
PROFESSIONAL MEDICAL PRODUCTS
MISURATORE DI PICCO DI FLUSSO
PEAK FLOW METER
SPIROMÈTRE DE CRÊTE
MEDIDOR DE FLUJO ESPIRATORIO MÁXIMO
MEDIDOR DE PICO DE FLUXO
PEAK-FLOW-METER
TOPPFLÖDESMÄTARE
ΡΟΟΜΕΤΡΟ
DL-F03 (33679)
DL-F04 (33680)
Taian Dalu Medical Instrument Co., Ltd.
West Part of Yitianmen Street, Hi-tech Zone,
Taian, 271000 Shandong, P.R. China
Made in China
Lotus NL B.V. Koningin Julianaplein 10
1e Verd, 2595AA, The Hague, Netherlands
Importato da / Imported by / Importé par / Importado por / Importado por
Importiert von / Importerad av / Εισαγωγή από:
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
M33679-M-Rev.0-11.21
0197

ENGLISH
Please read all the information in this manual before using the Peak Flow Meter.
Precaution:
1. The Peak Flow Meter is recommended for single patient use.
2. The Peak Flow Meter should be used under the supervision of a licensed healthcare professional.
3. If the PEF is used for longer than its specied life, the accuracy of the device may deteriorate.
Note: Patients symptoms take precedence over Peak Flow Meter readings.
Setting Action Zones
Zone markers may be set with a stylus by the healthcare professional to give personal boundaries for implementing
the asthma action plan.
To Use Peak Flow Meter
1. Slide pointer to the bottom of the scale.
2. Hold device horizontally in front of your mouth.
3. Inhale as deeply as possible.
4. Seal your lips rmly around the mouthpiece.
5. Blow out as HARD and as FAST as possible for a second or longer.
6. Take care that tongue or teeth do not obstruct the airow. A “spitting” action or coughing will give false readings.
7. Take note of the reading.
8. Repeat steps 1 to 7 three times and record the highest reading.
9. Take action according to your personal asthma management plan.
Cleaning
Clean the outer surfaces after use. We recommend using an ordinary alcohol wipe (IPA 70%). Pay special attention
to the mouthpiece area.
Indications for Use
Device measures peak expiratory ow (PEF) for use on adults and paediatrics, 5 years and older, in primary care, clinics, hospitals and at home, under the supervision of a healthcare professional. PEF can be used to monitor progress
in treatment of asthma, determine the severity of the asthma, check response to treatment during an acute asthma
episode, detect worsening lung function and avoid a possible severe asthma are-up.
Use in Clinic
When used for multiple subjects always use a disposable mouthpiece. The Peak ow meter should be externally
disinfected using an IPA wipe (70%) between each patient, and should be disposed of after 30 patients or 2 weeks,
whichever is the earlier.
Contraindications, Warnings, Cautions and Adverse Reactions
• This device is intended to help you assess how well your asthma is under control, by measuring your peak ow which
can then be monitored over time.
• Never attempt to dismantle the unit. This can cause faulty Peak Expiratory Flow scores.
• If you think that the device is not reading correctly, advise your healthcare professional immediately
• Any serious incident that has occurred in relation to the device should be reported to Taian Dalu or its Authorized
Representative and the Regulatory Authorities of the country.
Store
Avoid crushing the unit. Store in a clean dry place.
Disposal
The peak ow meter and its accessories constitute minimally soiled waste from human healthcare and should be
disposed of in line with local requirements.
Shelf Life
3 years
Technical Specications
Product: Peak Flow Meter
Model Number: DL-F03, DL-F04
Material: ABS plastic
Accuracy: ± 10L/min or ± 10% of the reading, whichever is greater
Repeatability: ±10 L/min or ± 5% of the reading, whichever is greater.
Frequency response: Prole A/B difference less than ±15 L/min or ±12%, whichever is greater
Highest resistance to ow: 205Pa/l/s @ 100 L/min
Measurement Range: DL-F03: 60~800L/min,
DL-F04: 50~400L/min

ENGLISH
Operating Temperature Range: Limits: 17-37°C.
