Page 1
COVID-19 Antigen Test Cassette
REF FI-NCP-502
English
Test cassettes
Package insert
Extraction Buffer
Sterile Swabs
Workstation
ID Card
Extraction tubes and tips(Optional)
Procedure Card
Timer
Fluorescence Immunoassay Analyzer
RT-PCR
(Nasopharyngeal Swab)
Positive
Negative
Positive
216 1 217
Negative
8
140
148
Total
224
141
365
Relative Sensitivity
96.43%(95%CI*: 93.08%~98.45%)
Relative Specificity
99.29%(95%CI*: 96.11%~99.98%)
Accuracy
97.53%(95%CI*: 95.37%~98.87%)
Description
Test Level
Adenovirus type 3
3.16 x 104 TCID50/ml
Adenovirus type 7
1.58 x 105 TCID50/ml
Human coronavirus OC43
1.0 x 106 TCID50/ml
Human coronavirus 229E
5.0 x 105 TCID50/ml
Human coronavirus NL63
1.0 x 106 TCID50/ml
Human coronavirus HKU1
1.0 x 106 TCID50/ml
MERS coronavirus Florida
1.17 x 104 TCID50/ml
Influenza A H1N1
3.16 x 105 TCID50/ml
Influenza A H3N2
1.0 x 105 TCID50/ml
Influenza B
3.16 x 106 TCID50/ml
Human Rhinovirus 2
2.81 x 104 TCID50/ml
Human Rhinovirus 14
1.58 x 106 TCID50/ml
Human Rhinovirus 16
8.89 x 106 TCID50/ml
Measles
1.58 x 104 TCID50/ml
Mumps
1.58 x 104 TCID50/ml
Parainfluenza virus 2
1.58 x 107 TCID50/ml
Parainfluenza virus 3
1.58 x 108 TCID50/ml
Respiratory syncytial virus
8.89 x 104 TCID50/ml
Arcanobacterium
1.0x108 org/ml
Candida albicans
1.0x108 org/ml
Corynebacterium
1.0x108 org/ml
Escherichia coli
1.0x108 org/ml
Moraxella catarrhalis
1.0x108 org/ml
Neisseria lactamica
1.0x108 org/ml
Neisseria subllava
1.0x108 org/ml
Pseudomonas aeruginosa
1.0x108 org/ml
Staphylococcus aureus subspaureus
1.0x108 org/ml
Staphylococcus epidermidis
1.0x108 org/ml
Streptococcus pneumoniae
1.0x108 org/ml
Streptococcus pyogenes
1.0x108 org/ml
Streptococcus salivarius
1.0x108 org/ml
Streptococcus sp group F
1.0x108 org/ml
Active Ingredient
Concentration
Mucin
2% w/v
Whole Blood
1% v/v
Sodium Chloride
5% w/v
Oxymetazoline
15%v/v
Zincum gluconium
5% w/v
peppermint
0.5% w/v
Fluconazole
5% w/v
Index of Symbols
Consult instructions
instructions for use
Authorized
representative in the
Community
Do not use if
package is damaged
instructions for use
Hangzhou AllTest Biotech Co., Ltd.
MedNet EC-REP GmbH
Borkstrasse 10
48163 Muenster
Germany
(Nasopharyngeal Swab)
Package Insert
COVID-19 Antigen Test Cassette is a fluorescence immunoassay for the
qualitative detection of SARS-CoV-2 Nucleocapsid protein antigens present in
human nasopharynx with the use of fluorescence Immunoassay Analyzer.
For professional in vitro diagnostic use only.
INTENDED USE】
【
The COVID-19 Antigen Test Cassette (Nasopharyngeal Swab) is based on
fluorescence immunoassay for the qualitative detection of SARS-CoV-2
Nucleocapsid protein antigens in nasopharyngeal swab specimens from
individuals with suspected SARS-CoV-2 infection in conjunction with clinical
presentation and the results of other laboratory tests.
