Page 1
Human Recombinant ADRB2 Adrenoceptors Stable Cell Line
Technical Manual No. TM0504 Version 06042010
I
Introduction ….…………………………………………………………………………….
1
II
Background………………….……………………………………………………………….
1
III
Representative Data………………………………………………………………………
2
IV
Thawing and Subculturing………………………………………………………………
2
V
References ……………………………………………………………………………….
3 Limited Use License Agreement…………………………………………………………
4
I. Introduction
Catalog Number: M00308
Cell Line Name: CHO-K1/ADRB2/Gα15
Gene Synonyms: BAR; B2AR; ADRBR; ADRB2R; BETA2AR; ADRB2
Expressed Gene: Genbank Accession Number NM_000024; no expressed tags
Host Cell: CHO-K1/Gα15
Quantity: 2 vials (3×106 per vial) frozen cells
Stability: 16 passages
Application: Functional assay for ADRB2 receptor
Freeze Medium: 45% culture medium, 45% FBS, 10% DMSO
Complete Growth Medium: Ham’s F12, 10% FBS
Culture Medium: Ham’s F12, 10% FBS, 200 μg/ml Zeocin, 100 μg/ml Hygromycin B
Mycoplasma Status: Negative
Storage: Liquid nitrogen immediately upon delivery.
II. Background
β-Adrenergic receptors (β-ARs) are members of the superfamily G-protein-coupled receptors that are stimulated by
naturally occurring catecholamines, epinephrine, and norepinephrine. As part of the sympathetic nervous system,
β-ARs have been shown to have important roles in cardiovascular, respiratory, metabolic, central nervous system,
and reproductive functions. Three distinct β-AR subtypes have been identified (β1-AR, β2-AR, and β3-AR). All
three of these β-AR subtypes are believed to signal by coupling to the stimulatory G-protein Gsα which leads to the
activation of adenylyl cyclase and accumulation of the second messenger cAMP. β1-ARs mediate cardiac
stimulation, β2-ARs mediate smooth muscle relaxation in the peripheral vasculature and respiratory system, and
β
-AR has been shown to have important roles in adipose tissue and gastrointestinal tract. In studies using
3
subtype-selective agonists and antagonists in the human heart, β2-AR stimulation leads to the activation of
adenylyl cyclase and contributes to both inotropic and chronotropic responses.
-1-
Page 2
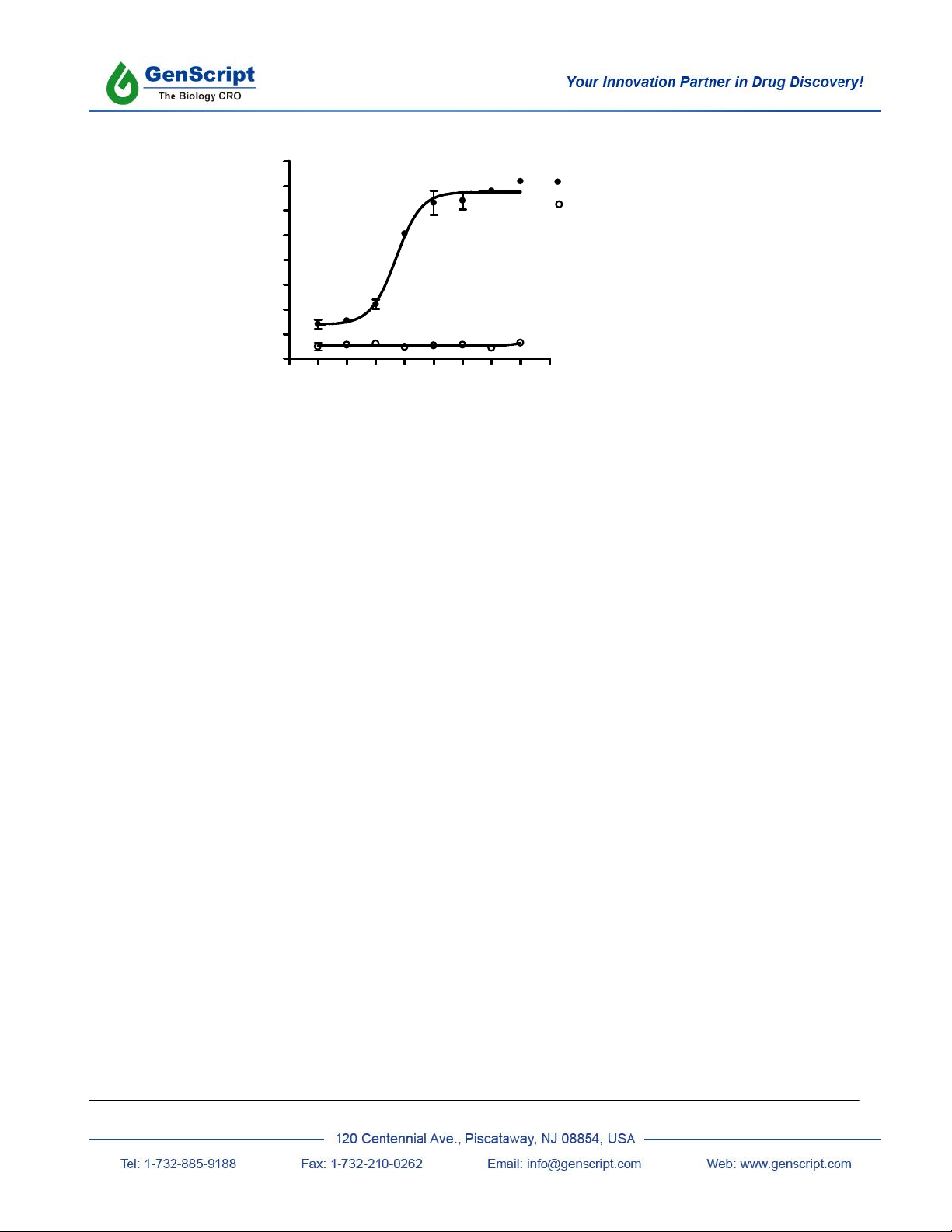
III. Representative Data
-14 -13 -12 -11 -10 -9 -8 -7 -6 -5
0
10
20
30
40
50
60
70
80
CHO-K1/ADRB2/G 15
CHO-K1/G 15
EC50 = 0.05 nM
Log[Isoproterenol] M
RFU
Figure Intracellular calcium response from CHO-K1/ Gα15 cells stably expressing human adrenergic receptor
ADRB2 and from untransfected control cells. Cells were loaded with Calcium-4 and then stimulated with the
indicated concentrations of isoproterenol. Calcium responses were recorded on a FlexStation3 plate reader. Data
represent the average +/- standard deviation of triplicate determinations.
