FOB Rapid Test Cassette (Feces)
REF TFO-602
English
Index of Symbols
Attention, see
instructions for use
Tests per
kit
Authorized
Representative
For in vitro
diagnostic use only
Use by
Do not reuse
Store between 2-30°C
Lot
Number
REF
Catalog #
Do not use if package is
damaged
Number:
145054803
Effective date:
2016-08-01
Method
Other Rapid Test
Total
Result
FOB Rapid Test Cassette
(Feces)
Results
Positive
Negative
Positive
205 6 211
Negative 5 800
805
Total Result
210
806
1016
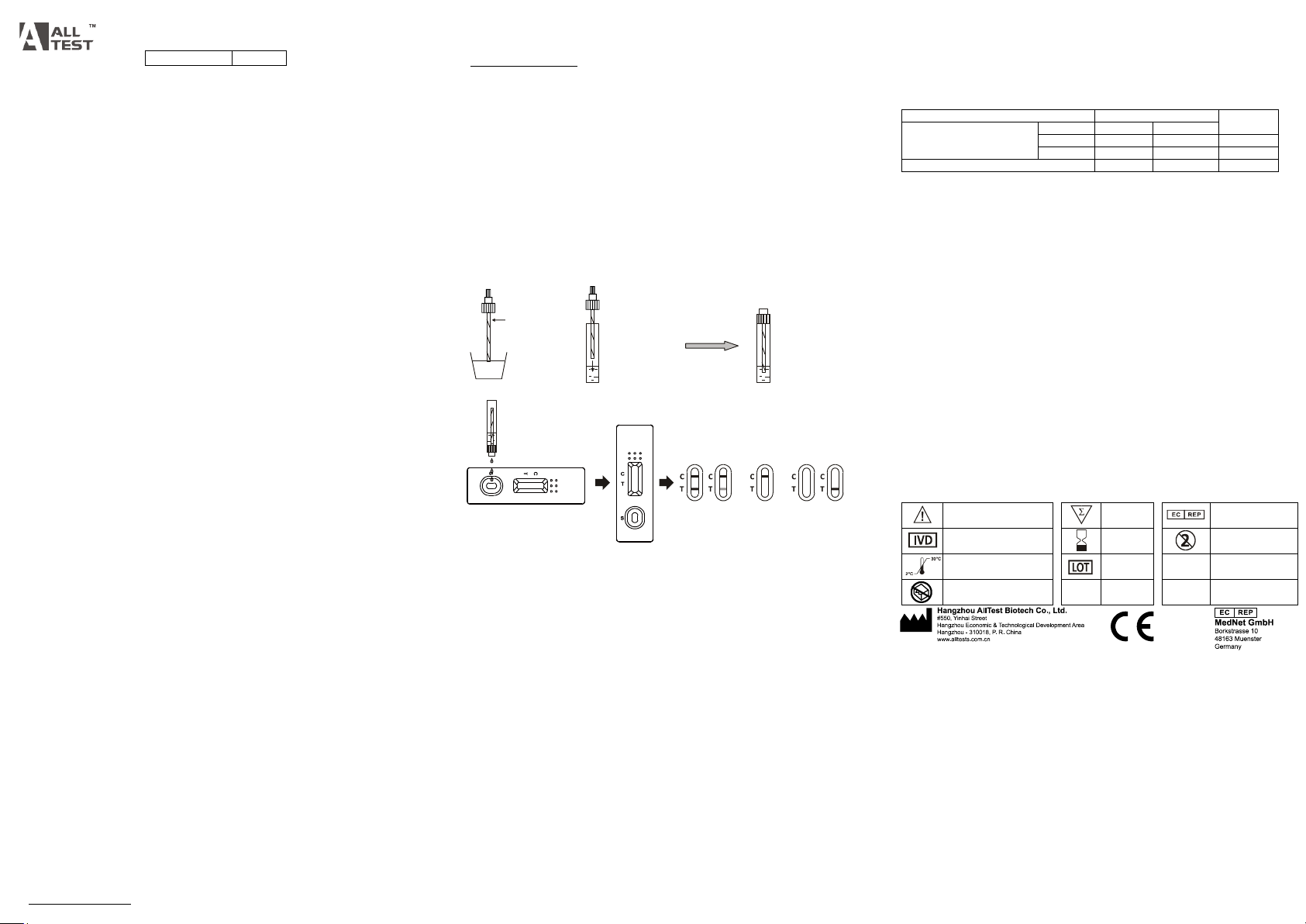
Collect the feces
Place the appllcator
into the dillution buffer
and mix well
2 Minutes
Leave the tube alone
Open the cap
3 Drops of Specimen
FOB
FOB
Positive Negative Invalid
Feces
Package Insert
A rapid, one step test for the qualitative detection of Human Occult Blood in feces.
