specimens:
Specimen collection: During the collection process, relevant personnel should be well protected to avoid direct contact
with the specimen. In case of accidental contact, timely disinfection should be carried out and necessary measures
should be taken.
Anterior nasal swab specimen collection: During sampling, the nasal swab head should be entirely inserted into the
nasal cavity until you feel resistance (about 2-3cm), and gently rotated 5 times. When it was removed, specimen
should be taken in the same way in the other nasal cavity to ensure the collection of enough specimens.
Specimen preservation:
After the specimen are
collected, please test
immediately after
sampling. Do not complete
the test over 1 hour.
Swab Left Nasal Cavity Swab Right Nasal Cavity
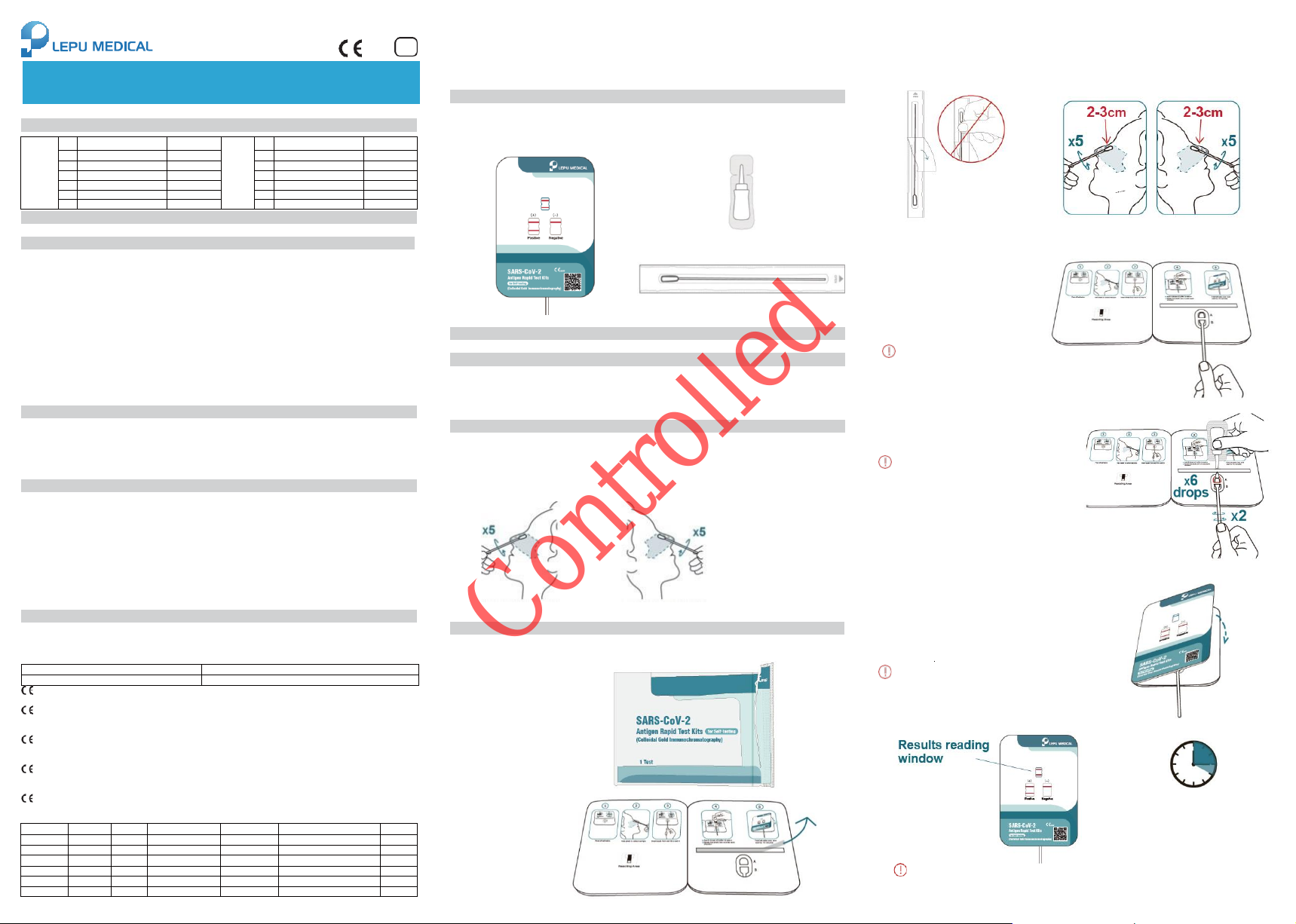
【Test Method】
Please read the instruction for use completely before performing any test, and use the reagents and specimens after
returning to room temperature.