Results are for the detection of SARS-CoV-2 Nucleocapsid protein antigens. An
antigen is generally detectable in upper respiratory specimens during the acute
phase of infection. Positive results indicate the presence of viral antigens, but
clinical correlation with patient history and other diagnostic information is
necessary to determine infection status. Positive results do not rule out
bacterial infection or co-infection with other viruses. The agent detected may
not be the definite cause of disease.
Negative results do not rule out SARS-CoV-2 infection and should not be used
as the sole basis for treatment or patient management decisions, including
infection control decisions. Negative results should be considered in the context
of a patient’s recent exposures, history and the presence of clinical signs and
symptoms consistent with COVID-19, and confirmed with a molecular assay, if
necessary for patient management.
SUMMARY】
【
The novel coronaviruses belong to the β genus. COVID-19 is an acute
respiratory infectious disease. People are generally susceptible. Currently, the
patients infected by the novel coronavirus are the main source of infection;
asymptomatic infected people can also be an infectious source. Based on the
current epidemiological investigation, the incubation period is 1 to 14 days,
mostly 3 to 7 days. The main manifestations include fever, fatigue and dry
cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are
found in a few cases.
PRINCIPLE】
【
The COVID-19 Antigen Test Cassette (Nasopharyngeal Swab) is a qualitative
membrane-based Fluorescence immunoassay for the detection of SARS-CoV-2
Nucleocapsid protein antigen in human nasopharyngeal swab specimen.
SARS-CoV-2 Nucleocapsid protein antibody is coated in test line region. During
testing, the specimen reacts with SARS-CoV-2 Nucleocapsid protein antibodycoated by fluorescent microspheres in the test; the mixture then migrates
upward on the membrane by capillary action and reacts with the SARS-CoV-2
Nucleocapsid protein antibody in test line region. The fluorescence
immunoassay analyzer detects the fluorescence signal value of a specific area
and calculates the result of the SARS-CoV-2 Nucleocapsid protein antigen in
the sample according to the algorithm on the ID card.
REAGENTS】
【
The test contains anti-SARS-CoV-2 Nucleocapsid protein antibody as the
capture reagent, anti-SARS-CoV-2 Nucleocapsid protein antibody as the
detection reagent.
PRECAUTIONS】
【
1. This package insert must be read completely before performing the test.
Failure to follow directions in package insert may yield inaccurate test
results.
2. For professional in vitro diagnostic use only.
3. Do not use after the expiration date indicated on the package. Do not use
the test if the foil pouch is damaged. Do not reuse.
4. Avoid cross-contamination of specimens by using a new specimen
collection container for each specimen obtained.
5. To obtain accurate results, do not use visually bloody or overly viscous
samples.
6. Do not eat, drink or smoke in the area where the specimens and tests are
handled. Handle all specimens as if they contain infectious agents.
Observe established precautions against microbiological hazards
throughout the procedure and follow standard procedures for proper
disposal of specimens. Wear protective clothing such as laboratory coats,
disposable gloves and eye protection when specimens are assayed.
7. Viral Transport Media (VTM) may affect the test result; extracted
specimens for PCR tests cannot be used for the test. Do not interchange or
mix reagents from different lots.
8. Humidity and temperature can adversely affect results.
9. Used testing materials should be discarded in accordance with local
regulations.
10. Read the entire procedure carefully prior to any testing.
11. The COVID-19 Antigen Test Cassette should only be used with the
Analyzer by approved medical professionals.
STORAGE AND STABILITY】
【
1. The kit should be stored at 4-30 °C until the expiry date printed on the
sealed pouch.
2. The test must remain in the sealed pouch until use.
3. Do not freeze.
4. Care should be taken to protect the components of the kit from
contamination. Do not use if there is evidence of microbial contamination or
precipitation. Biological contamination of dispensing equipment, containers
or reagents can lead to false results.