Design Limits: 10-40°C
Peak Flow Meter performance standards: EN ISO 23747:2015
Size/Weight
Model DL-F03: L155mm*W48mm*H28mm/ 55g
Model DL-F04: L155mm*W48mm*H28mm/ 55g
Package Contents
Peak Flow Meter, Instructions for Use
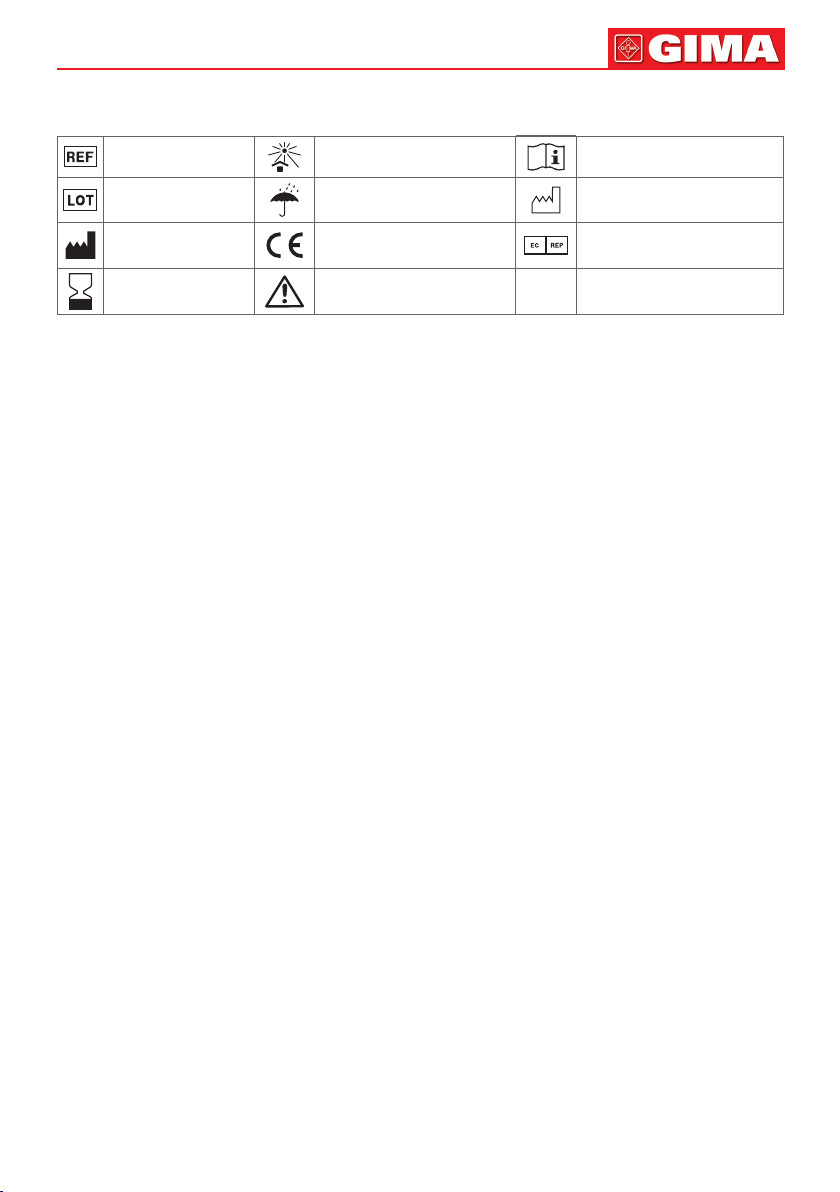
Symbols meaning
ENGLISH
Product code Keep away from sunlight
Lot number Keep in a cool, dry place Date of manufacture
Manufacturer
Expiration date
GIMA WARRANTY TERMS
The Gima 12-month standard B2B warranty applies.
Medical Device complies
with Directive 93/42/EEC
Caution: read instructions
(warnings) carefully
Consult instructions for use
Authorized representative
in the European community
 Loading...
Loading...