Page 1
r
Effective date: 2014-12-30 Number:
1155867904
H. pylori Rapid Test Device
(Whole Blood/Serum/Plasma)
Package Insert
English
A rapid test for the qualitative detection of antibodies to Helicobacter pylori (H. pylori) in whole blood,
serum, or plasma.
For professional in vitro diagnostic use only.
INTENDED USE
The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid chromatographic
immunoassay for the qualitative detection of antibodies to H. pylori in whole blood, serum, or plasma
to aid in the diagnosis of H. pylori infection in adults 18 years of age and older.
SUMMARY
H. pylori is a small, spiral-shaped bacterium that lives in the surface of the stomach and duodenum. It is
implicated in the etiology of a variety of gastrointestinal diseases, including duodenal and gastric ulcer,
non-ulcer dyspepsia and active and chronic gastritis.
used to diagnose H. pylori infection in patients with symptoms of gastrointestinal disease. Specimendependent and costly invasive diagnostic methods include gastric or duodenal biopsy followed by
urease testing (presumptive), culture, and/or histologic staining.3 Non-invasive techniques include the
urea breath test, which requires expensive laboratory equipment and moderate radiation exposure, and
serological methods.
with histologically confirmed H. pylori infection.
The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) is a simple test that utilizes a
combination of H. Pylori antigen coated particles and anti-human IgG to qualitatively and selectively
detect H. pylori antibodies in whole blood, serum, or plasma in just minutes.
4,5
Individuals infected with H. pylori develop antibodies which correlate strongly
1,2
Both invasive and non-invasive methods are
6,7,8
PRINCIPLE
The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative membrane based
immunoassay for the detection of H. pylori antibodies in whole blood, serum, or plasma. In this test
procedure, anti-human IgG is immobilized in the test line region of the test. After specimen is added to the
specimen well of the device, it reacts with H. pylori antigen coated particles in the test. This mixture migrates
chromatographically along the length of the test and interacts with the immobilized anti-human IgG. If the
specimen contains H. pylori antibodies, a colored line will appear in the test line region indicating a positive
result. If the specimen does not contain H. pylori antibodies, a colored line will not appear in this region
indicating a negative result. To serve as a procedural control, a colored line will always appear in the control
line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
REAGENTS
The test contains H. pylori antigen coated particles and anti-human IgG coated on the membrane.
PRECAUTIONS
• For professional in vitro diagnostic use only. Do not use after the expiration date.
• Do not eat, drink or smoke in the area where the specimens or kits are handled.
• Do not use test if pouch is damaged.
• Handle all specimens as if they contain infectious agents. Observe established precautions against
microbiological hazards throughout testing and follow the standard procedures for proper disposal of
specimens.
• Wear protective clothing such as laboratory coats, disposable gloves and eye protection when
specimens are being tested.
• The used test should be discarded according to local regulations.
• Humidity and temperature can adversely affect results.
STORAGE AND STABILITY
Store as packaged in the sealed pouch at room temperature or refrigerated (2-30°C). The test is stable
through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until
use. DO NOT FREEZE. Do not use beyond the expiration date.
SPECIMEN COLLECTION AND PREPARATION
• The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) can be performed using whole blood
(from venipuncture or fingerstick), serum, or plasma.
• To collect Venipuncture Whole Blood specimens: Collect anti-coagulated blood specimen (sodium
or lithium heparin, potassium or sodium EDTA, sodium oxalate, sodium citrate) following standard
laboratory procedures.
• To collect Fingerstick Whole Blood specimens:
• Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow to dry.
• Massage the hand without touching the puncture site by rubbing down the hand towards the
fingertip of the middle or ring finger.
• Puncture the skin with a sterile lancet. Wipe away the first sign of blood.
• Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site.
• Add the Fingerstick Whole Blood specimen to the test by using a capillary tube:
• Touch the end of the capillary tube to the blood until filled to the line. Avoid air bubbles.
• Place the bulb onto the top end of the capillary tube, then squeeze the bulb to dispense the whole
blood to the specimen well (S) of the test device.
• Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear, non-
hemolyzed specimens.
• Testing should be performed immediately after specimen collection. Do not leave the specimens at
room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up
to 3 days. For long term storage, specimens should be kept below -20°C. Whole blood collected by
venipuncture should be stored at 2-8°C if the test is to be run within 2 days of collection. Do not
freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
• Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed
and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
• If specimens are to be shipped, they should be packed in compliance with local regulations covering
the transportation of etiologic agents.
