
USER MANUAL
MNPG52-02 Edition 10/05/2011
Magnetotherapy model
MAG2000
I.A.C.E.R. Srl
www.iacer.it www.itechmedicaldivision.com

I.A.C.E.R. Srl
Via S. Pertini 24/A – 30030
Martellago (VE) ITALY
Tel. +39 041 5401356 – Fax +39 041 5402684
e-mail: iacer@iacer.it -
http://www.itechmedicaldivision.com

Summary
Summary 3
Introduction 4
Magnetotherapy 4
Technical Specifications 5
Declaration of conformity 5
Specifications 6
Purpose 6
Technical features 7
Labelling 8
Label details 9
Symbols description 9
Kit contents 10
How to use 10
Warnings 10
Electromagnetic interference 11
Contraindications and side effects 11
Quick use of the device with presetted parameters
11
Adjustable programs instructions 15
Stored programs list 18
Set up ( language selection) 20
Maintenance 20
Functioning control 20
Cleaning 20
Carriage and storage 21
Disposal 21
Troubleshooting 22
Assistance 22
Spare parts 23
Warranty 23
I.A.C.E.R. Srl 3 MNPG52-02

Introduction
Magnetotherapy
It’s a long time that low frequency and high intensity pulsed
electromagnetic fields have met maximum scientific consent in
chronic and degenerative diseases treatment.
Magnetotherapy uses low frequency and high intensity pulsed
electromagnetic fields induced by electric current on a bobbin;
due to its characteristics, the electromagnetotherapy is
universally recognized as the most suitable technique for the
treatment of the bony pathologies, in particular for the
osteoporosys.
Pulsed electromagnetic fields induce biological modifications on
biological membrane, cells and blood vessels with different
therapeutic effects: antiflogistic, antiedemigen and tissue repair.
In general magnetotherapy assures a good biostimulation in
order to re-establish correct cellular functions.
According to different authors experiences in osteoporosys a
considerable disease regression is evident from the sixth
treatment and moreover it’s evident an important increase of
BMD (Bone Mass Density). The magnetic field high value
(Gauss) generated by the device allows treatments in presence
of braces or plaster bandage.
MAG 2000 is a device able to satisfy home user needs thanks to
its high magnetic intensity and its use facility.
I.A.C.E.R. Srl 4 MNPG52-02

Technical
Specifications
Manufacturer
IACER S.r.l. is an Italian medical devices manufacturer (CE medical
certificate n° MED24021 issued by Cermet notified b ody n° 0476).
I.A.C.E.R. S.r.l.
Via S. Pertini, 24/a • 30030 Martellago (VE)
Tel. +39 0415401356 • Fax +39 0415402684
www.iacer.it • iacer@iacer.it
Declaration of conformity
IACER S.r.l., headquartered in Italy, via S. Pertini 24/A 30030
Martellago (VE), declares on its own responsibility that MAG2000 is
manufactured in conformity with Directive 93/42/EEC (MDD) dated 14
June 1993 (D. Lgs. 46/97 dated 24 February 1997 “Attuazione della
Direttiva 93/42/CEE concernente i dispositive medici), Annex II as
modified by Directive 2007/47/CE dated 5 September 2007 (D. Lgs.
37/2010 dated 25 January 2010).
Notified body: Cermet, Via di Cadriano 23 – 40057 Cadriano di
Granarolo (BO) Italy.
Certification Path: Annex II.
MAG2000 is a Class IIa equipment, with reference to Directive
93/42/EEC (MDD), annex IX rule 9 (and following modifications).
Martellago, 03/06/10 Legal Representative
Mario Caprara
I.A.C.E.R. Srl 5 MNPG52-02

Specifications
MAG 2000 has the following specifications:
• Class IIa equipment (Directive 93/42/CEE, Annexed IX, rule 9 and
following modifications);
• Class II applied part type BF (Classif. CEI EN 60601-1);
• Equipment not protected against liquid penetration;
• Equipment and accessories not subjected to sterilization;
• Use of the equipment is prohibited close to flammable substances
or in environments with high concentrations of oxygen;
• Continuous operating mode equipment;
• Equipment not suited to be used in external.
Purpose
Clinical purpose: Therapeutic
Use: Clinic/Hospital and domestic use
MAG2000 is indicated for the treatment, rehabilitation and functional
recovery of the following pathologies:
• wrist, hand, shoulder, foot, ankle and knee articulation
• skeletal motor apparatus
• arthrosis
• atrophies and muscular dystrophy
• bursitis
• bruises
• degeneration of locomotor apparatus
• sprains
I.A.C.E.R. Srl 6 MNPG52-02

