Page 1

PROFESSIONAL MEDICAL PRODUCTS
AEROSOL EOLO
EOLO NEBULIZER
NÉBULISEUR EOLO
EOLO NEBULIZADOR
INALADOR EOLO
EOLO INHALATOR
ΝΕΦΕΛΟΠΟΙΗΤΗΣ EOLO
M28091-M-Rev.9-09.20
28091
28097
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
Made in Italy
(spina UK / british plug / prise britannique / britische Socket /
enchufe británico /soquete britânico / βύσμα αγγλικού τύπου)
0476
IP21
Page 2

ENGLISH
Aerosol Eolo is a system for aerosol therapy, with 230V ~ / 50Hz power supply, intended for household
use.
The appliance is designed for continuous use.
The medical device is designed to be easy to transport and use and is indicated for nebulizing bronchodilators and antibiotics. Made of plastic housing with high heat and electrical insulation in accordance with the European safety standards.
12
GENERAL INSTRUCTIONS
• CAREFULLY READ THE MANUAL BEFORE USE
• FOR DRUG INHALATION ALWAYS FOLLOW MEDICAL ADVICE
• DO NOT DISASSEMBLE THE APPLIANCE. FOR ANY INTERVENTION, CONTACT THE TECHNICAL
SERVICE OF THE DISTRIBUTOR AND/OR GIMA’s TECHNICAL SERVICE
BASIC SAFETY STANDARDS
1.
When opening the packaging, check the integrity of the appliance by paying particular attention to the
presence of damage to plastic components which may disclose internal live parts of the appliance and
to breakage and/or stripping of the power cable. In this case, do not connect the plug to the socket.
Carry out these operations before each use.
2. Before connecting the appliance, always check that the electrical data indicated on the label and
the type of plug used correspond with the data of the power network to which it is intended to be
connected.
3. Do not leave the appliance plugged unnecessarily: disconnect the plug from the power network if
not used.
4. Comply with the safety standards for electrical appliances. In particular:
- Use only original accessories and components supplied by Gima S.p.A. in order to ensure utmost
efciency and security of the device.
- Never immerse the appliance in water.
- Place the appliance on at and stable surfaces in order to avoid obstructing the cooling openings
located on its sides.
- Do not use the appliance in environments with the presence of ammable anesthetic mixture with
air, oxygen, or nitrous oxide.
- Do not use the appliance with anesthetic and respiratory equipment.
- Avoid touching the appliance with wet hands.
- The use of this appliance by children and/or people with disabilities shall be closely monitored by
an adult with full mental capacity.
- Disconnect the appliance from the power supply if not used.
- Do not pull the power cable to disconnect the plug. Grab the plug with the ngers to pull it out from
the power network.
- Store and use the appliance in environments protected by atmospheric agents and away from
heat sources. After each use, it is recommended to store the device inside its box away from dust
and direct sunlight.
- In general, it is not recommended to use simple or multiple adapters and/or extension cables.
If their use is essential, it is necessary to use devices in compliance with the safety standards,
paying attention not to exceed the maximum power limits indicated on the adapters and on the
extension cables.
5. For repair works contact only Gima’s technical service or a technical center authorized by the manufacturer. The device requires the use of original spare parts. Failure to comply with the above may
compromise the safety of the appliance.
6. This appliance shall be solely intended for the use which was designed for and according
to the instructions of this manual. Therefore, it shall be used as aerosol therapy system. Any
other use is improper and therefore hazardous; the manufacturer shall not be deemed responsible
for damage caused by an improper use or if the appliance is used in electrical installations that do
not comply with the safety regulations in force.
7. The medical device requires specic precautions in terms of electromagnetic compatibility and
shall be installed and used according to the instructions provided along with the accompanying
Page 3

13
ENGLISH
documents: the Eolo device shall be installed and used away from portable and mobile RF communication devices (mobile phones, transceivers, etc.) which may affect the appliance.
8. Some components of the appliance are of small size and might be swallowed by children; keep the
device out of reach of children.