MATERIALS
Materials Provided
• Test devices • Droppers • Buffer
• Package insert
• Specimen collection containers • Lancets (for fingerstick whole blood only)
• Centrifuge • Time
• Heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
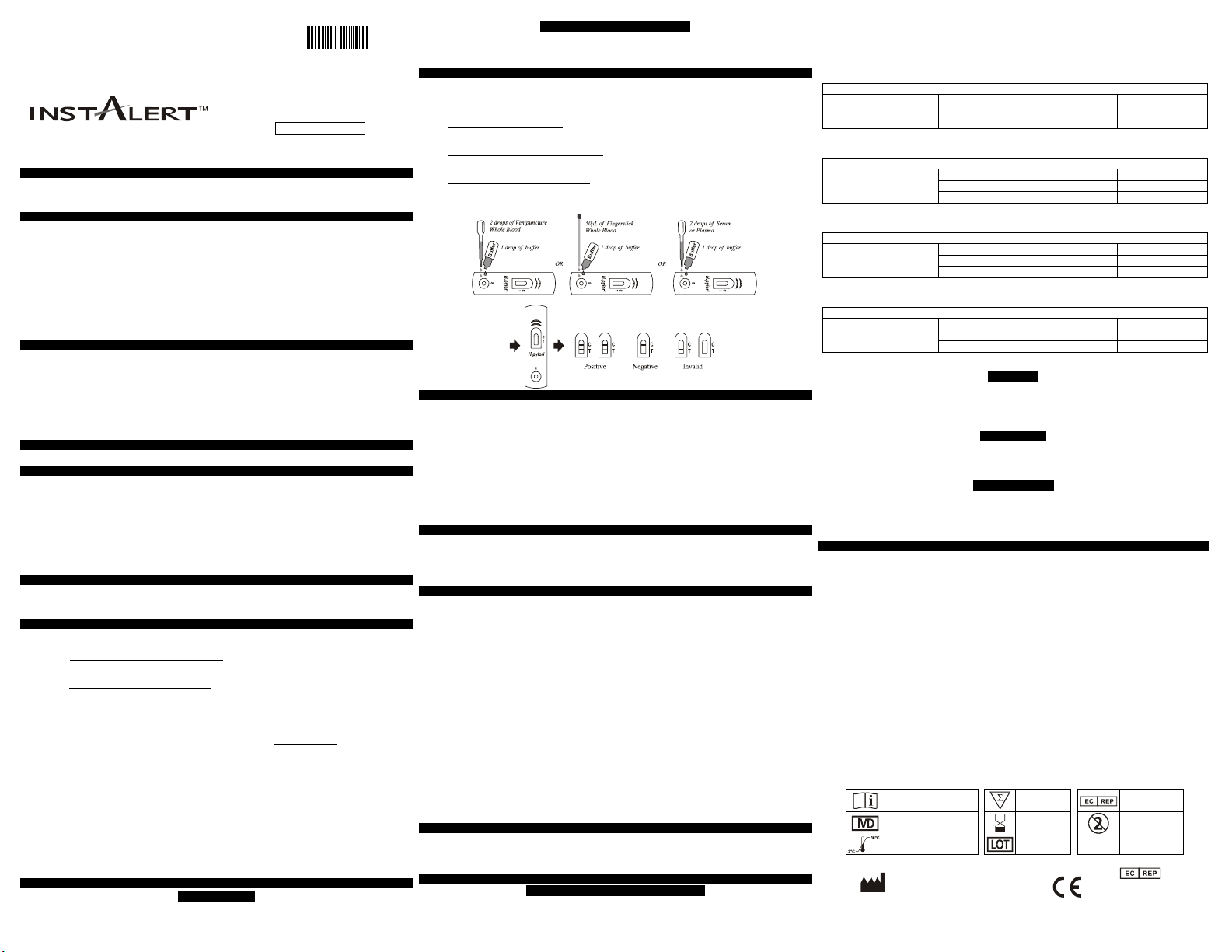
DIRECTIONS FOR USE
Materials Required But Not Provided
Allow the test, specimen, buffer, and/or controls to reach room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test device from the sealed
pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
For Serum or Plasma specimens: Hold the dropper vertically and transfer 2 drops of serum or
plasma (approximately 50 µL) to the specimen well (S) of the test device, then add 1 drop of buffer
to the specimen well (S). Start the timer. See illustration below.
For Venipuncture Whole Blood specimens: Hold the dropper vertically and transfer 2 drops of
whole blood (approximately 50 µL) to the specimen well (S) of the test device, then add 1 drop of
buffer and start the timer. See illustration below.