IV. Thawing and Subculturing
Thawing: Protocol
1. Remove the vial from liquid nitrogen tank and thaw the cells quickly in a 37°C water-bath.
2. Just before the cells are completely thawed, decontaminate the outside of the vial with 70% ethanol and
transfer the cells to a 15 ml centrifuge tube containing 9 ml of complete growth medium.
3. Pellet cells by centrifugation at 200 x g for 5 min, and discard the medium.
4. Resuspend the cells in complete growth medium.
5. Add 2 ml of the cell suspension per well in a 6-well plate.
6. Add Hygromycin B and Zeocin to concentrations of 100 μg/ml and 200 μg/ml respectively the following day
Subculturing: Protocol
1. Remove and discard culture medium.
2. Wash cells with PBS (pH=7.4) to remove all traces of serum that contains trypsin inhibitor.
3. Add 2.0 ml of 0.05% (w/v) Trypsin- EDTA (GIBCO, Cat No. 25300) solution to a 10 cm dish and observe the
cells under an inverted microscope until cell layer is dispersed (usually within 3 to 5 minutes).
Note: To avoid clumping, do not agitate the cells by hitting or shaking the dish while waiting for the cells to
detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
4. Add 6.0 to 8.0 ml of complete growth medium and aspirate cells by gently pipetting, centrifuge the cells 200 x
g for 5min, and discard the medium.
5. Resuspend the cells in culture medium, add appropriate aliquots of the cell suspension to new culture vessels.
6. Incubate cultures at 37°C.
Subcultivation Ratio: 1:3 to 1:8 weekly.
Medium Renewal: Every 2 to 3 days
-2-
Page 3

V. References
1. Emorine LJ, Marullo S, Briend-Sutren MM, et al. (1989) Molecular characterization of the human β3adrenergic receptor. Science, 245 : 1118-1121
2. Frielle, T., Collins, S., Daniel, K. W., et al. (1987)Cloning of the cDNA for the human beta 1-adrenergic
receptor. Proc. Natl. Acad. Sci. U. S. A., 84: 7920–7924
3. Lands, A. M., Arnold, A., McAuliff, J. P., et al. (1967) Differentiation of receptor systems activated by
sympathomimetic amines. Nature, 214: 597–598
4. Lands, A. M., Luduena, F. P., and Buzzo, H. J. (1967) Differentiation of receptors responsive to isoproterenol.
Life Sci., 6: 2241–2249
5. Brodde, O. E. beta_1-and beta2-adrenoceptors in the human heart: properties, function, and alterations in
chronic heart failure. (1991) Pharmacol. Rev, 43: 203–242
GenScript USA Inc.
120 Centennial Ave., Piscataway, NJ 08854
Tel: 732-885-9188, 732-885-9688
Fax: 732-210-0262, 732-885-5878
Email: info@genscript.com
Web: http://www.genscript.com
Research Use Only
-3-
Page 4

Limited Use License Agreement
This is a legal agreement between you (Licensee) and GenScript USA Inc. governing use of GenScript's stable cell
line products and protocols provided to licensee. By purchasing and using the stable cell line, the buyer agrees to
comply with the following terms and conditions of this label license and recognizes and agrees to such restrictions:
1) The products are not transferable and will be used at the site where they were purchased. Transfer to another
site owned by buyer will be permitted only upon written request by buyer followed by subsequent written
approval by GenScript.
2) The purchaser cannot sell or otherwise transfer (a) this product (b) its components or (c) materials made using
this product or its components to a third party.
3) The products sold by GenScript are for laboratory and animal research purposes only. The products are not to
be used on humans, for consumption, or for any unlawful uses.
GenScript USA Inc. will not assert against the buyer a claim of infringement of patents owned or controlled by
GenScript USA Inc. and claiming this product based upon the manufacture, use or sale of a clinical diagnostic,
therapeutic and vaccine, or prophylactic product developed in research by the buyer in which this product or its
components has been employed, provided that neither this product nor any of its components was used in the
manufacture of such product. For information on the use of this product for other purposes, contact Marketing
Department, GenScript USA Inc., 120 Centennial Avenue, Piscataway, New Jersey 08840, U.S.A. Phone: 1-732885-9188. Fax: 1-732-210-0262. Email: marketing@genscript.com.
-4-
 Loading...
Loading...