9. Keep the accessories out of reach of children. Children and dependents shall always use the medical device under the close supervision of an adult with full mental capacity. Keep the ampoule out
of reach of children under 36 months as it contains small parts which be may swallowed.
Do not leave the device unattended in areas accessible to minors and / or people with disa-
bilities.
10. Do not leave the device unattended in areas accessible to minors and / or people with limited mental
capacity as they may be strangled with the air hose.
11. The patient can come into contact with the medical device through the nebulizer / masks / mouthpiece and /or nosepiece. These components comply with the requirements of ISO 10993-1, therefore neither allergic reactions nor skin irritation can occur.
12. The product and its parts are biocompatible in accordance with the requirements of EN 60601-1.
13. The device is easy to use. No additional precautions other than the instructions of this manual of use
are required.
14. WARNING: Do not alter this appliance without the authorization of Gima S.p.A..
No electrical and / or mechanical part which the device consists of has been designed to be repaired
by the user.
Failure to do so can compromise the safety of the appliance.
15. The use of the medical device in environmental conditions other than those illustrated in this manual
may jeopardize the safety and the parameters of the appliance.
16. The materials used for the contact with drugs are thermoplastic polymers with high chemical stability and resistance.
Such materials have been tested with commonly-used medications (Salbutamol, Beclomethasone
Dipropionate, Acetylcysteine, Budesonide, Ambroxol) and no interaction has been reported. Nonetheless, given the variety and the ongoing evolution of the medicines used, potential interactions
cannot be ruled out. Therefore, it is recommended to:
- Use up the drug as quickly as possible after its opening.
- Always avoid prolonged contact of the drug with the container. Clean the container immediately
after its use.
- In the event of anomalous situations (e.g. softening or cracks) of the container, do not introduce
any solution and do not inhale. Contact the technical service, specifying the methods of use and
the type of drug used.
17. Remember to:
- use this appliance only with medicines prescribed by your doctor;
- perform the treatment by using only the accessory instructed by your doctor according to the
disease.
Under certain fault conditions, the packaging temperature can raise and there might be risk of
burns if the user comes into contact with such parts. In any event, the temperatures do not exceed
the limit of 105°C [221°F] (ref. Interpretation Sheet IEC 60601-1).
Gima S.p.A. cannot be held liable for accidental or indirect damage resulting from alterations of the device, repairs and/or unauthorized technical interventions, or damage to any of its
part due to accident, misuse and/or abuse.
Any unauthorized intervention on the device, even the slightest one, will immediately invalidate the warranty and does not ensure the compliance with the technical and safety requirements provided by Directive MDD 93/42/EEC (and subsequent amendments) and by the related
reference standards.
Page 4

PRODUCT SPECIFICATIONS
TYPE (Directive 93/42/EEC) Medical device Class IIa
MODEL Eolo
POWER SUPPLY 230V~ / 50Hz
ABSORBED POWER 170 VA
FUSE F 1 x 1.6A L 250V
MAXIMUM PRESSURE 250 kPa (2.5 Bar)
MAXIMUM FLOW (in the compressor) 14 L/min
OPERATING PRESSURE 110 kPa (1.10 Bar)
OPERATIONAL FLOW 5.0 L/min at 110 kPa
ATOMIZATION 0.40 ml/min (with 4ml solution NaCl 0.9%)
MMAD (measured in accordance
with EN 13544-1)
GSD 2.87
WEIGHT 1.65 Kg
SIZE 148 (L) x 223 (H) x 124 (P) mm
MAXIMUM NOISE LEVEL 55 dB (A)
FUNCTIONING Continuous
MINIMUM NEBULIZER VOLUME LEVEL 2ml
MAXIMUM NEBULIZER VOLUME LEVEL 6ml
OPERATING CONDITIONS Room temperature: 5 ÷ 40°C
STORAGE AND TRANSPORT
CONDITIONS
2.44
Percentage of humidity in the environment: 15 ÷ 93% RH
Atmospheric pressure: 700 ÷ 1060 hPa
Room temperature: -25 ÷ 70°C
Percentage of humidity in the environment: 0 ÷ 93% RH
Atmospheric pressure: 500 ÷ 1060 hPa
14ENGLISH
Copy of the EC Declaration of conformity can be requested to Gima S.p.A. - Via Marconi, 1 - 20060 –
Gessate, Milan (Italy)
MMAD = Mass Median Aerodynamic Diameter
GSD = Geometric Standard
Deviation
NB: The measures and the curves are not valid in the event of medications supplied in high viscosity
suspension.