SPECIMEN COLLECTION, TRANSPORT AND STORAGE】
【
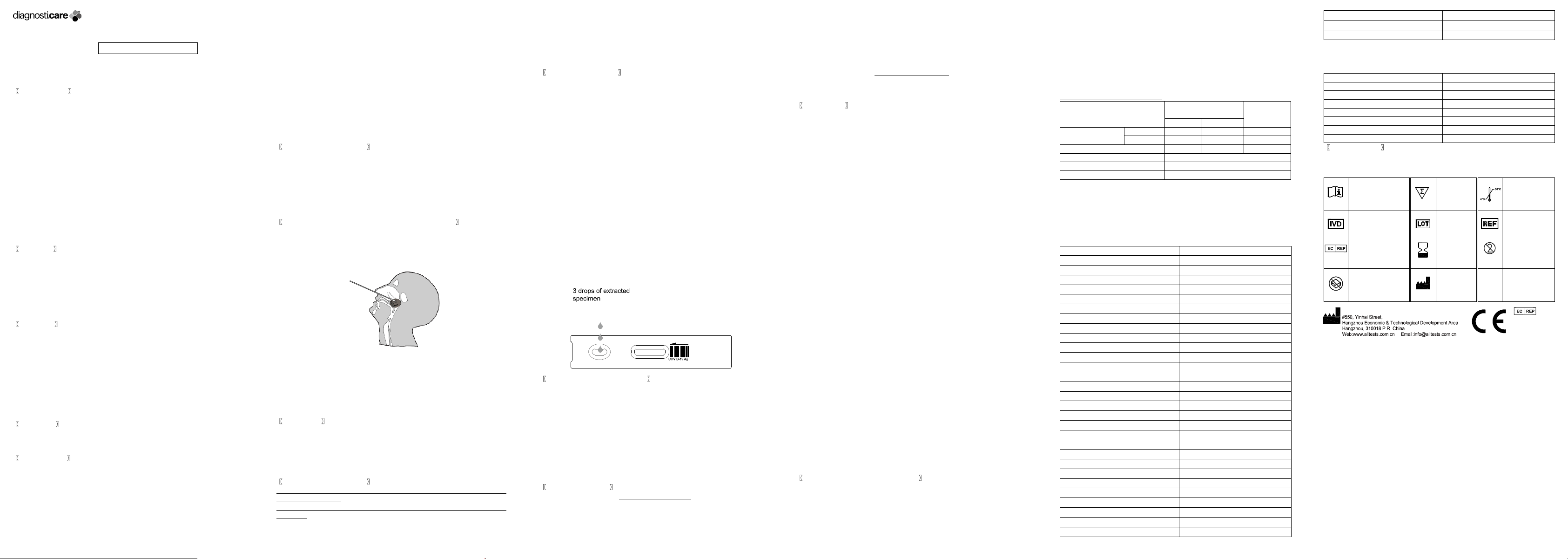
Specimen Collection
1. Insert a sterile swab into the nostril of the patient, reaching the surface of the
posterior nasopharynx.
2. Swab over the surface of the posterior nasopharynx.
3. Withdraw the sterile swab from the nasal cavity.
Caution: If the swab stick breaks during specimen collection, repeat specimen
collection with a new swab.
Specimen transport and storage
Specimens should be tested as soon as possible after collection.
If swabs are not been processed immediately, it is highly recommended the
swab sample is placed into a dry, sterile, and tightly sealed plastic tube for
storage. The swab specimen in dry and sterile condition is stable for 1 hour at
room temperature and 24 hours at 2-8 °C.
MATERIALS】
【
Materials Provided
Materials Required But Not Provided
【SPECIMEN PREPARATION】
Only the extraction buffer and tubes provided in the kit is to be used for swab
specimen preparation.
Please refer to the Procedure card for detailed information of Specimen
Extraction.
1. Place the swab specimen in the Extraction tube with Extraction Buffer. Rotate
the swab for approximately 10 seconds while pressing the head against the
inside of the tube to release the antigen in the swab.
2. Remove the swab while squeezing the swab head against the inside of the
Extraction tube as you remove it to expel as much liquid as possible from the
swab. Discard the swab in accordance with your biohazard waste disposal
protocol.
*NOTE: The storage of the specimen after extraction is stable for 2 hours at
room temperature or 24 hours at 2-8 °C.
