
MINIASPEED
BATTERY PRO
ISTRUZIONI D’USO
INSTRUCTION MANUAL
MANUEL D’INSTRUCTIONS
MONTAGE-UND GEBRAUCHSANWEISUNG
MANUAL DE INSTRUCCIONES
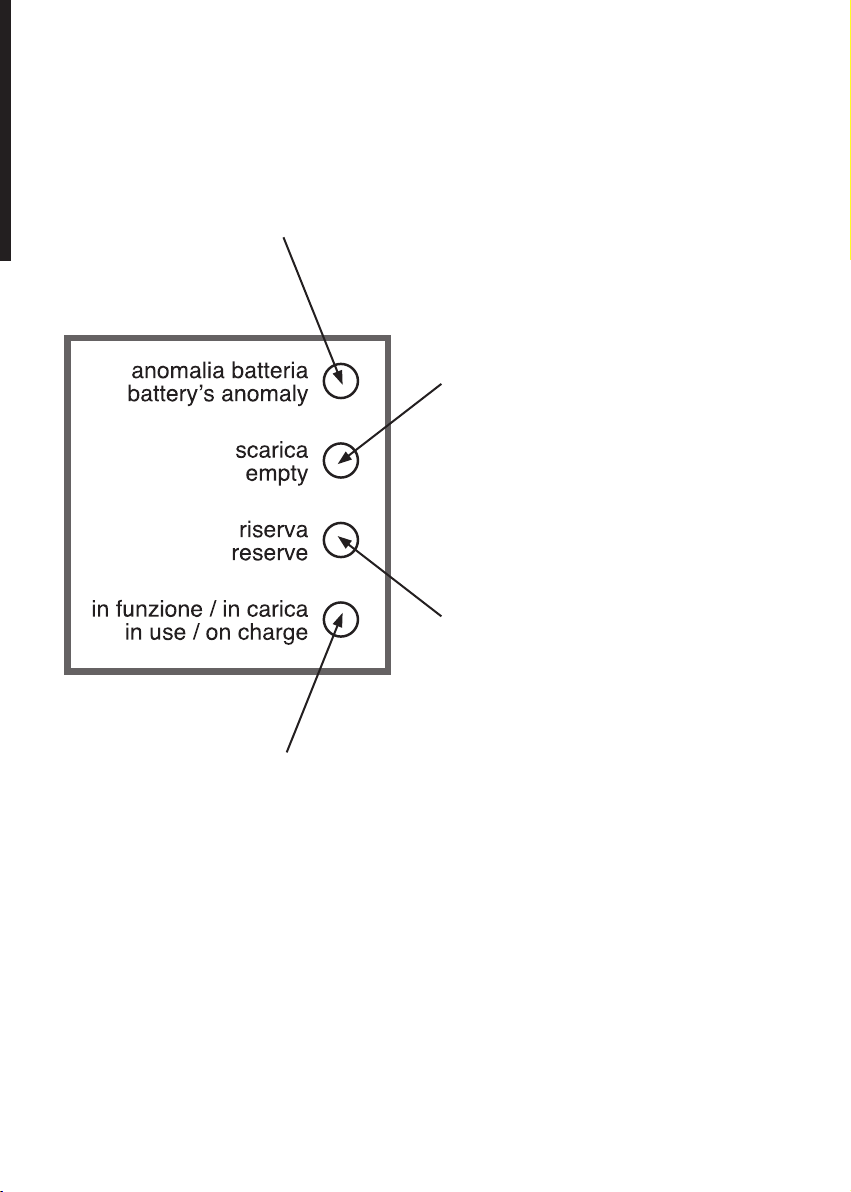
DESCRIZIONE FUNZIONAMENTO LED / LED OPERATION DESCRIPTION
DESCRIPTION DU FONCTIONNEMENT DES LED / BESCHREIBUNG DER FUNKTIONSWEISE DER LED /
DESCRIPCIÓN DEL FUNCIONAMIENTO DE LEDS
Led rosso lampeggiante: la batteria non è più in grado di fornire la massima autonomia, provvedere alla sua sostituzione / Flashing
red Led: the battery is no longer capable of supplying maximum autonomy; replace it. / Led rouge clignotante: la batterie n’est plus en
mesure d’assurer l’autonomie maximale, la remplacer. / Rote LED blinkt: Die Batterie ist nicht mehr in der Lage, maximale Autonomie zu
liefern. Batterie ersetzen./ Led rojo parpadeante: la batería ya no logra ofrecer la autonomía máxima; sustituirla.
Led rosso fisso: la batteria è scarica, utilizzare l’apparecchio
per 3 minuti massimo e provvedere alla ricaricare la batteria.
/ Red Led permanently on: the battery is flat: use the appliance
for a maximum of 3 minutes and re-charge the battery. / Led
rouge fixe: la batterie est déchargée, utiliser l’appareil pendant
3 minutes au maximum puis recharger la batterie. / Rote LED
leuchet kontinuierlich: Batterie entladen. Das Gerät maximal für
3 Minuten benutzen und die Batterie aufladen./ Led rojo fijo: la
batería está agotada, utilizar el aparato durante 3 minutos como
máximo y volver a cargarla.
Led giallo fisso: la batteria è in riserva (autonomia 10 minuti
circa), se possibile provvedere a ricaricarla. / Yellow Led permanently on: the battery is in reserve (around 10 minutes autonomy), se possible, recharge it. / Led jaune fixe: la batterie est
sur la réserve (10 minutes d’autonomie environ); la recharger si
possible. / Gelbe LED leuchet kontinuierlich: Batterie in Reserve
(Autonomie ca. 10 Minuten), Batterie aufladen. / Led amarillo
fijo: la batería se halla en reserva (autonomía de unos 10 minutos); si es posible volver a cargarla.