Page 5

15 ENGLISH
CLEANING OF THE APPLIANCE
The cleaning of the device shall be carried out with a soft and dry cloth and with non-abrasive detergents. Do not use excessively wet cloths as the contact of liquids with the electrical parts of the appliance may cause malfunctions or may be hazardous.
While cleaning the device, make sure that no liquids get inside the appliance and that the
power outlet is disconnected.
Wait for the device to dry off before storing or using it again.
MAINTENANCE
The Eolo device does not have any part that requires maintenance and/or lubrication.
Nonetheless, it is necessary to carry out some checks to verify the function and security of the appliance before each use. Remove the appliance from the box and always check that there is no visible
damage; particular attention should be given to any cracks in plastics which may leave some electrical
components exposed. Check also the integrity of the power cable which might have been damaged
during the previous use.
Then connect the cable to the power network and switch it on. Close the compression cap with one
nger and check that there are no disturbing noises which may be evidence of malfunction.
Check that the nebulizer has not suffered any breakage during the previous use (it was stored inappropriately or has suffered impacts). The appliance is protected by a protection fuse (F 1.6A L 250V)
not accessible from outside. Therefore, contact the technical staff authorized by the manufacturer for
its replacement.
Type of defect Cause Corrective action
1. Poor atomization. Clogged ampoule. Clean and disinfect the ampoule as set out in
2. Poor atomization. Clogged ampoule. If the washing had no positive outcome, replace
3. Lack of
atomization.
4. Slow atomization. Excessive oily drug. Dilute the drug with saline.
5. Noisy device. Prolonged use. Contact the reseller or Gima’s technical service.
Defects 1 - 2 - 3 -
4 - 5
Nozzle stuck.
None of the corrective
actions has been effective
the manual.
the ampoule.
Press hard the nozzle (cylindrical immersion
tube) placed inside the polycarbonate ampoule
bottom with a nger.
Contact the reseller or Gima’s technical service.
If the appliance still does not nebulize after having checked the above mentioned conditions, it is recommended to contact the reseller or Gima’s technical service.
BEFORE CARRYING OUT ANY CHECKS IN THE EVENT OF ANOMALIES OR MALFUNC-
TIONS, CONTACT GIMA’S TECHNICAL SERVICE. THE MANUFACTURER OFFERS NO GUARANTEE FOR THE APPLIANCES WHICH HAVE ASSESSED AS TAMPERED AFTER THE CHECKS
CARRIED OUT BY THE TECHNICAL SERVICE.
CLEANING AND WASHING OF ACCESSORIES
Before each use and / or after the cleaning operations, check the integrity of all accessories supplied
with the device. Turn off the device before each cleaning operation and disconnect the power cable
from the socket.
PREPARATION
1. Pull out the air hose from the nebulizer leaving it plugged to the air outlet pipe of the device.
2. Rotate the upper part of the nebulizer anticlockwise.
3. Disconnect the internal pisper at the bottom of the nebulizer with nger force.
Page 6

ENGLISH
16
CLEANING
Clean all the components of the nebulizer (except the air hose) before and after each use by choosing
one of the methods described below.
Method 1: Thoroughly clean the components for 5 minutes by using hot drinking tap water (around
40°C – 104°F) and neutral soap.
Method 2: Clean the components (except the air hose) by immersion in a solution of 60% of water and
40% of white vinegar. At the end of the operation, rinse with plenty of hot drinking water (around
40°C – 104°F).