• neuralgias
• periarthritis
• benign lesions and muscular tears
• tendinitis
MAG2000 is particularly suitable for the treatment and the care of the
osteoporosis and all the pathologies on bony tissue, for dermatologic
applications (varicose ulcers, phlebitis ulcers, arteropatic ulcers, acne,
burn sore, not healed wounds, cellulite, skin illnesses: in these cases
we recommend a low treatment intensity, lower than 40). Moreover the
device is indicated for tissue reconstruction.
Cellulite treatment is not inserted in the CE0476 marking of the device
device. Cellulite treatment can be used only for beauty purposes.
Thanks to its high magnetic field intensity MAG2000 is particularly
suitable for the treatment of bone fractures also with rigid bandages or
braces.
MAG2000 device is indicated both for professional (physiotherapists,
medics etc.) and for domestic user. In case of home therapy we
recommend using the device exclusively on medical/therapist
suggestion.
Expected lifetime: 5 years
Technical features
Power supply Power Supply MM1510 Series, 15VDC 1A
Max. absorbed current 0,5 A
Insulation class (CEI EN 60601-1) II
Applied part (CEI EN 60601-1) BF
Dimensions (mm) 180x110x50
Field intensity Adjustable on increasing level up to 100
Gauss (each channel) in P1-P20 programs.
Adjustable on increasing level up to 150
Gauss (each channel) in P21-P35 programs.
Squared wave frequency 1-100 Hz
Therapy time Adjustable by user
I.A.C.E.R. Srl 7 MNPG52-02

Label 1 (back)
Label 4
Label 3
Label 2
In frequency programs (from 21 to 35) maximum magnetic field
intensity is 150 Gauss for each channel with solenoids couple
applicator.
Intensity, frequency and time values are given with ±20% of
accuracy.
Environmental conditions of operation
Temperature from +5 to + 40 °C
Relative humidity from 30 to 80%
Pressure from 500 to 1060 hPa
Labelling
I.A.C.E.R. Srl 8 MNPG52-02

Label details
Label 1
Label 2 Label 3 Label 4
DC 15V/1A ON/OFF CH1 CH2
Symbols description
Attention, consult operating instructions
Product subject to WEEE regulations concerning separate waste
collection of electronic equipment
Class II equipment
Applied part type BF
Compliance with Directive 93/42/EEC and following modifications
Manufacturing date (month/year)
I.A.C.E.R. Srl 9 MNPG52-02

Kit contents
MAG2000 contains:
• N°1 MAG2000 device;
• N°1 power supply;
• N°1 user and maintenance manual;
• N°1 elastic therapeutic belt with 3 solenoids;
• N°1 carriage bag
Moreover solenoids couple and magnetotherapy carpet are available as
accessories.
Visit website www.iacer.it to obtain more information.
How to use
Warnings
Please read carefully the user manual before using MAG2000.
We recommend to visit magnetotherapy section on website www.iacer.it in
order to obtain other information.
Take care of what follows:
• Take care of position and meaning of the labels on MAG2000;
• Do not damage the therapeutic belt acting on connection cable and
avoid to roll up the cable around the belt or the device;
• Check the integrity of the power supply before use. Avoid the use in
case of damage to the case or to the wire;
• Avoid the use of MAG2000 to people not educated through the
reading of the manual;
• Avoid the use of MAG2000 contemporary with ointments containing
free ions of magnetic metals;
• Do not use the device in damp environments;
• Do not use in presence of inflammable agents;
• Do not wear metallic objects during therapy;
• Take care to place the green side of the therapeutic belt on the skin;
• Use only cables and applicators supplied by the Manufacturer.
Inadequate cables and applicators could damage the device and/or
could be hazardous for the patient;
• The user must periodically verify cables and applicators insulation and
control their integrity (eventually by contacting the manufacturer).
I.A.C.E.R. Srl 10 MNPG52-02