Led verde fisso: apparecchio in funzione, batteria carica. Led verde lampeggiante: batteria sotto carica. / Green Led permanently on:
appliance operating: battery charged. Green Led flashing: battery under charge. / Led verte fixe: appareil en marche, batterie chargée.
Led verte clignotante: batterie sous charge. / Grüne LED leuchet kontinuierlich: Gerät in Betrieb, Batterie geladen. Grüne LED blinkt:
Batterieaufladung. / Led verde fijo: aparato funcionando, batería cargada. Led verde parpadeante: batería en carga.
1

SCHEMA DI COLLEGAMENTO / CONNECTION DIAGRAM
SCHÉMA DE RACCORDEMENT / ANSCHLUSSPLAN
ESQUEMA DE CONEXIÓN
4
1f
1e
3
1d
1c
1b
1a
2
1
1. Aspiratore
1a. Presa per alimentatore multitensione e ricarica batteria
1b. LED di funzionamento
1c. Interruttore ON-OFF
1d. Regolatore di vuoto
1e. Presa entrata-aria INLET
1f. Vuotometro
2. Vaso da 1000 ml in policarbonato sterizzabile
2a. Tappo in polipropilene sterilizzabile
2b. Guida galleggiante in polipropilene sterilizzabile
2c. Corpo galleggiante in polipropilene sterilizzabile
2d. Valvola in gomma sterilizzabile
3. Filtro antibatterico monouso
4. Tubo in silicone sterilizzabile Ø 6x12 mm L 240 mm
5. Tubo in silicone sterilizzabile Ø 6x12 mm L 1300 mm
6. Regolatore manuale sterile monouso
7. Cannula sterile monouso
8. Sacca monouso
9. Cavo di alimentazione
10. Alimentatore multitensione
11. Cavo con spina accendisigari
12. Borsa con tracolla
1. Aspirator
1a. Multi-voltage power supply unit and battery charger socket
1b. Power LED
1c. ON-OFF switch
1d. Vacuum regulator
1e. Air INLET connection
1f. Vacuum gauge
2. 1000 ml vessel in sterilisable polycarbonate
2a. Sterilisable polypropylene cap
2b. Sterilisable polypropylene float guide
2c. Sterilisable polypropylene float body
2d. Sterilisable rubber valve
3. Disposable antibacterial filter
4. Sterilisable silicone tube Ø 6 x 12 mm L 240 mm
5. Sterilisable silicone tube Ø 6 x 12 mm L 1300 mm
6. Disposable sterile manual regulator
7. Disposable sterile cannula
8. Disposable bag
9. Power cord
10. Multi-voltage power supply unit
11. Cable with cigarette lighter plug
12. Bag with shoulder strap
2
1. Aspirateur
1a. Prise pour alimentation multi-tension
et recharge de la batterie
1b. LED de fonctionnement
1c. Interrupteur MARCHE/ARRÊT
1d. Régulateur de vide
1e. Prise d’arrivée d’air INLET
1f. Vacuomètre
2. Récipient de 1000 ml en polycarbonate
stérilisable
2a. Bouchon en polypropylène stérilisable
2b. Guide flottant en polypropylène
stérilisable
2c. Corps flottant en polypropylène
stérilisable
2d. Soupape en caoutchouc stérilisable
3. Filtre antibactérien jetable
4. Tuyau en silicone stérilisable Ø 6x12 mm
L 240 mm
5. Tuyau en silicone stérilisable Ø 6x12 mm
L 1300 mm
6. Régulateur manuel stérile jetable
7. Canule stérile jetable
8. Poche jetable
9. Câble d’alimentation
10. Alimentation multi-tension
11. Câble avec fiche allume-cigare
12. Sac bandoulière
1. Absauggerät
1a. Buchse für Mehrspannungsnetzteil und
Batterieaufladung
1b. Funktions-LED
1c. Schalter ON-OFF
1d. Vakuumregler
1e. Lufteinlassbuchse INLET
1f. Unterdruckmesser
2. 1000 ml-Sekretbehälter aus sterilisierba-
rem Polycarbonat
2a. Sterilisierbarer Polypropylendeckel
2b. Schwimmführung aus sterilisierbarem
Polypropylen
2c. Schwimmkörper aus sterilisierbarem
Polypropylen
2d. Sterilisierbares Gummiventil
3. Antibakterieller Einwegfilter