DIRECTIONS FOR USE】
【
Refer to Fluorescence Immunoassay Analyzer Operation Manual for the
complete instructions on use of the Test. The test should be conducted in room
temperature.
Allow the test, extracted specimen and/or controls to equilibrate to room
temperature (15-30°C) prior to testing.
1. Turn on the Analyzer power. Then according to the need, select “Standard
Test” or “Quick Test” mode.
2. Take out the ID card and insert it into the Analyzer ID Card Slot.
3. Remove the test cassette from the sealed foil pouch and use it within one
hour. Best result will be obtained if the test is performed immediately after
opening the foil pouch.
4. Invert the specimen collection tube and add 3 drops of the extracted
specimen (approx.75-100μl) to the specimen well(S) and then start the timer.
5. There are two test modes for Fluorescence Immunoassay Analyzer,
Standard Test mode and Quick Test mode. Please refer to the user manual
of Fluorescence Immunoassay Analyzer for details.
“Quick Test” mode: After 10 minutes of adding sample, Insert the test
cassette into the Analyzer, click “QUICK TEST”, fill the test information and
click "NEW TEST" immediately. The Analyzer will automatically give the test
result after a few seconds.
“Standard Test” mode: Insert the test cassette into the Analyzer immediately
after adding specimen, click “STANDARD TEST”, fill the test information and
click "NEW TEST" at the same time, The Analyzer will automatically
countdown 10 minutes. After the countdown, the Analyzer will give the result at
once.
INTERPRETATION OF RESULTS】
【
Results read by Fluorescence Immunoassay Analyzer.
The result of tests for SARS-CoV-2 Antigens is calculated by Fluorescence
Immunoassay Analyzer and display the result on the screen. For additional
information, please refer to the user manual of Fluorescence Immunoassay
Analyze.
NOTE: The test result of each specimen is given as Pos (+) or Neg (-) with
a Value. This value is calculated by dividing the signal obtained with
sample by cut-off value (S/C Ratio).
- Test results of Value ≥ 1.00 are considered positive for SARS-CoV-2
Antigen.
- Test results of Value < 1.00 are considered negative for SARS-CoV-2
Antigen.
QUALITY CONTROL】
【
Internal Quality Control
Each COVID-19 Antigen Test Cassette contains internal control that satisfies
routine quality control requirements. This internal control is performed each
time a sample is tested. This control indicates that the test cassette was
inserted and read properly by Fluorescence Immunoassay Analyzer. An invalid
result from the internal control causes an error message on Fluorescence
Immunoassay Analyzer indicating that the test should be repeated. An invalid
result from the internal control causes an “N/A” message on Fluorescence
Immunoassay Analyzer. Insufficient specimen volume or incorrect procedural
techniques are the most likely reasons for control failure. Review the procedure
and repeat the test with a new test. If the problem persists, discontinue using
the test kit immediately and contact your local distributor.
External Quality Control
Positive/Negative Controls are not included in this kit. However, in compliance
with Good Laboratory Practice (GLP) positive/negative controls are
recommended.
【
LIMITATIONS】
1
1. To obtain the best sensitive result, directly test patient specimens without
viral transport media is required for the testing. Proper specimen collection,
storage and transport are critical to the performance of this test.
2. The DIRECTIONS FOR USE and the DIRECTIONS FOR USE must be
followed closely when testing for the presence of SARS-CoV-2
Nucleocapsid protein antigens in the human nasopharyngeal specimens
from suspected individuals. For optimal test performance, proper sample
collection is critical. Failure to follow the procedure may give inaccurate
results. Viral Transport Media (VTM) may affect the test result; extracted
specimens for PCR tests cannot be used for the test.
3. The performance of the COVID-19 Antigen Test (Nasopharyngeal swab)
was evaluated using the procedures provided in this product insert only.
Modifications to these procedures may alter the performance of the test.