• In case of prolonged treatments (even up to 8 hours) we suggest to
use intensity lower than 50. In this case the efficacy is given by the
prolonged treatment time rather than maximum adjustable intensity;
• High magnetic field intensity (higher than 80/100 Gauss) are
recommended for short treatments (up to 2 hours) or in presence of
rigid bandages/braces.
WARNING. Disconnect the power supply from the main after each
treatment.
The manufacturer is considered responsible of the performances,
reliability and safety of the instrument only if:
• Possible additions, changes and/or reparations are effected from
authorized personnel.
• The electric plant of the environment in which MAG2000 is inserted it
is conforming to the national laws.
• The instrument is employed in conformity to the instructions contained
in this manual.
Electromagnetic interference
The device doesn’t produce and doesn’t receive interferences from other
equipments. However it’s opportune to use the instrument keeping it at
least at 3 meters from televisions, monitors, cellular telephones or any
other electronic equipment.
Contraindications and side effects
Patient in pregnancy, tuberculosis, juvenile diabetes, viral (in acute phase)
illnesses, mycosis, cardiopathic subjects, tumours, serious arrhythmias or
pacemaker carriers, children, metallic prosthesis carriers, acute infections,
epileptics (different medical prescriptions excepted).
No significant side effects are known of, nor are reported particular
contraindications for excessive time length using the device.
Quick use of the device with presetted parameters
We recommend to read the following instructions for an easy and quick
use of MAG2000:
I.A.C.E.R. Srl 11 MNPG52-02

1. Connect the applicator (or the applicators) to the device, by
connecting the applicator cable to one of two plugs (CH1-CH2)
placed on device upper side;
2. Connect the power supply cable to the main, then connect the power
supply plug to the circular connector placed on device upper side,
near to ON/OFF switch.
3. Connect the power supply plug to the main (110-230 VAC, 50-60
Hz);
4. Move the ON/OFF switch, placed on device upper side, to the ON
position: I-TECH logo and programs menu will be displayed on
screen;
5. Select the therapy program running through programs menu, using
and buttons and put yourself on desired program;
6. Press OK. Screen will display base setting with therapy time (2
hours) and magnetic field intensity (50 int.). These mean values
are recommend by IACER in order to start immediately the
treatment.
7. Press button highlighting magnet icon on left down position;
I.A.C.E.R. Srl 12 MNPG52-02

8. Press OK key. The device will start the treatment and on screen will
be displayed the magnet icon with magnetic field flux. Display green
light indicates that therapy is running.
9. At the end of therapy the screen will display automatically the menu
programs.
Attention: it’s possible to stop temporary the therapy at any time
pressing OK key at least for 2 seconds. Press again OK key to continue
the treatment. During pause time green led turns off till the treatment
restarts.
Attention: it’s possible to get out from the treatment at any time
pressing once key: screen will display the base setting (step 6). By
pressing again key screen will display the programs menu (step 5).
Attention: MAG2000 recognizes applicators correct connection. During
the treatment screen displays connection state under the magnet icon.
The presence of symbol near to the channel number (1 or 2) is an
indicator of correct connection and applicator recognition. The symbol X
near to the channel number (1 or 2) indicates a not correct connection
of applicator, or its absence or even its incorrect functioning (see
“Functioning control” paragraph).
Therapeutic belt and solenoids couple positioning
Here below a list of main positions for the therapeutic belt and for the
solenoids couple.
Wrap the belt around the area to be treated ( or position the belt on the
area, for example in vertebral column treatment). During this phase take
care to place the green side of the therapeutic belt on the skin.
The professional solenoids couple have to be placed in opposite
positions on the area to be treated. Also in this case take care to place
the green side of the therapeutic belt on the skin.
I.A.C.E.R. Srl 13 MNPG52-02

Ankle
Shoulder
Knee
Hip
Wrist
Back (lumbar)
Elbow
Tibia/fibula
Knee
Cervical
Ankle
Shoulder
I.A.C.E.R. Srl 14 MNPG52-02
Femur head
Femur