4. Sterilisierbarer Silikonschlauch Ø 6x12
mm L 240 mm
5. Sterilisierbarer Silikonschlauch Ø 6x12
mm L 1300 mm
6. Steriler Einweghandregler
7. Sterile Einwegkanüle
8. Einwegbeutel
9. Netzkabel
10. Mehrspannungsnetzteil
11. Kabel mit Zigarettenanzünderstecke
12. Schultertasche
1. Aspirador
1a. Enchufe para alimentador multitensión
y recarga batería
1b. LED de funcionamiento
1c. Interruptor ON-OFF
1d. Regulador de vacío
1e. Toma de entrada-aire INLET
1f. Vacuómetro
2. Recipiente de 1000 ml en policarbonato
esterilizable
2a. Tapón de polipropileno esterilizable
2b. Guía flotador de polipropileno
esterilizable
2c. Cuerpo flotador de polipropileno
esterilizable
2d. Válvula de goma esterilizable
3. Filtro antibacteriano desechable
4. Tubo de silicona esterilizable Ø 6x12 mm
L 240 mm
5. Tubo de silicona esterilizable Ø 6x12 mm
L 1300 mm
6. Regulador manual estéril desechable
7. Cánula estéril desechable
8. Bolsa desechable
9. Cable de alimentación
10. Alimentador multitensión
11. Cable con toma de mechero
12. Bolsa con bandolera
Schema di collegamento con Vaso - fig. 1
Connection with vessel - fig. 1
Schéma de raccordement au récipient - fig. 1
Anschlussplan mit Sekretbehälter - Abb. 1
Esquema de conexión con Recipiente - fig. 1
4
Fig - Abb. 1
5
3
2a
2d
2c
2b
2
Schema di collegamento con Sacca monouso - fig. 2
Connection with disposable bag - fig. 2
Schéma de raccordement avec poche jetable - fig. 2
Anschlussplan mit Einwegbeutel - Abb. 2
Esquema de conexión con Bolsa desechable - fig. 2
5
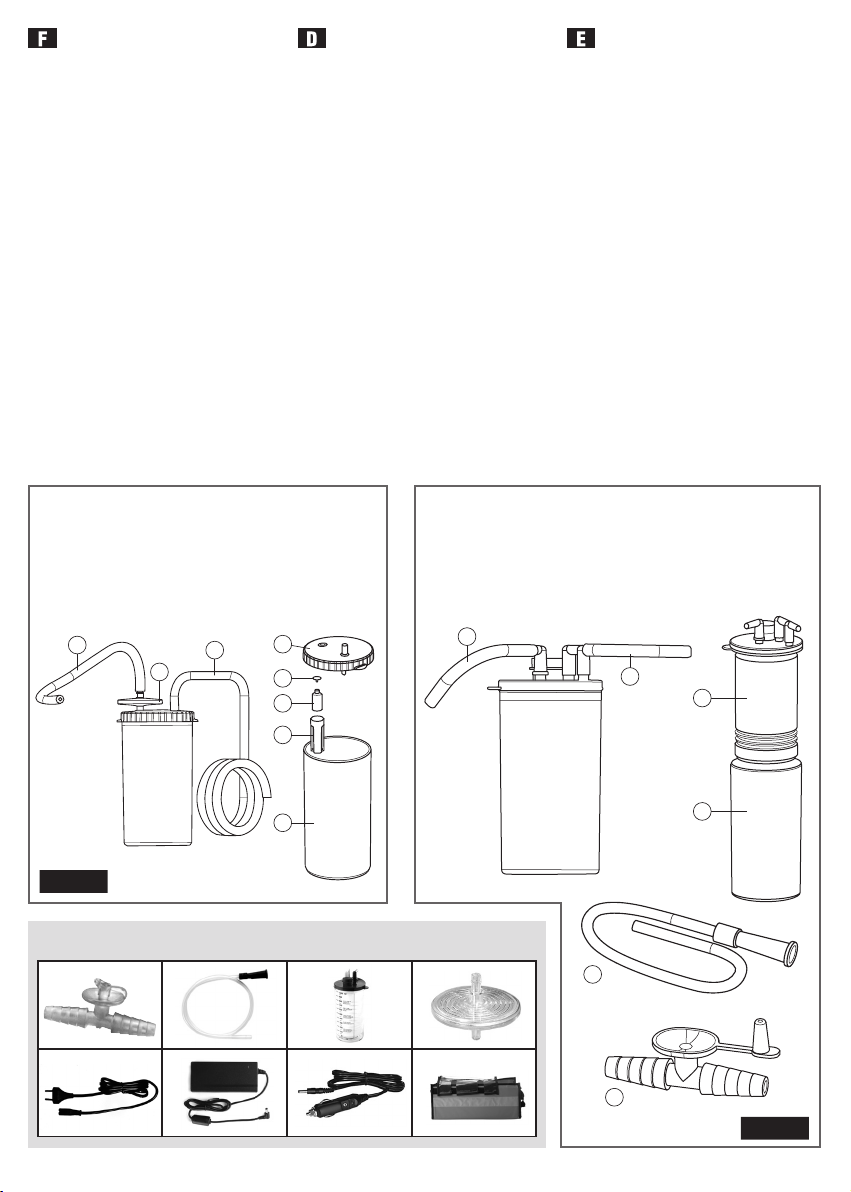
ACCESSORI / ACCESSORIES / ACCESSOIRES / ZUBEHÖR / ACCESORIOS
6. 7. 8. 3.
9. 10. 11 . 12.
4
8
2
7
6
Fig - Abb. 2
3

EN
The MINIASPEED BATTERY PRO aspirator, specific for secretion removal, is a medical and surgical device suitable for use in the home,
in clinics, and also in field facilities and on vehicles. This model is equipped with a 12 V rechargeable battery, which enables the device to
operate with no mains power available. It can also be powered via a 12 V cigarette lighter socket. The device generates a vacuum for aspira
tion to extract bodily fluids through a disposable cannula connected to a collection vessel, where they are collected for appropriate disposal.
Its use must be prescribed by a physician. For correct operation and a longer service life, carefully follow the use and cleaning instructions.
The device includes a vacuum regulator, vacuum gauge and 1000 ml vessel with a safety device that prevents liquid from entering the suc
tion pump, which would cut out the suction flow. It never needs lubrication. It is sturdy, silent, reliable, easy to handle and simple to operate.
The MINIASPEED BATTERY PRO aspirator includes the following accessories: 1000 ml vessel with safety device, power cord with multi-
voltage power supply, power cord with cigarette lighter plug, short sterilisable silicone connection tube, long sterilisable silicone connec
tion tube, disposable, sterile cannula, disposable, sterile manual regulator, disposable bag and a disposable antibacterial filter.
N.B.: Only use genuine accessories supplied by 3A Health Care; the accessories supplied with the device by the manufac
turer have been tested and proven compliant with the latest current safety standards. WARNING! Correct operation of the
device is not guaranteed in the event of use of accessories other than those supplied.