4. The COVID-19 Antigen Test Cassette (Nasopharyngeal swab) is for in vitro
diagnostic use only. This test should be used for detection of SARS-CoV-2
Nucleocapsid protein Antigens in human nasopharyngeal specimens as an
aid in the diagnosis of patients with suspected SARS-CoV-2 infection in
conjunction with clinical presentation and the results of other laboratory
tests. Neither the quantitative value nor the rate of increase in the
concentration of SARS-CoV-2 Nucleocapsid protein antigens can be
determined by this qualitative test.
5. The COVID-19 Antigen Test Cassette (Nasopharyngeal Swab) will only
indicate the presence of SARS-CoV-2 Antigens in the specimen and should
not be used as the sole criteria for the diagnosis of SARS-CoV-2 infections.
6. The results obtained with the test should be considered with other clinical
findings from other laboratory tests and evaluations.
7. If the test result is negative or non-reactive and clinical symptoms persist. It
is recommended to re-sample the patient a few days later and test again or
test with a molecular diagnostic device to rule out infection in these
individuals.
8. The test will show negative results under the following conditions: The
concentration of the novel coronavirus antigens in the sample is lower than
the minimum detection limit of the test. Negative results do not rule out
SARS-CoV-2 infection, particularly in those who have been in contact with
the virus or with symptom onset .Follow-up testing with a molecular
diagnostic should be considered to rule out infection in these individuals.
9. Excess blood or mucin on the swab specimen may interfere with test
performance and may yield a false positive result.
10. The accuracy of the test depends on the quality of the swab sample. False
negatives may result from improper sample collection or storage.
11. Positive results of COVID-19 may be due to infection with non-SARS-CoV-2
coronavirus strains or other interference factors.
12. The formulation of extraction buffer in the kit may inactivate cells and virus.
So the specimen in the extraction buffer is not suitable for culture.
PERFORMANCE CHARACTERISTICS】
【
1. Precision
Within-run and Between-run precision has been determined by using three
specimens of COVID-19 standard control. Three different lots of COVID-19
Antigen Test (Nasopharyngeal Swab) have been tested using negative, SARSCoV-2 Antigen weak Positive and SARS-CoV-2 Antigen Strong Positive. Ten
replicates of each level were tested each day for 3 consecutive days. The
specimens were correctly identified >99% of the time.
2. Clinical Performance
The COVID-19 Antigen Test Cassette (Nasopharyngeal Swab) has been
evaluated with specimens obtained from the patients. RT-PCR
(Nasopharyngeal Swab) is used as the reference method for the COVID-19
Antigen Test Cassette (Nasopharyngeal Swab). Specimens were considered
positive if RT-PCR (Nasopharyngeal Swab) indicated a positive result.
Specimens were considered negative if RT-PCR (Nasopharyngeal Swab)
indicated a negative result.
Nasopharyngeal Swab Specimen
COVID-19 Antigen Test Cassette
Total
COVID-19 Antigen
*Confidence Intervals
3. Limitation of Detection
The COVID-19 Antigen Test Cassette (Nasopharyngeal Swab) can detect out
SARS-CoV-2 heat-inactivated virus strain as low as 1 x 10
2
TCID50/ml.
4. Cross Reactivity (Analytical Specificity)
No cross-reactivity or interference was observed with the following
microorganisms when tested at these concentrations presented in the table
below.
TCID50 = Tissue Culture Infectious Dose is the dilution of virus that under the
conditions of the assay can be expected to infect 50% of the culture vessels
inoculated.
4. Endogenous Interfering Substances
【BIBLIOGRAPHY】
1. Westgard JO, Barry PL,Hunt MR, Groth T. A multi-rule Shewhart for quality
control in clinical chemistry, Clinical Chemistry 1981;27:493-501
for use or consult
electronic
In vitro diagnostic
medical device
European
and consult
Contains
sufficient for
<n> tests
Batch code
Use-by date
Manufacturer
Temperature limit
Catalogue
number
Do not re-use
Statement: Information about manufacturer of sterile swab is placed on the
packaging.
Distributed in Italy by PM2 Services Srl, Corso Mazzini 38- Largo Marchi, 36071
Arzignano (VI) - info@pm2services.it
Number: F145106100
Revision date: 2022-09-14
 Loading...
Loading...