For professional in vitro diagnostic use only.
【INTENDED USE】
The FOB Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for
the qualitative detection of Human Occult Blood in feces.
【SUMMARY】
Many diseases can cause hidden blood in the feces. This is also known as Fecal
Occult Blood (FOB), Human Occult Blood, or Human Hemoglobin. In the early stages,
gastrointestinal problems such as colon cancer, ulcers, polyps, colitis, diverticulitis,
and fissures may not show any visible symptoms, only occult blood. Traditional
guaiac-based methods lack sensitivity and specificity, and also have diet restrictions
prior to testing.
The FOB Rapid Test Cassette (Feces) is a rapid test to qualitatively detect low levels
of Fecal Occult Blood. The test uses a double antibody sandwich assay to selectively
detect Fecal Occult Blood at 40ng/ml or higher, or 4.8μg/g feces. In addition, unlike
guaiac assays, the accuracy of the test is not affected by the diet of the patients.
1,2
【PRINCIPLE】
The FOB Rapid Test Cassette (Feces) is a qualitative, lateral flow immunoassay for
the detection of Human Occult Blood in feces. The membrane is precoated with antihemoglobin antibody on the test line region of the test. During testing, the specimen
reacts with the particle coated with anti-hemoglobin antibody. The mixture migrates
upward on the membrane chromatographically by capillary action to react with antihemoglobin antibody on the membrane and generate a colored line. The presence of
this colored line in the test line region indicates a positive result, while its absence
indicates a negative result. To serve as a procedural control, a colored line will always
appear in the control line region, indicating that the proper volume of specimen has
been added and membrane wicking has occurred.
【REAGENTS】
The test contains anti-hemoglobin antibody particles and anti-hemoglobin antibody
coated on the membrane.
【PRECAUTIONS】
For professional in vitro diagnostic use only. Do not use after expiration date.
The test should remain in the sealed pouch until use.
Do not eat, drink or smoke in the area where the specimens or kits are handled.
Handle all specimens as if they contain infectious agents. Observe established
precautions against microbiological hazards throughout all procedures and follow
the standard procedures for proper disposal of specimens.
Wear protective clothing such as laboratory coats, disposable gloves and eye
protection when specimens are assayed.
The used test should be discarded according to local regulations.
Humidity and temperature can adversely affect results.
【STORAGE AND STABILITY】
The kit can be stored at room temperature or refrigerated (2-30°C). The test cassette
is stable through the expiration date printed on the sealed pouch. The test cassette
must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond the
expiration date.
【SPECIMEN COLLECTION AND PREPARATION】
1. Specimens should not be collected during or within three days of a menstrual
period, or if the patient suffers from bleeding hemorrhoids or blood in the urine.
2. Alcohol, aspirin and other medications taken in excess may cause gastrointestinal
irritation resulting in occult bleeding. Such substances should be discontinued at
least 48 hours prior to testing.
3. No dietary restrictions are necessary before using the FOB Rapid Test Cassette.
【MATERIALS】
• Test cassettes • Specimen collection tubes with extraction buffer • Package insert
• Specimen collection containers • Timer • Droppers
【DIRECTIONS FOR USE】
Allow the test, specimen, buffer and/or controls to reach room temperature (1530°C) prior to testing.
1. To collect fecal specimens:
Collect sufficient quantity of feces (1-2 mL or 1-2 g) in a clean, dry specimen
collection container to obtain maximum antigens (if present). Best results will be
obtained if the assay is performed within 6 hours after collection. Specimen
collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long
term storage, specimens should be kept below -20℃.
2. To process fecal specimens:
• For Solid Specimens:
Materials Provided
Materials Required But Not Provided
Unscrew the cap of the specimen collection tube,then randomly stab the specimen
collection applicator into the fecal specimen in at least 3 different sites to collect
approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal
specimen.
• For Liquid Specimens:
Hold the dropper vertically, aspirate fecal specimens, and then transfer 3 drops
(approximately 120 μL) into the specimen collection tube containing the extraction
buffer.