At the end of the cleaning operations, rinse thoroughly by removing excess water and allow to air-dry
in a clean spot.
DO NOT BOIL OR AUTOCLAVE THE AIR HOSE AND THE MASKS.
DO NOT WASH THE ACCESSORIES IN THE DISH WASHER.
WASHING
Where diseases with risk of infection and microbial contamination are present, the end user shall carry
out the washing operations properly. The washing procedure can be performed only if the components
have been previously cleaned (see cleaning section).
For the washing procedure, the following operations shall be carried out:
- Fill a container of suitable size to hold every individual component with drinking water and disinfect-
ant (hypochlorite solution easily available in pharmacies) by complying with the proportions indicated
on the disinfectant packaging.
- The immersion time in the solution is indicated on the packaging of the hypochlorite solution accord-
ing to the concentration chosen for the preparation of the solution.
- Rinse with plenty of warm drinking water until removing any trace of solution, dry and store in a dry
and dust-free place.
- Dispose the solution used according to the instructions provided by the manufacturer of the disinfect-
ant.
SUPPLIED ACCESSORIES
ACCESSORIES
HI-FLO accessories kit
(HI-FLO ampoule, Adult Mask, Children Mask,
Air hose, Mouthpiece and Nosepiece)
1 – Air hose
2 – Ampoule lower part
3 – Nebulizer Nozzle
4 – Ampoule upper part
5 – Mouthpiece
6 – Adult Mask
7 – Children Mask
8 – Nosepiece (non-invasive)
Use only the original accessories intended and indicated by the Manufacturer.
NEBULIZER: For each individual patient, it is recommended to use the nebulizer
for 6 months or up to 120 applications. The nebulizer shall be replaced after a long
period of inactivity, if it is deformed or broken or if the nozzle is clogged by a dry
medicine or medicine in powder form, etc.
Use the Nosepiece only if prescribed by the doctor. NEVER PUT the bifurcations into the nose but
place them as closely as possible.
7
4
6
3
2
8
5
1
Page 7

17
Where diseases with risk of infection and microbial contamination are present, it is recommended to use the accessories and the spray ampoule individually (always seek medical advice).
The device is equipped with a lter which removes any impurities of the air inhaled from the compressor. Check the conditions of the lter on a periodical basis or if the device is no longer efcient. If the
lter is too dirty, it shall be replaced.
AIR FILTER REPLACEMENT: The air lter shall be replaced every 25 hours of operation or when it is too
dirty. For the replacement, lift the lter from its seat and replace it with a new one.
The masks and the air hose shall be replaced when there is evidence of deterioration of their materials.
Expected useful life: More than 1500 operating hours (or 5 years) in accordance with the standard test
and operating conditions. Expected shelf life: up to 5 years from the date of manufacture.
ENGLISH
INSTRUCTIONS FOR USE
• Check the device before each use in order to detect malfunctions and / or damage due to transport
and / or storage.
• When inhaling, the patient must sit upright in a relaxed position at a table and not on an armchair to
avoid compressing the airways and thus compromising the effectiveness of the treatment.
• It is recommended not hold the device in the hands during the therapy and/or avoid prolonged con-
tacts with the casing of the appliance.
WARNING: Place the appliance on at and stable surfaces in order to avoid obstructing the
cooling openings located on its sides.
• Extract the power supply cable and insert the plug into the
mains socket. It is recommended to unwind the entire length
of the power supply cable to prevent dangerous overheating. If the power supply cable is damage and must be replaced contact the Gima technical service;
• Open the nebulizer 2 by unscrewing the lid;
• Pour the medicine prescribed by the doctor into the nebuliz-
er;
• Re-close the nebulizer, re-screwing the lid;
• Connect air pipe 5 to the air exit well 4;
• Connect the other end of the pipe to the connection in the
lower part of the nebulizer;.
• Ensure that the supplied air lter (6) is present;
• Connect the desired accessory to the nebulizer: child mask
or adult mask, mouth-piece or nosepiece;
• Press switch 1 on position I to proceede with nebulization;.