-
-
-
-
Battery-powered medical / surgical aspirator intended for use in the field and/or in vehicles. It may also be used in home care and/or outpatient settings.
Medical purposes: This product is intended for use for the aspiration of body fluids.
Intended users of the product:
• Legally certified medical personnel (doctors, nurses and therapists).
• For home treatment or home care under the guidance of medical personnel.
• Users must also be able to understand the operation of the medical device, and the contents of the instruction manual, in general terms.
Intended patients for the product: People who need to remove body fluids (saliva, blood, etc.).
Environment: This product is intended for use in the field and/or on a vehicle. It may also be used in home care and/or outpatient settings.
Expected duration: Duration may vary based on the operating environment. The lifetime of the device is 5 years and that of the collection
vessel and the silicone tubes 1 year or 30 sterilisation cycles. The cannula, manual flow regulator, antibacterial filter and bag are disposable
devices and must therefore be replaced after each application. Frequent use of the product may shorten the duration.
Precautions for use: The warnings and cautions described in the instruction manual must be observed.
IMPORTANT WARNINGS
This is a medical device and must be used by qualified staff. It must be operated as indicated in this user manual. It is
important for the operator to read and understand the information on use and maintenance of the unit. If you have any
questions, contact your dealer. MICROBIAL CONTAMINATION: in case of illnesses with a risk of infection or microbial
contamination, the accessories should be thoroughly cleaned and sterilised after each use.
The manufacturer has made every effort to ensure that all its products are of the highest quality and safe. Nevertheless, as with all electrical
devices, make sure to follow all basic safety standards.
• Children and persons who are not self-sufficient may only use the device under the strict supervision of a responsible adult who
has read this manual. Some device components are small enough for children to swallow. Therefore, keep the device out of reach of
children.
• Due to their lengths, the power cord and connection tube could create a strangulation hazard.
• The device must always be used by specifically trained staff, who have read this manual.
• This device should only be used for its intended purpose as an aspirator for home and clinical use. Any other use shall be considered
inappropriate and therefore hazardous. The manufacturer shall not be liable for any consequences arising from inappropriate use.
• Never use power adapters with voltages other than the voltage shown on the power supply unit (rating plate). Keep the power cord away
from hot surfaces.
• The device should not be used in the presence of flammable anaesthetic mixtures with air, oxygen or nitrous oxide.
• Never handle the power supply unit with wet hands. Never use the device (with power supply unit connected) close to water.
• Never immerse the device in any liquid. Do not wet the device. If the device falls into water, disconnect the power supply from the power
outlet before recovering the device.
• Do not use the device if the plug or power cord are worn or wet (immediately send it back to your dealer).
• Only authorised personnel may perform maintenance and/or repair work on this device. Unauthorised repairs void the warranty.
• Ensure that all connections and vessel closing are performed carefully to avoid suction losses.
• Do not overturn the vessel while connected to the device in operation, as liquid may be sucked inside the device, causing pump damage.
If this occurs, immediately switch the aspirator off. Then drain and clean/sterilise the vessel. Send the device to your dealer.
• Tripping of the safety device stops the suction. Drain the vessel and perform the cleaning/sterilisation operations.
• The disposable cannula and manual regulator are disposable sterile products. They must be replaced after every use.
• Check the expiry date of the cannula on the original packaging. Check the integrity of the sterile packaging. If expired or damaged,
9
INTENDED USE

replace it immediately.
• The disposable antibacterial filter must be replaced after every use.
• Never use the battery charger with other devices or for uses other than that described in this manual. Never use the MINIASPEED
BATTERY PRO with other power supply units.
• The cigarette lighter plug cord has a safety fuse, which can be inspected in case of malfunction.
• Use of the device in ambient conditions other than those specified in the manual may seriously impair its safety and technical cha
racteristics.
• In the event of aspiration without vessel and/or antibacterial filter, or if you suspect that solid or liquid substances have entered the
aspiration circuit, send the device to customer service.
• Always place the device in a vertical position on an unobstructed, stable and flat surface before use.
-
Before each use, ensure that all accessories are perfectly clean according to the instructions in the “CLEANING AND DISINFECTION
INSTRUCTIONS FOR USE
OPERATIONS” section.
1. Operation using cigarette lighter plug cord:
1.1 For use on vehicles, connect the power cord (11.) to the socket on the device (1a.).
1.2 Check the charge status of the vehicle’s battery before using the device.
1.3 Connect the device as shown in the connection diagram on page 2.
1.4 Use the vacuum regulator (1d.) to set the level of vacuum required (bar/KPa). Turn the knob clockwise, in the “+” direction, to obtain
a stronger vacuum or anticlockwise, in the “-“ direction, for a weaker vacuum. These levels may be read on the vacuum gauge (1f).
Important: the vacuum values on the control decal are purely for guidance. Always refer to the vacuum gauge rea
ding.
1.5 Start the device using the “ON-OFF” switch (1c.) (solid green indicator light on).
1.6 After use, switch off the device, disconnect the cigarette lighter plug cord (11.) and clean the device as described in the “CLEANING
AND DISINFECTION PROCEDURES” section.
1.7 If the cigarette lighter plug fuse blows, replace it with a delayed 6.3 A-250 V fuse, size Ø 6.3 x 30 mm, by unscrewing the tip of the
cigarette lighter plug.
2. Operation using the battery and/or multi-voltage power supply unit:
2.1 The device is supplied with the battery partially charged. We therefore recommend charging it before use.
2.2 Charge the battery, with the device off, by connecting the multi-voltage power supply unit (10.) to the device’s socket (1a.) and to
mains power using the power cord (9.). Maximum charging time 6 hours, which provides autonomy of about 45 minutes.