1.Wash and dry hands.
Then take out test card
from outer package.
2.Place test card flat
on table, remove
cover-layer of
adhesive.
3. Take out swab from stick-end, refer to standard anterior nasal swab specimen collection to collect
specimen: The nasal swab head should be entirely inserted into the nasal cavity until you feel
resistance (about 2-3cm), and gently rotated 5 times. When it was removed, specimen should be
taken in the same way in another nasal cavity to ensure the collection of enough specimens.
*The length of anterior nasal cavity of users may be different in different regions, 2~3cm is only for reference. It is
recommended for user to insert swab until feel resistance.
Open swab package stick end Swab left nasal cavity Swab right nasal cavity
Note: Do not touch the swab head. Note: Sampling in both nasal cavity sample is required
4. Insert the swab head into well A
from the bottom of well B.
Keep the card flat on
5. Add 6 drops of the Sample Treatment
Solution to well A. Then rotate the swab for 2
rounds, each direction in the buffer.
Keep the card flat on
Note: False negative results may occur if the
sample swab is not turned before closing the
test card.
Note: Do not rotate the swab while dropping
the sample
Rotate clockwise and counterclock- wise twice
6.Fold the left side over, fit two sides together
completely, start timing.
Keep the card flat on table
7. Wait for the appearance of purple-red line. Test results should be read within 15-20 minutes.
15-20 min
Note: False results can occur if the card is
disturbed/moved.
Note: False results can occur if the test results
are read before 15minutes or over 20 minutes.
Keep the card flat on table Do not move the test card
8. After test, put the test card, swab, and sample treatment solution bottle into outer package and
seal it tightly. Dispose the bag in medical waste container according to local laws and regulations.

【Interpretation of Test Results】
preservatives)
Positive (+): A purple-red band appears in the Control Line (C) and Test Line (T).
A positive test result means that you may have Corona Virus Disease 2019 (COVID-19). It is important to inform your
health care provider of the results. Your healthcare provider will work with you to further confirm the diagnosis of
COVID-19 and determine how best to care for you based on your test result(s) along with your medical history,
symptoms and other related medical tests.
Negative (-): Only the Control Line (C) shows a
purple-red band. No purple-red band appears in the
A negative test result means that proteins from SARS-CoV-2 which causes COVID-19 were not found in your
sample.
Negative results are presumptive and confirmation with a molecular assay, if necessary, for patient management
may be performed. Negative results should be consider ed in the con text of an in dividual’s recen t exposures,
history and the presence of clinical signs and symptoms consistent with COVID-19.
Invalid: If “no purple-red band appears in Control Line (C)” and “a blue band appears in the Control Line (C)”, it
indicates that the operation process is incorrect or the test paper has been damaged. In this case, please read the
instruction manual carefully again and retest with a new test paper. If the problem persists, please stop using this
batch of products immediately and contact your local supplier.
Test Line (T).
【Limitations of the testing method】
1. The test results of this product should be combined with other clinical information and comprehensively judged
by physicians, and should not be used as the only criterion.
2. This product is only used to determine the novel coronavirus (SARS-CoV-2) antigen in the specimen.
3. A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
4. False negative results may occur if a specimen is improperly collected or handled.
5. Invalid results may occur if inadequate sample treatment buffer is used (e.g., <6 drops). False negative results
may occur if excessive sample treatment buffer is used (e.g., > 6 drops).
6. False negative results may occur if specimen swabs are not twirled within the test card.
7. False negative results may occur if swabs are stored in their paper sheath after specimen collection.
8. False negative results are more likely after seven days or more of symptoms.
9. This test detects both viable (live) and non-viable SARS-CoV-2. Test performance depends on the amount of
virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same
sample.
10. The performance of the SARS-CoV-2 Antigen Rapid Test Kits for Self-testing (Colloidal Gold Immunochro-
matography) was evaluated using the procedures provided in this product insert only. Modifications to these
procedures may alter the performance of the test.
11. The presence of high concentration mupirocin may interfere with the product and may cause false positive
results.