• On completing of nebulization, press the switch on position
0 and remove the plug from the socket;
• Wash the nebulizer and its accessories as indicated in the
cleaning charter;
• Place the cable and accessories inside the box.
5
4
1
3
2
6
Always use the nebulizer facing upwards in order to prevent any substances and / or the medicine from
leaking out of the nebulizer during normal use.
In the event of overlling, empty the ampoule, clean it and repeat the operation. After having poured
the medicine, screw the top again to the bottom and repeat the operations as specied in section
“instructions for use”.
WARNING: The power cable plug is the separation component from the power network; even though
the device has the power on / power off switch, the power plug shall be kept accessible once the
appliance is in use in order to allow an additional method of disconnection from the power network.
Page 8
ENGLISH
NEVER INHALE IN HORIZONTAL POSITION.
DO NOT TILT THE NEBULIZER OVER 60°.
18
ELECTROMAGNETIC INTERFERENCE RISKS AND POTENTIAL
CORRECTIVE MEASURES
This section contains information on the device compliance with EN 60601-1-2 (2015). Eolo is a medical device suitable for household use.
Group ranking and CISPR category: group 1, category B
Avoid using this device close to or overlapped on other appliances because it could not work
properly. If such use is necessary and inevitable, special precautions shall be adopted so that the
electromedical device works properly in its standard conguration (for instance, by steadily and visually
checking the absence of anomalies or malfunctions).
The use of accessories, transducers and cables other those supplied by the manufacturer of the
appliance may cause an increase in the electromagnetic emissions and/or a reduction in the electromagnetic immunity of the device, thus causing a malfunction.
Portable and mobile radio communication devices (mobile phones, transceivers, including peripheral devices like antennas cables and external antennas, etc.) may affect the medical device and should
not be used close to (at more than 30cm from any part of the device, including cables), adjacent to or
overlapped on the medical device. If such use is necessary and inevitable, special precautions shall be
adopted so that the electromedical device works properly in its standard conguration (for instance, by
steadily and visually checking the absence of anomalies or malfunctions).
The tables below provide information on the EMC characteristics (Electromagnetic Compatibility) of this
electromedical appliance.
Guide and declaration of the manufacturer – Electromagnetic Emissions
The Eolo aerosol can be used in the following electromagnetic environment. The Customer and/or the
user of the Eolo aerosol shall make sure that the appliance is used in such environment.
Emission test Conformity Guide to the electromagnetic
Radiated emissions /
Conductions CISPR11
Radiated emissions /
Conductions CISPR11
Harmonic currents
EN 61000-3-2
Voltage uctuations /
icker EN 61000-3-3
Group 1 The Eolo aerosol uses RF energy only for Inter-
Class [B] The Eolo aerosol is designed for use in any envi-
Class [A]
Compliant
environment
nal function. Therefore, its RF emissions are very
low and do not cause any interference with other
nearby electronic appliances.
ronment, including households and those directly
connected to the public power distribution grid
which supplies power to environments intended
for domestic use.
Page 9
19
ENGLISH
Guide and declaration of the manufacturer – Electromagnetic Immunity
The Eolo aerosol can be used in the following electromagnetic environment. The Customer and/or
the user of the Eolo aerosol shall make sure that the appliance is used in such environment.
Immunity test Standard of proof Level of
conformity
Electrostatic
discharge (ESD) EN 61000-4-2
± 8kV contact
± 15kV air
The appliance
does not alter
its status
Guide to the electromagnetic
environment
Floors should be in wood, cement
or ceramics. If oors are covered
by synthetic material, the relative
humidity should be at least 30%
Fast transient / burst
EN 61000-4-4
Surge EN 61000-4-5 ± 0.5kV e± 1kV
Voltage dips, short
outages and
voltage variations
EN 61000-4-11
Network frequency
magnetic eld
EN 61000-4-8
± 2kV power supply
± 1kV signal cables
The appliance
does not alter
its status
The appliance
differential mode
does not alter
its status
5% UT (>95% dip
-- The power supply should be that
in UT) per 0.5 cycle
40% UT (60% dip
in UT) per 5 cycles
70% UT (30% dip
in UT) per 25 cycles
<5% UT (>95% dip
in UT) per 5 s
30 A/m The appliance
does not alter
its status
The power supply should be that
of a typical commercial premise
or hospital.