2.3 Operation with internal battery only:
Start the device using the “ON-OFF” switch (1c.) (solid green indicator light on). If the green light goes out during use (1b), and the
yellow light comes on, there will be about 10 minutes of battery charge left (reserve level). Then, terminate use as soon as possible. If
use cannot be stopped, continue until the red indicator light comes on (battery flat). If this occurs, do not use the device for more
than 3 minutes to avoid damaging the battery. To continue using the device with the battery flat (red light on), connect the
multi-voltage power supply unit (10.) (as described in point 2.2). After use, turn the switch (1c.) to “O” (OFF) and leave the power supply
(10.) connected to the device’s socket (1a.) to charge the battery. The power supply (10.) should be left connected when the device is
not in use to ensure an optimal battery charge level.
2.4 For aspiration procedures, see points 1.3, 1.4 and 1.5.
N.B.: when the battery power drops below a set level the aspirator shuts down to avoid damage to the battery. If abso
lutely necessary, the user can restart the aspirator for 1 minute by pressing the ON/OFF button again.
2.5 After use, switch off the device, disconnect the power cord (9.) from the mains socket and disconnect the power supply unit (10.) from
the device. Perform the cleaning operations as described in the “CLEANING AND DISINFECTION PROCEDURES” section.
2.6 Use only genuine 3A accessories.
3. Secretion collection vessel – 1000ml
The 1000 ml collection bottle (2.) supplied with the aspirator can be used in two ways: as a collection vessel which can be sterilised as
shown in figure 1 or as a collection vessel with disposable bag (8.) as illustrated in figure 2.
3.1 Sterilisable secretion vessel (2.):
The secretion vessel set consists of an overflow valve, a vessel in clear material (polycarbonate) and a blue plug (2a.). Fit the antibacterial
filter (3.) straight into the plug (2a.); it will only fit into the hole marked VACUUM/VUOTO. The antibacterial filter also protects the
aspiration circuit from any contaminating agents sucked in during use. Do not use the aspirator without the antibacterial filter,
because from a bacteriological point of view, it becomes dangerous for the patient. Keep the device vertical to
allow the overflow to function correctly.
All the components of the vessel can be sterilised using a conventional system in an autoclave at a temperature of 121°C, or by boiling
for 10 minutes. We recommend replacing the complete vessel at every 30 sterilisation cycles. Do not overturn the vessel during use,
in order to prevent the intervention of the non-return valve (2b/2c/2d); should this occur, switch the aspirator off and detach the tube
connected to the antibacterial filter. Never use the aspirator without the secretion collection vessel and/or without the antibacterial filter.
3.1.1 Connection: connect one end of the short, sterilisable silicon tube (4.) to the antibacterial filter connector (3.) and insert this
latter into the “VACUUM” hole of the blue top (2a.); connect the other end to the “INLET” connector (1e.) of the aspirator. Connect one
end of the long sterilisable silicon tube (5.) to the PATIENT/PAZIENTE” connector of the blue top (2a.); to the other end, connect the
disposable, sterile manual regulator (6.) and connect the disposable, sterile cannula (7.) to this latter.
10
-
-

3.2 Secretion collection vessel with single use bag (8.):
The aspirator can be used with the 1000 cc re-usable transparent secretion collection vessel (2.) and with the single use bag (8.)
supplied. In this case the antibacterial filter is integrated in the single-use bag, therefore the antibacterial filter (3.) and the blue lid with
the valve (2a.) should not be used. The filter embedded in the bag, also prevents the reflux of the liquids sucked towards the aspirator
when it is full, or when it is inadvertently turned over. In this case to restore the device to normal operation, the single use
bag shall be replaced. For the cleaning and disinfecting operations of the tubes (4. and 5.) and vessel (2.), sterilise the single
parts in autoclave at a maximum temperature of 121°C, or by boiling them for 10 minutes. The bag is single use and it MUST be
replaced after each use. The bag must be completely inserted in the vessel in order to prevent any vacuum losses.
N.B.: maximum disposable bag usage vacuum: -0.75 bar (75 kPa).
3.2.1 Connection: connect one end of the short sterilisable silicon tube (4.) to the yellow rubber holder (VACUUM) of the bag (8.)
and the other end to the “INLET” input (1e.) of the aspirator. Connect one end of the long sterilisable silicon tube (5.) to the red rubber
holder (PATIENT) of the bag (8.) and connect the sterile single-use manual regulator (6.) and the single-use sterile cannula (7.) to the
other end.
N.B.: only use the single use bags supplied by 3A - Code 3AC461.
N.B.: If using chemical disinfectants, carefully follow the manufacturer’s instructions.
• The cannula and the manual regulator are sterile, disposable products and must be replaced after every application.
• The disposable antibacterial filter must be replaced after every use.
• Never wash the device under running water or by immersion. Clean the outside of the device using only a cloth dampened with a nonabrasive detergent.
TECHNICAL SPECIFICATIONS OF THE ACCESSORIES:
• Disposable antibacterial filter - code 3A1385
• 1000 ml collection vessel in polycarbonate, complete with cap - code 3AC286
• Silicone tube Ø 6 x 12 mm L 240 mm - code 3A476
• Silicone tube Ø 6 x 12 mm L 1300 mm - code 3A561
• Disposable sterile cannula CH14 - code 3A4167
• Disposable manual regulator - code 3A560
• 1 litre disposable bag - code 3AC461
PROBLEMS, CAUSES and SOLUTIONS
PROBLEMS POSSIBLE CAUSES SOLUTIONS
CLEANING AND DISINFECTION PROCEDURES
Excessive noise.