12. Positive test results do not rule out co-infections with other pathogens.
13. Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
14. Negative results do not rule out COVID-19 infection and it may be necessary to obtain additional testing with a
molecular assay, if needed for patient management.
15. Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
【Internal Quality Control 】
The product has a Test Line (T) and a Control Line (C) on the surface of the test card. Neither the Test Line (T) nor
the Control Line (C) is visible in the result window before applying a specimen. The control line is used for procedural
control and should always appear if the test procedure is performed properly and the test reagents of the control line
are working.
【Product Performance Index 】
1.Determination of the Limit of Detection
SARS-CoV-2 Antigen Rapid Test Kits limit of detection (LoD) was determined by evaluating different
concentrations of inactivated SARS-CoV-2 Virus culture medium. Negative natural nasal swab specimens were eluted in
6 drops of sample treatment solution. 20 Swab eluates were combined and mixed thoroughly to create a clinical
matrix pool to be used as the diluent. Inactivated SARS-CoV-2 Virus culture medium was diluted in this natural nasal
swab matrix pool to generate virus dilutions for testing.
The bottom line can be very faint Any pink/purple line visible here is positive.
The testing was performed according to the test procedure, with the virus dilutions applied directly onto the Swab to
prepare the contrived nasal swab samples.
The LoD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentra - tion
at which at least 19 out of 20 replicates tested positive). Based on this test condition, the laboratory experiment data
showed that the SARS-CoV-2 Antigen Rapid Test Kits forSelf-testing (Colloidal Gold Immunochromatography) LoD
in natural nasal swab matrix was confirmed 200 TCID50/mL.
2.Analysis of specificity
2.1 Cross-reaction:
concentration presented in the table below.
Human coronavirus HKU1 recombinant N protein 50μg/mL
MERS coronavirus recombinant N protein 50μg/mL
2.2 Interfering substances:
concentration presented in the table below.
3. Clinical performance
The clinical performance study for SARS-CoV-2 Antigen Rapid Test Kit was conducted in Germany. A total of 222
clinical samples were used to perform the test. The positive and negative samples were all confirmed by PCR. The
diagnostic sensitivity and diagnostic specificity of the product was 95.9% (90.8-98.2%) and 100% (96.3-100.0%)
respectively.
Results with correlation to Ct value of the positive samples were shown in the table below.
Ct Value Diagnostic sensitivity 95%CI
≤ 25 97.0 % 84.7-99.5%
≤ 30 96.2 % 88.3-98.7%
≤ 36 95.9 % 90.8- 98.2%
* All the data above only represent the results of this clinical performace study in Germany.
No cross-reactivity was seen with the following microorganisms when tested at the