The power supply should be that
of a typical commercial premise
or hospital.
of a typical commercial premise
or hospital.
If the user of the Eolo aerosol requires the appliance to work continuously, it is recommended to
use it with an uninterruptible power supply.
The power supply should be that
of a typical commercial premise
or hospital.
Note: UT is the value of the supply voltage
Guide and declaration of the manufacturer – Electromagnetic Immunity
The Eolo aerosol can be used in the following electromagnetic environment. The Customer and/or
the user of the Eolo aerosol shall make sure that the appliance is used in such environment.
Immunity
test
Conducted
immunities
EN 61000-4-6
Level set out by
EN 60601-1-2
3Vrms 150kHz
to 80MHz
(for non-lifesupporting appliances)
Level of
conformity
V1 =
3 V rms
Electromagnetic Environment - Guide
Portable and mobile RF communication
devices should be used no closer than the
separation distance from any part of the
Eolo device, including cables, calculated
from the equation applicable to the freRadiated
immunity
EN 61000-4-3
10 V/m 80MHz to
2.7GHz
(for non-life-equipment
appliances)
E1 =
10 V / m
quency of the transmitter.
Recommended separation distances
d= [3.5 / V1] √P
d= [12 / E1] √P 80MHz to 800MHz
d= [23 / E1] √P 800MHz to 2,7GHz
P is the maximum rated output power of
the transmitter in Watt (W) according to
the manufacturer of the transmitter and d
is the recommended separation distance
calculated in meters (m).
Page 10
ENGLISH
20
The eld strengths from xed RF transmit-
ters, as established in an electromagnet-
ic survey of the sitea, could be less than
the level of conformity of each frequency
rangeb.
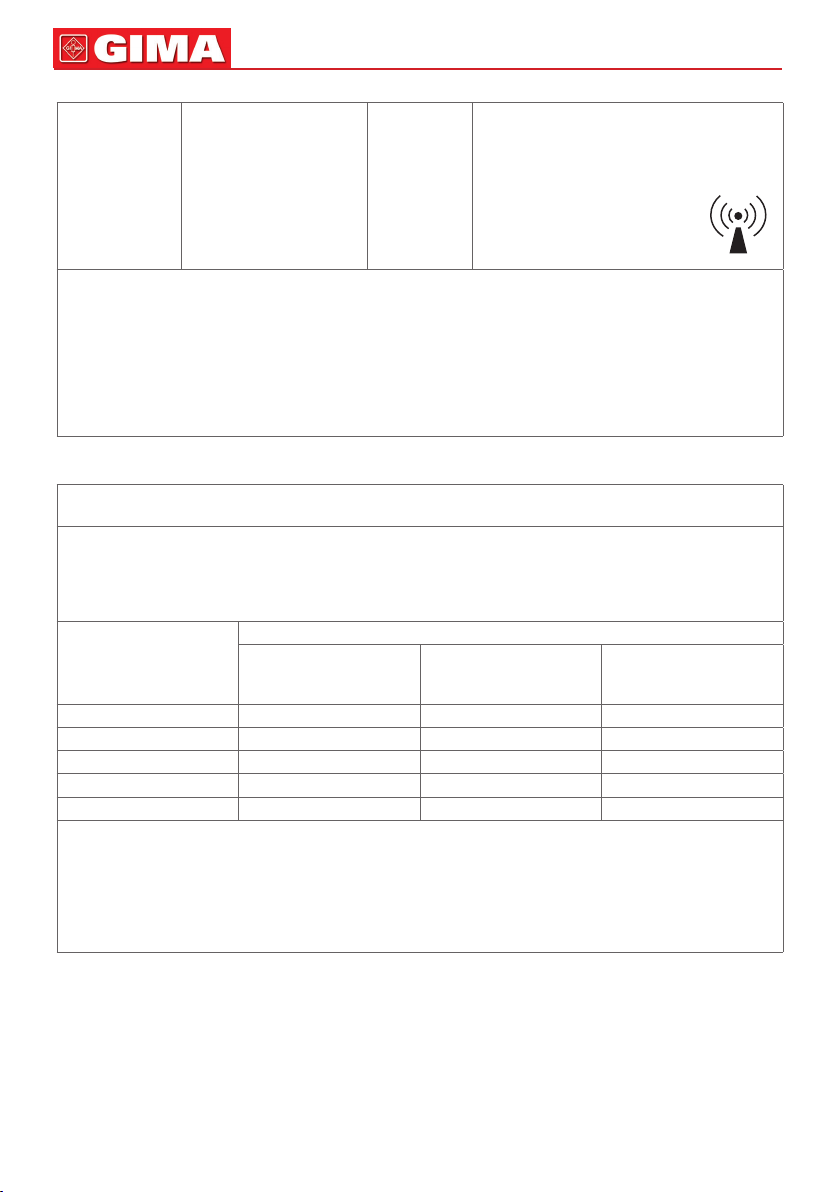
It is possible to check the in-
terference close to the appli-
ances labelled with the follow-
ing symbol:
a. The eld strengths for xed transmitters like base stations of radiotelephones (mobiles and cord-
less) and terrestrial mobile radio networks, amateur radio appliances, AM and FM radio transmitters and TV transmitters cannot be theoretically and accurately predicted. In order to determine an
electromagnetic environment caused by xed RF transmitters, an electromagnetic survey of the site
should be taken into account. If the eld strength measured in the place where the Eolo appliance is
used exceeds the applicable level of conformity hereinabove, the normal operation of the appliance