The unit switches on but does not aspirate
The vacuum rate cannot be regulated
When the appliance is switched on, the
protection fuse always trips
The vacuum gauge does not work Liquid penetrating the pneumatic circuit. Send to the assistance circuit
Damaged pump or blockages in the internal
aspiration circuit
- Damaged pump
- Vacuum regulator fully open.
Connection tubes disconnected and/or badly
connected, broken connection tubes. Container not in a vertical position, full, or defective
overflow valve. Possible blockage of
the hydraulic circuit inside the unit.
Damage to the internal hydraulic system or
blockage of the connection tubes to the aspiration unit.
Pump probably damaged or in shortcircuit. Send to the assistance circuit
Send to the assistance circuit
- Send to the assistance circuit.
- Check the position of the vacuum regulator. Check the connections and the integrity of the tubes. Position the container in a
vertical position, check the overflow valve
(blocked) and/or replace the silicon tubes.
Send to the assistance circuit
Note: if you experience faults or malfunctioning problems different to those listed above, always and exclusively
contact authorised assistance centres.
11

Electric two-cylinder piston compressor with lifetime lubrication. Metal casing with epoxy powder coating
TECHNICAL SPECIFICATIONS
Risk class under Directive 93/42/EEC: IIa
Suction class: High vacuum / Low flow
Voltage: 12VDC 45 W
Power consumption: 3.75A
Adjustable vacuum level: from 0 to -0.85 bar (-85 KPa)
Vacuum gauge precision class: 2.5% according to UNI EN 837
Air flow rate: 28 L/min operating without restrictions +/- 10%
Temporary use: maximum 45 minutes
Dimensions: 37 (length) x 10 (width) x 24 (height) cm
Weight: 5.8 kg approx.
Noise level: 58 dBA approx.
Multi-voltage switching battery charger: PRI: 100-240V~ 50-60Hz
SEC: 14V DC 4.29 A
Internal battery: 12VDC 4.5 Ah lead, hermetic
Battery charge duration: 45 minutes at maximum suction power
Cigarette lighter plug cord fuse: F6.3 A-250 V, delayed; dimensions Ø 6.3 x 30 mm
Operating conditions: Temperature: min. 0°C max. 40°C – Air humidity: min 10% maximum 95%
Storage conditions: Temperature: min. -10°C max. 50°C – Air humidity: min. 10% max. 95%
Atmospheric pressure for operation/storage: min. 690 hPa - max. 1060 hPa
Type BF device
SYMBOLS USED
Class II device
It is compulsory to carefully read the instructions before using this device
Switch on
Switch off (or battery on charge)
Direct current
Alternating current
Never use the device when taking a bath or a shower
The device contains a sealed lead-acid battery. It must be disposed of in accordance with current regulations on the
disposal of toxic-harmful waste.
The device’s casing is protected against solids 12.5 mm or more in diameter, against vertically falling drops of water and against
IP21
finger access to hazardous parts.
Ethylene oxide sterilisation
Disposable
3A HEALTH CARE S.r.l.
Via Marziale Cerutti, 90F/G
25017 Lonato del Garda (BS) - ITALY
Compliant with Medical Devices Directive 93/42/EEC
12

Electromagnetic Compatibility
Compliance levels according to EN 60601-1-2:2015 standard
- ESD immunity: 15 kV air, 8 kV contact (EN 61000-4-2)
- Burst immunity: 2 kV/100 kHz (EN 61000-4-4)
- Surge immunity (EN 61000-4-5): 1 kV common mode /2 kV differential mode
- Magnetic field (EN 61000-4-8): 30 A/m
- Immunity to rf currents in the 150 kHz-80 MHz range (EN 61000-4-6) 3 V modulation 80% 1 kHz
- RF emissions, CISPR 11: Class B
- Harmonics emissions, EN 61000-3-2: Class A
Rf field immunity (EN 61000-4-3):
Field (V/m) Frequency Modulation
3 80MHz 2700MHz 1kHz AM 80%
27 380MHz 390MHz 18Hz PM 50%
28 430MHz 470MHz 18Hz PM 50%
9 704MHz 787MHz 217Hz PM 50%
28 800MHz 960MHz 18Hz PM 50%
28 1700MHz 1990MHz 217Hz PM 50%
28 2400MHz 2570MHz 217Hz PM 50%
9 5100MHz 5800MHz 217Hz PM 50%
Warnings:
Although compliant with the EN 60601-1-2 standard, the MINIASPEED BATTERY PRO medical device may interfere with other devices
in the vicinity. The device must not be used in proximity to or stacked on top of other equipment. Install the device well away from other
equipment that emits high frequencies (short waves, microwaves, electric scalpels, cell phones).
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are under control. The customer
or user can help prevent electromagnetic interference by maintaining a minimum distances between mobile and portable RF communication equipment (transmitters) and the medical device as recommended below, according to the maximum output power of the radio
communication equipment.
Rated maximum
output power
of transmitter
(W)
from 150 kHz to 80 MHz
Separation distance (m) in relation to transmitter frequency
d = 1.2 √P
from 80 MHz to 800 MHz
d = 1.2 √P
from 800 MHz to 2.5 GHz
d = 2.3 √P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters with rated maximum output power not listed above, the recommended separation distance d in metres (m) may be
determined using the equation adopted for the transmitter frequency, where P is the maximum rated output power of the transmitter in
Watts (W) stated by the transmitter manufacturer.
Notes:
(1) At 80 MHz and 800 MHz the highest frequency range applies.