Vertebral column
Lumbar
Suggestions for a correct use:
• In P1-P20 programs a longed treatment with an intensity higher
than 60 can heat the 3 solenoids belt and this aspect makes
therapy less comfortable: we recommend to space out
treatments and to not go over 2 consecutive hours of therapy;
• In P21-P35 programs we recommend to use the professional
solenoids couple (available as optional accessories) if you want
to adjust an intensity higher than 100 and a treatment time
longer than 2 hours;
• Do not adjust intensity higher than 50 if you use
magnetotherapy carpet (optional accessory) for prolonged
treatments;
Adjustable programs instructions
With MAG2000 you can adjust time therapy and magnetic field intensity
parameters as indicated in the following steps:
1. Connect the applicator (or the applicators) to the device by
connecting applicator cable to one of two plugs (CH1-CH2) placed
on device upper side;
2. Connect the power supply cable to the main, then connect the power
supply plug to the circular connector placed on device upper side,
near to ON/OFF switch;
3. Connect the power supply plug to the main (110-230VAC, 50-60
Hz);
4. Move the ON/OFF switch, placed on device upper side, to the ON
position: I-TECH logo and programs menu will be displayed on
screen;
I.A.C.E.R. Srl 15 MNPG52-02
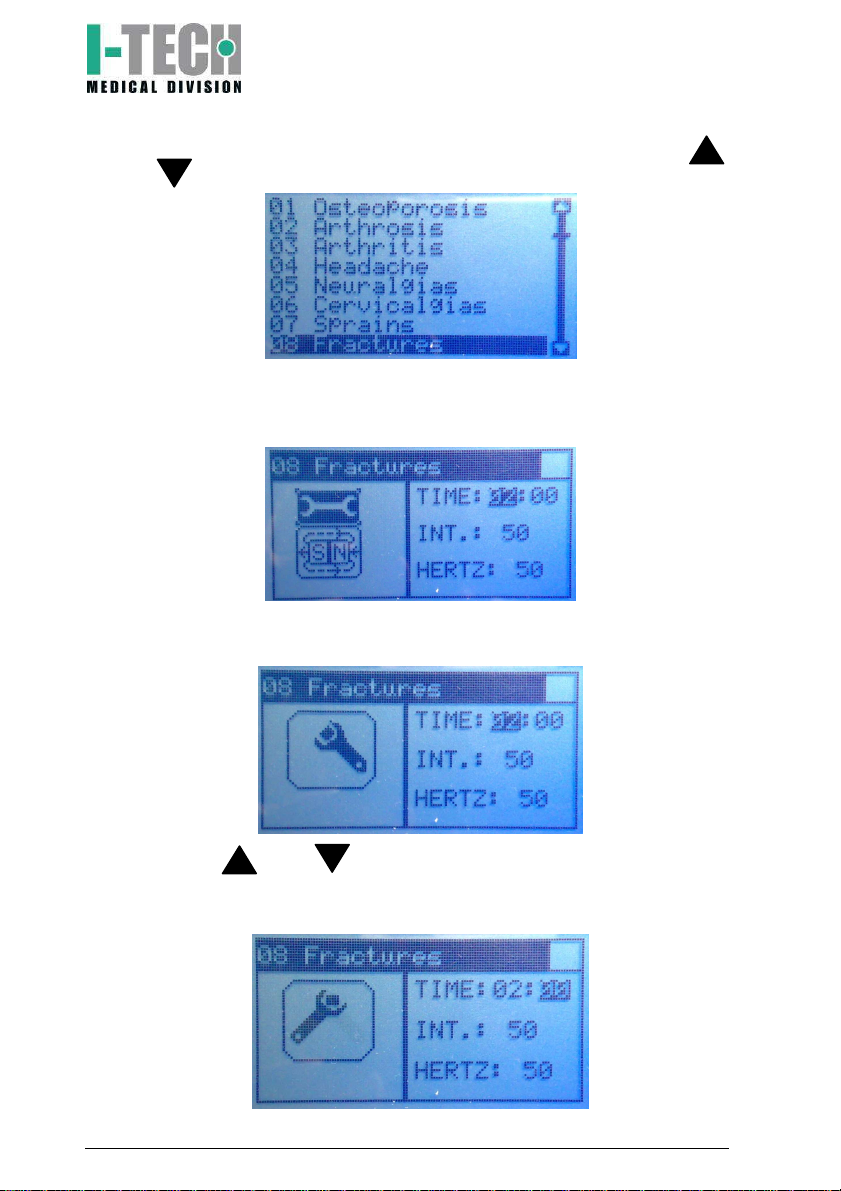
5. Select therapy program running through programs menu using
and buttons and put yourself on desired program;
6. Press OK. Screen will display base setting with therapy time (2
hours) and magnetic field intensity (50 int.). These parameters can
be modified as follows:
a) Press OK button: screen displays a moving key icon on the left
side;
b) Press and key to adjust the desired therapy hours
(from 0 to 24) and confirm by pressing OK key. Screen will
highlight the therapy minutes;
I.A.C.E.R. Srl 16 MNPG52-02