Potential Cross-Reactant Test Concentration
Human coronavirus OC43 10 5 TCID50/mL
Human coronavirus 229E 10 6 TCID50/mL
Human coronavirus NL63 10 5 TCID50/mL
adenovirus 10 6 TCID50/mL
Human metapneumovirus(hMPV) 10 6 TCID50/mL
Parainfluenza virus 1 10 7 TCID50/mL
Parainfluenza virus 2
Parainfluenza virus 3 10 6 TCID50/mL
Parainfluenza virus 4 10 7 TCID50/mL
Influenza A 10 6 TCID50/mL
Influenza B 10 6 TCID50/mL
Enterovirus(EV68) 10 7 TCID50/mL
Respiratory syncytial virus
Rhinovirus 10 6 TCID50/mL
Measles virus 10 5 TCID50/mL
Varicella zoster virus 10 5 TCID50/mL
Haemophilus influenzae 10
Chlamydia pneumoniae 10 6 CFU/mL
Legionella pneumophila 10
Mycobacterium tuberculosis 10
Streptococcus pneumoniae 10
Streptococcus pyogenes 10
Bordetella pertussis 10
Mycoplasma pneumoniae 10
Candida albicans 10
Staphylococcus epidermidis 10
Staphylococcus aureus
Pneumocystis giraldii 10 6 CFU/mL
Staphylococcus salivarius 10
Combined human nasal Lotion
Potential Interfering substances Test Concentration
Oxazole (nasal spray) 15%
Sodium chloride (containing
Strepsils (flurbiprofen 8.75mg) 5%
Throat candy (Mint) 5%
Naso GEL (NeilMed) 5%
No interference was seen with the following substances when tested at the
Mucin 0.5%
Human whole blood
HAMA 60 ng/mL
Biotin 1.2μg/mL
Benzocaine 2 mg/mL
Zanamivir 18μg/mL
Ribavirin 25μg/mL
Lopinavir 20μg/L
Ritonavir 18μg/mL
Acetylsalicylic acid 2 mg/dL
Ibuprofen 25 mg/dL
Tobramycin 16μg/mL
Phenylephrine 15%
Fluticasone 5%
Beclomethasone 2μg/mL
Budesonide 4ng/mL
Mometasone 2ng/mL
10 5 TCID50/mL
10 5 TCID50/mL
7
CFU/mL
7
CFU/mL
7
CFU/mL
7
CFU/mL
7
CFU/mL
7
CFU/mL
8
CFU/mL
7
CFU/mL
7
CFU/mL
7
10
CFU/mL
7
CFU/mL
/
4%
10 mg/mL
【Warnings and Precautions】
1. For in vitro diagnostic use only. The product can be used for self-testing.
2. Do not eat or smoke while handling specimens.
3. The temperature and humidity of the experimental environment should be avoided to be too high, the reaction
temperature should be 15-30°C and the humidity should be below 70%.
4. The packaging bag contains desiccant, do not eat.
5. It is recommended to test in a well-lit environment.
6. Before testing, please wash hands or wear clean gloves.
7. Please do not use the test card with damaged card bag packaging, unclear marking or beyond the expiration
date.
8. A test card should be used within 1 hour after it is taken out from the aluminum foil bag.
9. Users shall take samples according to the instruction manual. Inadequate or inappropriate sample collection
may yield error results and retesting with a new test may be required. Particular attention needs to be paid to
appropriate sample collection technique.
10. Remove the covering layer of double-sided adhesive to prevent liquid splashing before testing.
11. Do not drop the dilution buffer into the wrong well.
12. In the process of testing, the test card should be placed on a horizontal table, and it should not be moved.
13. If the buffer solution makes contact with the skin or eye, wash/ flush with a large volume of water. If skin
irritation, rash or other abnormal reaction occurs, please get medical advice/attention.
14. Avoid splashing or aerosol formation of specimen and buffer.
15. All users have to read the instruction prior to performing a test.
16. Do not mix or interchange different specimens.
17. Do not mix reagent of different lots or those for other products.
18. To avoid contamination, do not touch the head of provided swab when opening the swab pouch.
19. To avoid cross-contamination, do not reuse the sterilized swabs for specimen collection.
20. Do not dilute the collected swab with any solution except for the provided extraction buffer.
21. Keep foreign substances away from the test during the testing process. Contact with foreign substances,
specifically bleach, may result in an incorrect test result.
22. Nasal swabs are not recommended for anyone who is prone to nosebleeds or has had facial or head
injury/surgery in the last 6 months.
23. Patients with severe allergic rhinitis may have false positive results.
24. For patients with severe dry nasal mucosa, the sample volume may be insufficient due to the serious shortage of
nasal secretions, resulting in inaccurate results.
25. Do not refrigerate or use after the expiration date (see packaging bag for expiration date).
26. Dispose of used specimens, test cards and other waste into waste container in accordance with relevant local
laws and regulations.
27. It is suggested that the test should be performed in the company of people with normal vision for abnormal
color vision users.
【Explanation of Symbols】
DO NOT USE IF
PACKAGE IS DAMAGED
DO NOT REUSE
TEMPERATURE LIMIT
MANUFACTURER
CONSULT
INSTRUCTIONS FOR USE
USE-BY DATE
DATE OF MANUFACTURER
BATCH CODE
KEEP AWAY FROM SUNLIGHT
IN VITRO DIAGNOSTIC
MEDICAL DEVICE
AUTHORIZED
REPRESENTATIVE
IN THE EUROPEAN
COMMUNITY
【Basic Information】
【Date of Approval and Revision of the Manual】
Approved on 01 April 2022;
Version number: CE-EN-CG36-In-002 A3
Beijing Lepu Medical Technology Co., Ltd.
Building 7-1, No.37 Chaoqian Road, Changping District, 102200 Beijing, China
Tel: +86-10-80123964
Email: lepuservice@lepumedical.com Web: en.lepumedical.com
Lepu Medical (Europe) Cooperatief U.A.
Abe Lenstra Boulevard 36, 8448 JB, Heerenveen,The Netherlands Tel: +31-515-573399 Fax:
+31-515-760020
0197
KEEP DRY
CE MARK
CATALOGUE NUMBER
 Loading...
Loading...