(2) These guidelines might not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
13

PROCEDURA DI SMALTIMENTO (Dir.2012/19/Ue-RAEE) Il simbolo posto sul fondo dell’apparecchio indica la raccolta separata delle apparecchiature
PROCEDURA DI SMALTIMENTO (Dir.2012/19/Ue-RAEE) Il simbolo posto sul fondo dell’apparecchio indica la raccolta separata delle apparecchiature
elettriche ed elettroniche. Al termine della vita utile dell’apparecchio, non smaltirlo come rifiuto municipale solido misto ma smaltirlo presso un centro
di raccolta specifico situato nella vostra zona oppure riconsegnarlo al distributore all’atto dell’acquisto di un nuovo apparecchio dello stesso tipo ed
adibito alle stesse funzioni. Questa procedura di raccolta separata delle apparecchiature elettriche ed elettroniche viene effettuata in visione di una
politica ambientale comunitaria con obiettivi di salvaguadia, tutela e miglioramento della qualità dell’ambiente e per evitare effetti potenziali sulla salute
umana dovuti alla presenza di sostanze pericolose in queste apparecchiature o ad un uso improprio delle stesse o di parti di es se. Attenzione! Uno
smaltimento non corretto di apparecchiature elettriche ed elettroniche potrebbe comportare sanzioni.
PROCEDURA DI SMALTIMENTO (Dir.2012/19/Ue-RAEE) Il simbolo posto sul fondo dell’apparecchio indica la raccolta separata delle apparecchiature
elettriche ed elettroniche. Al termine della vita utile dell’apparecchio, non smaltirlo come rifiuto municipale solido misto ma smaltirlo presso un centro
di raccolta specifico situato nella vostra zona oppure riconsegnarlo al distributore all’atto dell’acquisto di un nuovo apparecchio dello stesso tipo ed
adibito alle stesse funzioni. Questa procedura di raccolta separata delle apparecchiature elettriche ed elettroniche viene effettuata in visione di una
politica ambientale comunitaria con obiettivi di salvaguadia, tutela e miglioramento della qualità dell’ambiente e per evitare effetti potenziali sulla salute
umana dovuti alla presenza di sostanze pericolose in queste apparecchiature o ad un uso improprio delle stesse o di parti di es se. Attenzione! Uno
smaltimento non corretto di apparecchiature elettriche ed elettroniche potrebbe comportare sanzioni.
ENTSORGUNGSVERFAHREN (RICHTLINIE 2012/19/Ue-Weee) Das Symbol auf dem Boden des Geräts gibt die getrennte Müllsammlung der elektrischen
und elektronischen Ausrüstungen an. Am Ende der Lebensdauer vom Gerät es nicht als gemischter fester Gemeindenabfall, sondern es bei einem
spezifischen Müllsammlungszentrum in Ihrem Gebiet entsorgen oder es dem Händler zurückgeben, wenn Sie ein neues Gerät desselben Typ mit
denselben Funktionen kaufen. Diese Prozedur getrennter Müllsammlung der elektrischen und elektronischen Ausrüstungen wird im Hinblick auf eine
zukünftige gemeinsame europäische Umweltschutzpolitik vorgenommen, welche darauf zielen wird, die Umwelt zu schützen und sichern, als auch die
Umweltqualität zu verbessern und potentielle Wirkungen auf die menschliche Gesundheit wegen der Anwesenheit von gefährlichen Stoffen in diesen
Vorrichtungen oder Missbrauch derselben oder von Teilen derselben zu vermeiden. Vorsicht! Die fehlerhafte Entsorgung von elektrischen und elektronischen
Vorrichtungen könnte Sanktionen mit sich bringen.
elettriche ed elettroniche. Al termine della vita utile dell’apparecchio, non smaltirlo come rifiuto municipale solido misto ma smaltirlo presso un centro
di raccolta specifico situato nella vostra zona oppure riconsegnarlo al distributore all’atto dell’acquisto di un nuovo apparecchio dello stesso tipo ed
adibito alle stesse funzioni. Questa procedura di raccolta separata delle apparecchiature elettriche ed elettroniche viene effettuata in visione di una
politica ambientale comunitaria con obiettivi di salvaguadia, tutela e miglioramento della qualità dell’ambiente e per evitare effetti potenziali sulla salute
umana dovuti alla presenza di sostanze pericolose in queste apparecchiature o ad un uso improprio delle stesse o di parti di es se. Attenzione! Uno
smaltimento non corretto di apparecchiature elettriche ed elettroniche potrebbe comportare sanzioni.
DISPOSAL PROCEDURE (Dir. 2012/19/Ue-WEEE) The symbol on the bottom of the device indicates the separated collection of electric and electronic
ENTSORGUNGSVERFAHREN (RICHTLINIE 2012/19/Ue-Weee) Das Symbol auf dem Boden des Geräts gibt die getrennte Müllsammlung der elektrischen
equipment. At the end of life of the device, do not dispose it as mixed solid municipal waste, but dispose it referring to a sp ecific collection centre
und elektronischen Ausrüstungen an. Am Ende der Lebensdauer vom Gerät es nicht als gemischter fester Gemeindenabfall, sondern es bei einem
located in your area or returning it to the distributor, when buying a new device of the same type to be used with the same functions. This procedure
spezifischen Müllsammlungszentrum in Ihrem Gebiet entsorgen oder es dem Händler zurückgeben, wenn Sie ein neues Gerät desselben Typ mit
of separated collection of electric and electronic devices is carried out forecasting a European environmental policy aiming at safeguarding, protecting
denselben Funktionen kaufen. Diese Prozedur getrennter Müllsammlung der elektrischen und elektronischen Ausrüstungen wird im Hinblick auf eine
and improving environment quality, as well as avoiding potential effects on human health due to the presence of hazardous substances in such equipment
zukünftige gemeinsame europäische Umweltschutzpolitik vorgenommen, welche darauf zielen wird, die Umwelt zu schützen und sichern, als auch die
or to an improper use of the same or of parts of the same. Caution! The wrong disposal of electric and electronic equipment may involve sanctions.