c) Press and key to adjust the desired therapy minutes
(from 0 to 59) and confirm by pressing OK key. Screen will
highlight treatment intensity;
d) Press and key to adjust the treatment intensity (from 5
to 100 Gauss on P1-P20 programs, from 5 to 150 Gauss on
P21-P35 programs) and confirm by pressing OK key;
7. Display will show the step 6 screen highlighting the key icon on the
left side: press key to highlight the magnet icon;
8. Press OK: the device will start the treatment displaying on screen
the magnet icon with magnetic field flux. Green light indicates that
therapy is running.
9. At the end of therapy the screen will display automatically the menu
programs.
Attention: it’s possible to stop temporary the therapy at any time
pressing OK key at least for 2 seconds. Press again OK key to continue
I.A.C.E.R. Srl 17 MNPG52-02

1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
the treatment. During pause time green led turns off till the treatment
restarts.
Attention: it’s possible to get out from the treatment at any time by
pressing once key, screen will display the base setting (step 6). By
pressing again key screen will display programs menu (step 5).
Attention: MAG2000 recognizes applicators correct connection. During
the treatment screen displays connection state under the magnet icon.
The presence of symbol near to the channel number (1 or 2)
indicates correct connection and applicator recognition. The symbol X
near to the channel number (1 or 2) indicates a not correct connection
of applicator, or its absence or even its incorrect functioning (see
“Functioning control” paragraph).
Stored programs list
Pre-adjusted values Recommended values
N° Pathology Hz Time
hours
Osteoporosis 50 2 - 6 30 24 hours
Arthrosis 12 2 - 6 20 24 hours
Arthritis 25 2 - 6 20 24 hours
Headache 3 2 - 6 15 24 hours
Neuralgias 25 2 - 6 15 24 hours
Cervicalgias 12 2 - 6 15 24 hours
Sprains 50 2 - 6 15 24 hours
Fractures 50 2 - 6 30 24 hours
Cicatrizations 50 2 - 6 20 24 hours
Ulcers 50 2 - 6 20 24 hours
Intercostal
Neuralgia
Lumbalgy 25 2 - 6 15 24 hours
Sciatalgy 25 2 - 6 15 24 hours
Trigeminal
Neuralgias
12 2 - 6 20 24 hours
12 2 - 6 15 24 hours
Treatment
cycles
Treatment
interval
I.A.C.E.R. Srl 18 MNPG52-02

15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
Varicose Ulcers 50 2 - 6 20 24 hours
Periarthritis 50 2 - 6 20 24 hours
Coxartrosis 50 2 - 6 20 24 hours
Muscular atrophy 25 2 - 6 20 24 hours
Muscular
contracture
Osteonecrosis 50 2 - 6 20 24 hours
Treat. 1 Hz 1 free free 24 hours
Treat. 3 Hz 3 free free 24 hours
Treat. 5 Hz 5 free free 24 hours
Treat. 10 Hz 10 free free 24 hours
Treat. 15 Hz 15 free free 24 hours
Treat. 20 Hz 20 free free 24 hours
Treat. 30 Hz 30 free free 24 hours
Treat. 40 Hz 40 free free 24 hours
Treat. 50 Hz 50 free free 24 hours
Treat. 60 Hz 60 free free 24 hours
Treat. 70 Hz 70 free free 24 hours
Treat. 80 Hz 80 free free 24 hours
Treat. 90 Hz 90 free free 24 hours
Treat. 100 Hz 100 free free 24 hours
Autoscan* * 2 - 6 20 24 hours
12 2 - 6 15 24 hours
*Autoscan program allows to adjust the desired therapy time then
it will start automatically a frequency cycle from 10 Hz to 100 Hz
with a time therapy of 5 minutes for each frequency. It’s an ideal
program for the regeneration of both hard tissues (bones) and soft
tissues (tendons, ligaments) in the same treatment.
Therapy duration values are recommended by I.A.C.E.R. S.r.l. however
the user can adjust the time as he prefers. MAG2000 uses therapy time
values, working frequency values and field intensity values coming from
scientific and medical literature, as result of well known sperimentations
and clinical evaluations (Barker - Lunt 1983, Bassett – Pawluk – Pilla
1974, Bassett - Valdes – Hernandez 1982).
I.A.C.E.R. Srl 19 MNPG52-02