For Fingerstick Whole Blood specimens: Fill the capillary tube and transfer approximately 50 µL of
fingerstick whole blood to the specimen well (S) of the test device, then add 1 drop of buffer and start
the timer. See illustration below.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 15 minutes.
INTERPRETATION OF RESULTS
POSITIVE:* Two lines appear. One colored line should be in the control line region (C) and another
apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration
of H. pylori antibodies in the specimen. Therefore, any shade of color in the test line region (T) should
be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line
region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural
techniques are the most likely reasons for control line failure. Review the procedure and repeat the test
with a new test. If the problem persists, discontinue using the test kit immediately and contact your
local distributor.
(Please refer to the illustration above)
QUALITY CONTROL
An internal procedural control is included in the test. A colored line appearing in the control line
region (C) is an internal positive procedural control. It confirms sufficient specimen volume, adequate
membrane wicking and correct procedural technique.
Control standards are not supplied with this kit; however, it is recommended that positive and negative controls
be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
1. The H. pylori Rapid Test Device (Whole Blood/ Serum/Plasma) should be used only to evaluate patients
with clinical signs and symptoms suggestive of gastrointestinal disease and is not intended for use with
asymptomatic patients.
2. The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) is for in vitro diagnostic use only. The
test should be used for the detection of H. pylori antibodies in whole blood, serum or plasma specimens
only. Neither the quantitative value nor the rate of increase in H. pylori antibody concentration can be
determined by this qualitative test.
3. The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) will only indicate the presence of H. pylori
antibodies in the specimen and should not be used as the sole criteria for the diagnosis of H. pylori infection.
4. Grossly hemolysed samples will yield invalid results. Strictly follow the Package Insert instructions to
obtain accurate results.
5. A positive result does not allow one to distinguish between active infection and colonization by H. pylori.
6. A positive result only indicates the presence of IgG antibody to H. pylori and does not necessarily indicate
that gastrointestinal disease is present.
7. A negative result indicates that IgG antibody to H. pylori is not present or is below the detection limit of the
test.
8. As with all diagnostic tests, all results must be interpreted together with other clinical information
available to the physician.
9. Literature references have suggested cross reactivity of IgG antibody with a closely related organism,
Borrelia burgdorferi. Performance of this assay has not been evaluated with this organism. Therefore, the
specificity of this test device is not known if this organism is encountered.
10.If the test result is negative and clinical symptoms persist, additional testing using other clinical methods
is recommended. A negative result does not at any time preclude the possibility of H. pylori infection.
11.This assay has not been established for patients under 18 years of age.
H. pylori infection is present worldwide and has been shown to correlate with age, ethnic background,
family size, and socioeconomic class.
2% annually.10 Eighty to 100% of individuals with signs and symptoms of other gastrointestinal
conditions such as duodenal ulcers are reported to be positive for H. pylori infection.11
The H. pylori Rapid Test Device (Whole Blood/Serum/Plasma) has been evaluated with specimens
obtained from a population of symptomatic and asymptomatic individuals who presented for
endoscopic examination. Culture and/or Histology of biopsy specimens served as the reference method.
PERFORMANCE CHARACTERISTICS
Clinical Sensitivity, Specificity and Accuracy
LIMITATIONS
EXPECTED VALUES
9
In the United States, the incidence of infection may increase 1-
Of the 321 fresh clinical samples collected, 136 were considered biopsy positive and 185 clinical
specimens were considered biopsy negative. Biopsy “positive” was defined as either or both culture
and histology are positive and biopsy “negative” was defined as both culture and histology negative.
The results for each sample matrix are summarized below.
H. pylori
Test Device
Sensitivity = 121/136 = 89% (82%-94%)* Specificity = 164/185 = 89% (83%-93%)*
Accuracy = 285/321 = 89% (85%-92%)*
H. pylori
Test Device
Sensitivity = 120/136 = 88% (81%-93%)* Specificity = 164/185 = 89% (83%-93%)*
Accuracy = 284/321 = 88% (84%-92%)*
H. pylori
Test Device
Sensitivity = 54/62 = 87% (76%-94%)* Specificity = 76/88 = 86% (77%-93%)*
Accuracy = 130/150 = 87% (80%-92%)*
H. pylori
Test Device
Sensitivity = 119/136 = 88% (81%-93%)* Specificity = 163/185 = 88% (83%-92%)*
Accuracy = 282/321 = 88% (84%-91%)* *Denotes 95% Confidence Interval
Three physicians’ offices were used to conduct an evaluation of the H. pylori Rapid Test Device (Whole
Blood/Serum/Plasma). Personnel with various educational backgrounds performed the testing. Each
physician’s office tested a randomly coded panel of samples consisting of negative (20), low positive (20)
and medium positive (20) for three days. The results obtained had a >99% correlation with the expected
results.