3. Tighten the cap onto the specimen collection tube, then shake the specimen
collection tube vigorously to mix the specimen and the extraction buffer. Leave the
tube alone for 2 minutes.
4. Bring the pouch to room temperature before opening it. Remove the test cassette
from the foil pouch and use it within one hour. Best results will be obtained if the test
is performed immediately after opening the foil pouch.
5. Hold the specimen collection tube upright and open the cap onto the specimen
collection tube. Invert the specimen collection tube and transfer 3 full drops of the
extracted specimen (approximately 120 μL) to the specimen well (S) of the test
cassette, then start the timer. Avoid trapping air bubbles in the specimen well (S).
See illustration below.
6. Read results at 5 minutes after dispensing the specimen. Do not read results after
10 minutes.
7. Note: If the specimen does not migrate (presence of particles), centrifuge the
extracted specimens contained in the extraction buffer vial. Collect 120 μL of
supernatant, dispense into the specimen well (S) of a new test cassette and start
afresh following the instructions mentioned above.
【INTERPRETATION OF RESULTS】
POSITIVE:* Two lines appear. One colored line should be in the control line region (C)
and another apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the
concentration of Fecal Occult Blood present in the specimen. Therefore, any shade of
color in the test line region (T) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in
the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect
procedural techniques are the most likely reasons for control line failure. Review the
procedure and repeat the test with a new test. If the problem persists, discontinue
using the test kit immediately and contact your local distributor.
(Please refer to the illustration above)
【QUALITY CONTROL】
Internal procedural controls are included in the test. A colored line appearing in the
control region (C) is an internal valid procedural control. It confirms sufficient specimen
volume and correct procedural technique.
Control standards are not supplied with this kit; however, it is recommended that
positive and negative controls be tested as a good laboratory practice to confirm the
test procedure and to verify proper test performance.
【LIMITATIONS】
1. The FOB Rapid Test Cassette (Feces) is for in vitro diagnostic use only.
2. The FOB Rapid Test Cassette (Feces) will only indicate the presence of Fecal
Occult Blood, the presence of blood in feces does not necessarily indicate
colorectal bleeding.
3. As with all diagnostic tests, all results must be considered with other clinical
information available to the physician.
4. Other clinically available tests are required if questionable results are obtained.
【EXPECTED VALUES】
The FOB Rapid Test Cassette (Feces) has been compared with another leading
commercial rapid test. The correlation between this two system is 98.9%
【PERFORMANCE CHARACTERISTICS】
The FOB Rapid Test Cassette (Feces) has been compared with another leading
commercial rapid test using clinical specimens.
Relative sensitivity: 97.6% (95%CI*: 94.5%~99.2%);
Relative specificity: 99.3% (95%CI*: 98.4%~99.7%);
Accuracy: 98.9% (95%CI*: 98.1%~99.5%). *Confidence Intervals
The FOB Rapid Test Cassette (Feces) can detect levels of Fecal Occult Blood as low
as 40ng/ml or 4.8 μg/g feces.
Within-run precision has been determined by using 15 replicates of three specimens:
40ng/ml, 200ng/ml and 10μg/ml positive specimens. The specimens were correctly
identified >99% of the time.
Between-run precision has been determined by 15 independent assays on the same
three specimens: 40ng/ml, 200ng/ml and 10μg/ml positive specimens. Three different
lots of the FOB Rapid Test Cassette (Feces) have been tested using these specimens.
The specimens were correctly identified >99% of the time.
The FOB Rapid Test Cassette (Feces) is specific to human hemoglobin. Specimens
containing the following substances were diluted in the extraction buffer to a
concentration of 1.0 mg/ml, and tested on both positive and negative controls with no
effect on test results: Bovine hemoglobin, Chicken hemoglobin, Pork hemoglobin,
Goat hemoglobin, Horse hemoglobin, Rabbit hemoglobin and Turkey hemoglobin.
Accuracy
Sensitivity
Precision
Intra-Assay
Inter-Assay
Cross-reactivity
【BIBLIOGRAPHY】
1. Simon JB. Occult Blood Screening for Colorectal Carcinoma: A Critical Review,
Gastroenterology, 1985; 88: 820.
2. Blebea J, Mcpherson RA. False-Positive Guaiac Testing With Iodine, Arch
PatholLab Med, 1985;109:437-40.
 Loading...
Loading...