Page 1

GE Power & Water
Fact Sheet
Water & Process Technologies
Analytical Instruments
Sievers Technology Transfer Protocols
A QSO Product for Upgrading from Legacy Sievers TOC Analyzers
to the 900 and 500 RL Models
In the drug industry, implementing changes
or change management is often discussed
in terms of resources, documentation, and
time spent on the manufacturing process
or the nal product. However, changing
from legacy technology to new technology,
in addition to implementing a process for
new TOC analyzers, requires a structured
procedure and tools.
To help with this objective, GE Analytical
Instruments has released Technology Transition Protocols that facilitate the change
from the Sievers 400 ES to the 500 RL, and
from the Sievers 800 to the 900 Series Laboratory TOC Analyzers. Since the transition to a new TOC
analyzer can take a signcant time commitment, GE’s
unique validation protocols (tools) can greatly accelerate
this conversion.
Aligning with the Current Best
Practices Process
By denition, the International Conference of
Harmonization (ICH) describes a form of technology
transfer in ICH Q10 as “activities or validation steps
associated with the transfer of process knowledge
or data between departments, manufacturing sites,
or related technologies/ equipment.” However,
this document states that “this
knowledge forms the basis for the
manufacturing process, control
strategy, process validation approach
and ongoing continual improvement.”
It is important to note, though, that
without a clear path in transitioning to
new TOC technologies, this can be a
multifaceted process.
In addition to ICH Q10, the United
States Pharmacopeia (USP) has come
forward in USP <1058> Analytical
Instrument Qualication to provide
additional guidance by highlighting
a transition to new technology associated with
analytical instruments. USP <1058> suggests that this
type of transition involves a form of “change control”
that should evaluate elements of design, as well as
operational and performance qualication. Figure 1
highlights GE Analytical Instruments’ rationale and
justication for developing these transition protocols.
Key Components of GE’s
Transition Protocols
TOC instruments have historically been employed to satisfy
compendial requirements involved in the measurement of
organic contamination in pharmaceutical waters. When
Figure 1. The Transition Process for New TOC Technologies
Page 2
transitioning to “newer or updated versions” of these
analyzers, regulatory bodies have suggested that a series
of protocols be conducted when transferring to the new
instruments, technologies, or methods in order to ensure
that the new approach is suitable for the intended use per
current Good Manufacturing Practices (cGMPs).
The overall intent of GE Analytical Instruments’ Technology
Transfer Protocols is to align with ICH best practices that
the “new method, instrument, or equipment needs to
demonstrate that it performs equivalently to, or better
than, the previous validated method, instrument, or
equipment.” To achieve this goal, the protocols have been
designed emphasizing:
• “Like-for-like” methods through an instrumentation technology comparison
• Similarities in system suitability results to satisfy
compendial specications
• An equivalency study of accuracy and precision
verication to satisfy regulatory guidelines
Table 1 summarizes each element, scope, justication
and rationale for the transition process incorporated in
these specic protocols.
Quality System Optimization (QSO)™ —
Accelerating the “Change” Process
The new transition protocols are part of GE Analytical
Instruments’ Quality System Optimization (QSO)
program. In 2010, QSO was introduced to the
pharmaceutical and biopharmaceutical industries as
a risk-based, scientic approach designed to eciently
implement TOC analyzers for real-time release (RTR)
testing of pharmaceutical water. QSO provides the
framework for transitioning TOC testing from the
laboratory to the production oor within an integrated
compliance foundation. This type of “platform” was
used to design these transition protocols to accelerate
the process of changing from legacy technology to a
new technology.
Validation Services and Additional Products
In addition to these documents, GE Analytical Instruments
oers a one-day on-site validation service and specic
reference standards to ensure an ecient transition
to new TOC technology. Furthermore, these transition
protocols can be fully integrated into GE Analytical
Instruments VSPs and on-site validation services oering.
To explore these and other GE Analytical Instrument
products, please visit www.geinstruments.com or contact
your local representative.