Sera containing known amounts of IgG antibodies to H. pylori have been tested with C. jejuni, C. fetus,
C. coli, P. aeruginosa and E. coli. No cross-reactivity was observed, indicating that the H. pylori Rapid
Test Device (Whole Blood/ Serum/ Plasma) has a high degree of specificity for human serum IgG
antibodies to H. pylori.
No interference with the H. pylori Rapid Test Device (Whole Blood/Serum/ Plasma) results was
observed in samples containing high levels of hemoglobin (up to 1000 mg/dL), bilirubin (up to 1000
mg/dL) and human serum albumin (up to 2000 mg/mL). The test results were also unaffected when the
hematocrit was altered ranging from 20% to 67%. 600mg/dL triglyceride concentration sample did not
interfere with test performance.
1. Marshall, BJ, McGechie, DB, Rogers, PAR and Glancy, RG. Pyloric Campylobacter infection and
gastroduodenal disease. Med. J. Australia. (1985), 149: 439-44.
2. Soll, AH. Pathogenesis of peptic ulcer and implications for therapy. New England J. Med. (1990), 322: 909-16.
3. Hazell, SL, et al. Campylobacter pyloridis and gastritis I: Detection of urease as a marker of bacterial
colonization and gastritis. Amer. J. Gastroenterology. (1987), 82(4): 292-96.
4. Loffeld, RJLF, et al. Usefulness of several commercial enzyme-linked immunoassays for detection of Helicobacter
pylori infection in clinical medicine. Euro. J. Gastroen. Hepa. (1993) 5:333-37.
5. Cutler, AF, et al. Accuracy of invasive and non-invasive tests to diagnose Helicobacter pylori infection.
Gastroenterology.(1995), 109: 136-141.
6. Ansorg, R, Von Recklinghausen, G, Pomarius, R and Schmid, EN. Evaluation of techniques for isolation,
subcultivation and preservation of Helicobacter pylori. J. Clin. Micro. (1991), 29:51-53.
7. Pronovost, AP, Rose, SL, Pawlak, J, Robin, H and Schneider, R. Evaluation of a new immunodiagnostic assay for
Helicobacter pylori antibody detection: Correlation with histopathological and microbiological results. J. Clin.
Micro. (1994), 32: 46-50.
8. Megraud, F, Bassens-Rabbe, MP, Denis, F, Belbouri, A and Hoa, DQ. Seroepidemiology of Campylobacter pylori
infection in various populations. J. Clin. Micro. (1989), 27: 1870-3.
9. Lotfeld, R.J.L.F., E. Slobberingh, J.P. Van Spreeuwel, J.A. Flendrig, & J.W. Arends. The prevalence of anti-
Helicobacter (Campylobacter) pylori antibodies in patients and healthy blood donors. J. Med. Microbiol. (1991),
32:105-109.
10.Graham, D.Y. H.M. Malaly, D.G. Evans, D.J. Evans, Jr., P.D. Klein, & E. Adam. Epidemiology of Helicobacter
pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status.
Gastroenterology. (1991), 100:1495-1501.
11.Perez-Perez, G, Dworkin, B, Chodos, J, Blaser, M. Campylobacter pylori antibodies in humans. Annals of Internal
Med. (1988), 109:11-17.
Manufacturer
H. pylori Rapid Test Device vs. Biopsy/Histology
Method Biopsy/Histology
Method Biopsy/Histology
Method Biopsy/Histology
Method Biopsy/Histology
SERUM
Results Positive Negative
Positive 121 21
Negative 15 164
PLASMA
Results Positive Negative
Positive 120 21
Negative 16 164
FINGERSTICK
Results Positive Negative
Positive 54 12
Negative 8 76
VENOUS WHOLE BLOOD
Results Positive Negative
Positive 119 22
Negative 17 163
POL Studies
Cross-Reactivity
Interference Studies
BIBLIOGRAPHY
Consult instructions for
use
For in vitro
diagnostic use only
Store between 2-30°C Lot Number
Innovacon, Inc.
9975 Summers Ridge Road
San Diego, CA 92121, USA
Index of Symbols
Tests per kit
Use by Do not reuse
REF
30175 Hannover, Germany
Authorized
Representative
Catalog #
MDSS GmbH
Schiffgraben 41
 Loading...
Loading...