Set up ( language selection)
Move the ON/OFF switch, placed on device upper side, to the ON
position. Immediately after keeping pressed the button until the
language list appears on the display. Release the button: select the
chosen language by using the buttons.
Press OK key to confirm your selections.
Maintenance
Functioning control
Mag2000 equipment offers a magnet in order to control the device
functioning.
Control procedure:
1. switch on the device in according to user manual safety prescriptions;
2. start a treatment in according to user manual instructions;
3. get the magnet and put it near to applicator;
4. check magnet vibration (it will be proportional to selected treatment
frequency).
Please contact the manufacturer in case of magnet vibration absence.
Cleaning
Clean the equipment from the dust using a dry soft cloth.
Resistant stains can be removed using a sponge soaked in solution of
water and alcohol (20%).
We recommend to disconnect the applicator from the device before
cleaning the elastic therapeutic belt with 3 solenoids or the circular cases
of professional solenoids couple.
• Extract the cable with 3 solenoids by removing the 2 silver studs
through a screwdriver or open the circular cases through lateral
zip.
I.A.C.E.R. Srl 20 MNPG52-02

• Clean the tissue using water and mild soap and wait for the
complete drying before reconnecting the applicators.
ATTENTION: always respect the applicators polarity paying attention to
insert the bobbins with the side indicated by + symbol turned to the green
side of elastic belt (therapeutic side)
Carriage and storage
Carriage precautions
MAG2000 is a portable device, so it does not need any particular carriage
precautions.
However we recommend to put away MAG2000 and its accessories in
their own bag after every treatment.
We recommend to not roll up power supply and applicators cables.
Storage precautions
MAG 2000 is protected till following environmental conditions:
Outside of the packaging
Temperature from +5 to + 40 °C
Rel. humidity from 30 to 80%
Pressure from 500 to 1060 hPa
Inside of the packaging
Temperature from –10 to +55 °C
Rel. humidity from 10 to 90%
Pressure from 500 to 1060 hPa
Disposal
The equipment is subjected to WEEE regulations (see the symbol
on the label) concerning separate waste collection: when disposing
this product, please use the designed areas for disposing electronic
waste or contact the manufacturer.
I.A.C.E.R. Srl 21 MNPG52-02

Troubleshooting
If it is used in accordance with the instructions of the user manual,
MAG2000 does not need a particular regular maintenance.
If you find any malfunctioning using MAG2000, please follow these
instructions:
• check the main integrity by connecting a running device to the same
main;
• check the connection with power supply and connection cables
integrity;
• check the correct connection between MAG2000 and the applicator
(or the applicators);
• check all the operations have been done properly;
• every two years we suggest a complete check of device (contact the
manufacturer or locator dealer).
If you find any problems contact immediately the National Distributor or the
manufacturer at the following address:
I.A.C.E.R. S.r.l.
Via S. Pertini, 24/a • 30030 Martellago (VE) - ITALY
Tel. +39 0415401356 • Fax +39 0415402684
www.iacer.it • iacer@iacer.it
Assistance
Every intervention on device must be performed by manufacturer. For
any assistance intervention contact the National Distributor or the
manufacturer at the following address:
I.A.C.E.R. S.r.l.
Via S. Pertini, 24/a • 30030 Martellago (VE) - ITALY
Tel. +39 0415401356 • Fax +39 0415402684
www.iacer.it • iacer@iacer.it
You can get any technical documentation on spare parts but only prior
business authorization.
I.A.C.E.R. Srl 22 MNPG52-02
Spare parts
For original spare parts contact the National Distributor or the
manufacturer at following address:
I.A.C.E.R. S.r.l.
Via S. Pertini, 24/a • 30030 Martellago (VE) - ITALY
Tel. +39 0415401356 • Fax +39 0415402684
www.iacer.it • iacer@iacer.it
To preserve product warranty, functionality and product safety we
recommend to use only original spare parts.
Warranty
Make reference to the national laws for any warranty conditions by
contacting the national distributor (or directly the manufacturer IACER).
MAG2000. All rights reserved. MAG2000 and logos are owned by I.A.C.E.R
Srl and are registered.
I.A.C.E.R. Srl 23 MNPG52-02

 Loading...
Loading...