Page 1
Fact Sheet
Find a contact near you by visiting www.gewater.com and clicking on “Contact Us”.
* Trademark of General Electric Company; may be registered in one or more countries.
©2013, General Electric Company. All rights reserved.
FSsmPharmaElements_EN.doc Sep-13
Membrane
Thin film membrane (TFM*)
Model
Average
permeate flow
gpd (m3/day)
Minimum
rejection
PHARMA NF2 3838C30
1,3
1,800 (6.8)
96.0%
PHARMA RO3 3840C30
1,2
1,650 (6.2)
98.5%
PHARMA RO3 8038C35
1,2
6,750 (25.5)
98.5%
Model
Spacer
mil (mm)
Active area
ft2 (m2)
Part
number
PHARMA NF2 3838C30
30 (0.76)
78 (7.2)
1232576
PHARMA RO3 3840C30
30 (0.76)
77 (7.2)
1223957
PHARMA RO3 8038C35
35 (0.89)
315 (29.2)
3021475
PHARMA UF1 3840C30
30 (0.76)
80 (7.4)
1207089
Model2
Dimensions, inches (cm)
Boxed
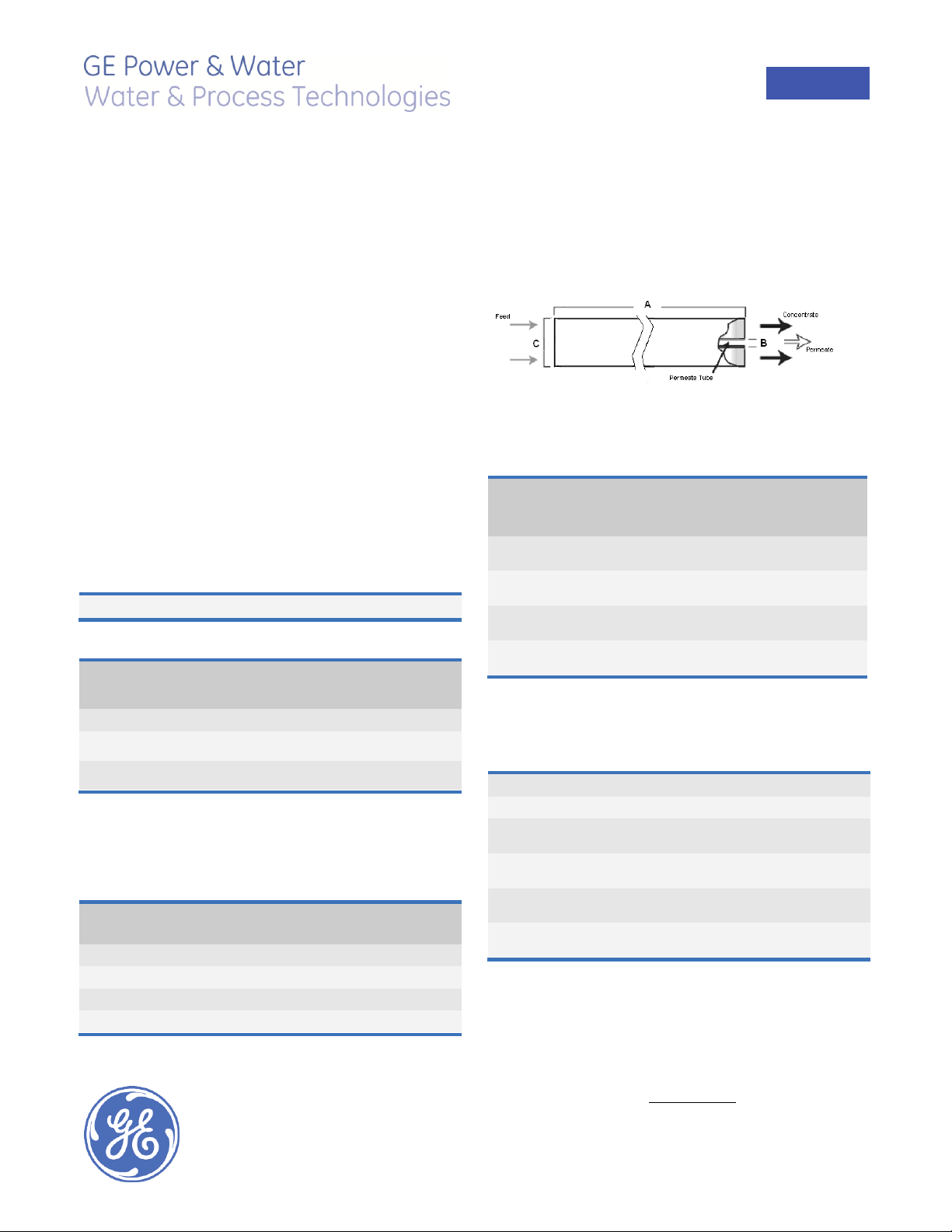
A
B1
C
Weight
lbs (kg)
PHARMA NF2 3838C30
38.00
(96.5)
0.833
(2.12)
3.79
(9.6)
7
(3.2)
PHARMA RO3 3840C30
38.75
(98.4)
0.833
(2.12)
3.79
(9.6)
7
(3.2)
PHARMA RO3 8038C35
38.0
(96.5)
1.125
(2.86)
7.91
(20.1)
29
(13.2)
PHARMA UF1 3840C30
38.75
(98.4)
0.833
(2.12)
3.79
(9.6)
7
(3.2)
Typical Operating Flux
5-20GFD (8-34 LMH)
Maximum Operating Pressure
600 psi (4,137 kPa)
Maximum Temperature
Continuous operation: 122°F (50°C)
Clean-In-Place (CIP): 122°F (50°C)
pH Range
Continuous operation: 3.0-10.0,
Clean-In-Place (CIP): 2.0-10.5
Maximum Pressure Drop
Over an element: 15psi (103kPa)
Per housing: 60 psi (414 kPa)
Chlorine Tolerance
500+ ppm-hours,
Dechlorination recommended
Pharma Elements
Concentration and Purification of Pharmaceutical Ingredients
The Pharma elements enable the purification and
concentration of high value organic molecules used
in the synthesis of pharmaceutical ingredients.
These elements are used in applications requiring
stringent sanitary procedures.
The Pharma elements feature a patented Durasan*
cage outer wrap, a selection of feed spacers and
polysulfone parts.
The Pharma elements comply with:
• FDA Regulations relevant sections of 21CFR
• EU Framework 1935/2004/EC
Figure 1: Element Dimensions Diagram (Female)
Table 2: Dimensions and Weight
Table 1: Element Specification
1
Average salt rejection after 24h operation. Individual flow rate may vary ±25%.
2
Testing conditions: 2,000ppm NaCl solution at 425psi (2,930kPa) operating pres-
sure, 77°F (25°C), pH6.5 and 15% recovery.
3
Testing conditions: 2,000ppm MgSO4 solution at 110psi (760kPa) operating
pressure, 77 °F (25°C), 15 % recovery.
1
Internal diameter.
² These elements are bagged dry before shipping.
Table 3: Operating and CIP parameter
 Loading...
Loading...