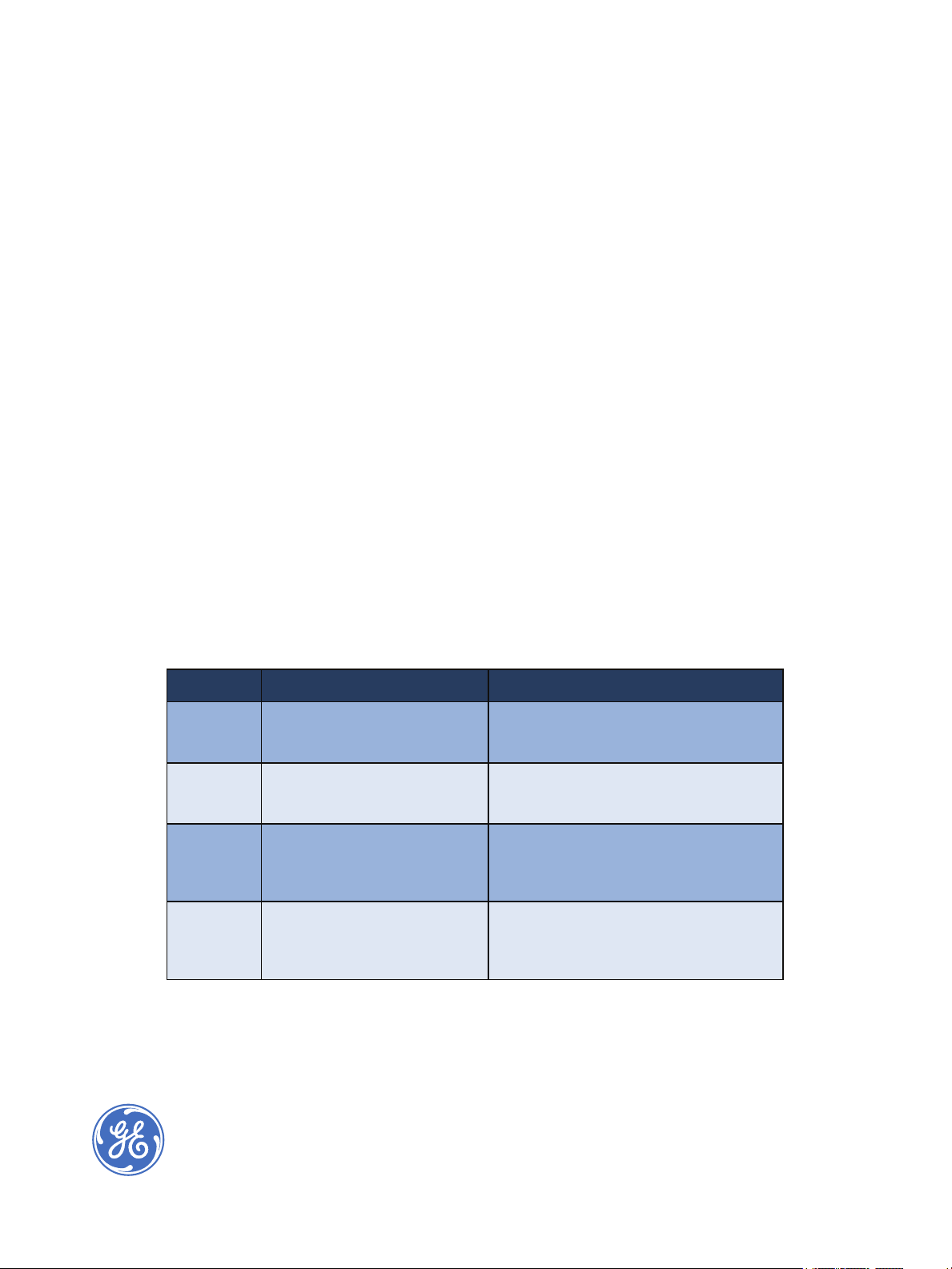
Table 1. GE Analytical Instruments’ Transition Protocols for the On-Line and Laboratory Analyzers
Elements Scope Justification & Rationale
Design Demonstration of ‘like-for-like’
technology comparison
Operation Comparison test of the operational
suitability of the two analytical
instruments
Performance Equivalency test of validation
characteristics for performance of
the two analytical instruments
Transfer Documentation to summarize both
methods are ‘like-for-like’ in design,
operation, and performance
The Americas
GE Analytical Instruments
6060 Spine Road
Boulder, CO 80301-3687 USA
T +1 800 255 6964
T +1 303 444 2009
F +1 303 444 9543
geai@ge.com
Europe/Middle East/Africa
GE Analytical Instruments
Unit 3, Mercury Way
Urmston, Manchester
UK M41 7LY
T +44 (0) 161 864 6800
F +44 (0) 161 864 6829
geai.europe@ge.com
www.geinstruments.com
© 2011. General Electric Company. All rights reserved.
Compare oxidation and detection techniques
based on the design of the TOC technology
and vendor specifications
Demonstrate that the transition to the new
instrumentation is suitable and operates
under actual conditions of use
Justify that the transition to the new
instrumentation provides comparable results
based on accuracy and precision with the
same check standards
The ‘like-for-like’ technology incorporated in
both instruments and results from the
comparison tests further facilitates the
transfer from one technology to another.
Asia Pacic
GE Analytical Instruments
7/F, Building 2, No. 5 Hua Tuo Rd.
ZhangJiang Hi-Tech Park, Pudong
Shanghai, China 201203
T +(8621) 38777735
F +(8621) 38777469
geai.asia@ge.com
300 00236 Rev A
MC11-188
 Loading...
Loading...