Umweltqualität zu verbessern und potentielle Wirkungen auf die menschliche Gesundheit wegen der Anwesenheit von gefährlichen Stoffen in diesen
Vorrichtungen oder Missbrauch derselben oder von Teilen derselben zu vermeiden. Vorsicht! Die fehlerhafte Entsorgung von elektrischen und elektronischen
PROCÉDURE D’ÉLIMINATION (Dir. 2012/19/Ue-WEEE) Le symbole placé sur le fond de l’appareil indique la récolte séparée des appareils électriques
Vorrichtungen könnte Sanktionen mit sich bringen.
et électroniques. A la fin de la vie utile de l’appareil, il ne faut pas l’éliminer comme déchet municipal solide mixte; il faut l’éliminer chez un centre de
récolte spécifique situé dans votre zone ou bien le rendre au distributeur au moment de l’achat d’un nouveau appareil du même type et prévu pour les
DISPOSAL PROCEDURE (Dir. 2012/19/Ue-WEEE) The symbol on the bottom of the device indicates the separated collection of electric and electronic
mêmes fonctions. Cette procédure de récolte séparée des appareils électriques et électroniques se réalise dans une vision d’une politique de sauvegarde,
equipment. At the end of life of the device, do not dispose it as mixed solid municipal waste, but dispose it referring to a sp ecific collection centre
protection et amélioration de la qualité de l’environnement et pour éviter des effets potentiels sur la santé humaine dus à la présence de substances
located in your area or returning it to the distributor, when buying a new device of the same type to be used with the same functions. This procedure
dangereuses dans ces appareils ou bien à un emploi non autorisé d’elles ou de leurs parties. Attention! Une élimination incorrecte des appareils
of separated collection of electric and electronic devices is carried out forecasting a European environmental policy aiming at safeguarding, protecting
électriques pourrait impliquer des pénalités.
and improving environment quality, as well as avoiding potential effects on human health due to the presence of hazardous substances in such equipment
or to an improper use of the same or of parts of the same. Caution! The wrong disposal of electric and electronic equipment may involve sanctions.
ENTSORGUNGSVERFAHREN (RICHTLINIE 2012/19/Ue-Weee) Das Symbol auf dem Boden des Geräts gibt die getrennte Müllsammlung der elektrischen
und elektronischen Ausrüstungen an. Am Ende der Lebensdauer vom Gerät es nicht als gemischter fester Gemeindenabfall, sondern es bei einem
PROCÉDURE D’ÉLIMINATION (Dir. 2012/19/Ue-WEEE) Le symbole placé sur le fond de l’appareil indique la récolte séparée des appareils électriques
spezifischen Müllsammlungszentrum in Ihrem Gebiet entsorgen oder es dem Händler zurückgeben, wenn Sie ein neues Gerät desselben Typ mit
et électroniques. A la fin de la vie utile de l’appareil, il ne faut pas l’éliminer comme déchet municipal solide mixte; il faut l’éliminer chez un centre de
denselben Funktionen kaufen. Diese Prozedur getrennter Müllsammlung der elektrischen und elektronischen Ausrüstungen wird im Hinblick auf eine
récolte spécifique situé dans votre zone ou bien le rendre au distributeur au moment de l’achat d’un nouveau appareil du même type et prévu pour les
zukünftige gemeinsame europäische Umweltschutzpolitik vorgenommen, welche darauf zielen wird, die Umwelt zu schützen und sichern, als auch die
mêmes fonctions. Cette procédure de récolte séparée des appareils électriques et électroniques se réalise dans une vision d’une politique de sauvegarde,
Umweltqualität zu verbessern und potentielle Wirkungen auf die menschliche Gesundheit wegen der Anwesenheit von gefährlichen Stoffen in diesen
protection et amélioration de la qualité de l’environnement et pour éviter des effets potentiels sur la santé humaine dus à la présence de substances
Vorrichtungen oder Missbrauch derselben oder von Teilen derselben zu vermeiden. Vorsicht! Die fehlerhafte Entsorgung von elektrischen und elektronischen
dangereuses dans ces appareils ou bien à un emploi non autorisé d’elles ou de leurs parties. Attention! Une élimination incorrecte des appareils
Vorrichtungen könnte Sanktionen mit sich bringen.
électriques pourrait impliquer des pénalités.
PROCEDIMIENTO DE ELIMINACIÓN (Dir.2012/19/Ue-RAEE) El símbolo colocado en el fondo del aparato indica la recogida separada de los equipos
eléctricos y electrónicos. Al término de la vida útil del aparato, no eliminar como residuo municipal sólido mixto sino eliminarlo en un centro de recogida
específico colocado en vuestra zona o entregarlo al distribuidor a la hora de comprar un nuevo aparato del mismo tipo y destinado a las mismas
funciones. Este procedimiento de recogida separada de los equipos eléctricos y electrónicos se realiza con el propósito de una política del medioambiente
comunitaria con objetivos de salvaguardia, defensa y mejoramiento de la calidad del medioambiente y para evitar efectos potenciales en la salud de
los seres humanos debido a la presencia de sustancias peligrosas dentro de estos equipos o a un uso inapropiado de los mismos o de algunas de
sus partes. Cuidado! Una eliminación no correcta de equipos eléctricos y electrónicos podría conllevar sanciones.
3A HEALTH CARE S.r.l.
Via Marziale Cerutti, 90F/G - 25017 Lonato del Garda (BS) - Italy
tel. +39 030 9133177 - fax +39 030 9919114
e-mail: mail@3-a.it - www.3-a.it
3A3993 rev. 02 - 11/2021
3A3992 rev. 00 - 02/2018
 Loading...
Loading...