should be kept under watch. If abnormal performances are noted, additional measures could be
necessary, such as a different direction or positioning of the appliance.
b. The eld strength on a frequency range between 150 kHz and 80 MHz should be less than 10 V/m.
Recommended separation distances between portable and mobile radio communication appliances and the monitor
The Eolo aerosol is designed to operate in an electromagnetic environment in which the RF radiated
interferences are kept under control. The customer or the user of the Eolo appliance can contribute to
prevent electromagnetic interferences by ensuring a minimum distance between portable and mobile
RF communication devices (transmitters) and the Eolo appliance as recommended below, according
to the maximum rated output power of the radio communication devices.
Maximum rated
output power
of the transmitter W
Separation distance at transmitter frequency m
150KHz to 80MHz
d= [3.5 / V1] √P
80MHz to 800MHz
d= [12 / E1] √P
800MHz to 2,7GHz
d= [23 / E1] √P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
The recommended separation distance d in meters (m) for transmitters with maximum rated output
power not specied above can be calculated from the equation applicable to the frequency of the
transmitter, in which P is the maximum rated output power of the transmitter in Watt (W) according to
the manufacturer of the transmitter.
Note 1: At 80 MHz and 800 MHz the separation distance for the highest frequency range applies.
Note 2: These guidelines could not apply to all conditions. The electromagnetic propagation is affect-
ed by the absorption and reection of premises, objects and individuals.
Page 11

21
SYMBOLS
ENGLISH
Caution: read instructions
(warnings) carefully
Keep in a cool, dry place
Manufacturer
Product code Lot number
Medical Device complies with Directive
93/42/EEC
WEEE disposal
Class II applied
Temperature limit Humidity limit
Atmospheric pressure limit
Disposal: The product must not be disposed of along with other domestic waste. The users
must dispose of this equipment by bringing it to a specic recycling point for electric and electronic equipment.
GIMA WARRANTY TERMS
The Gima 12-month standard B2B warranty applies.
Follow instructions for use
Keep away from sunlight
Date of manufacture
Serial number
Type BF applied part
IP21
Covering Protection rate
 Loading...
Loading...