Page 1
Human Recombinant Free Fatty Acid Receptor 1 Stable Cell Line
Technical Manual No. TM0420 Version 06042010
I
Introduction ….…………………………………………………………………………….
1
II
Background……………………………………………………………………………….
1
III
Representative Data………………………………………………………………………
2
IV
Thawing and Subculturing………………………………………………………………
2
V
References ……………………………………………………………………………….
3 Limited Use License Agreement…………………………………………………………
4
I. Introduction
Catalog Number: M00273
Cell Line Name: CHO-K1/FFA1/Gα15
Expressed Gene: GenBank Accession Number NM_005303; no expressed tags
Host Cell: CHO-K1
Quantity: 2 vial (3×106 per vial) frozen cells
Stability: 16 passages
Applications: Functional assays for FFA1 receptor (GPR40)
Freeze Medium: 45% culture medium, 45% FBS, and 10% DMSO
Complete Culture Medium: Ham’s F12, 10% FBS
Culture Medium: Ham’s F12, 10% FBS, 200 μg/ml Zeocin, 100 μg/ml Hygromycin B
Mycoplasma Status: Negative
Storage: Liquid nitrogen immediately upon delivery
II. Background
Free fatty acid G protein coupled receptor family consists of four members and plays significant roles in nutrition
regulation. GPR40 (FFA1) and GPR120 are activated by medium and long-chain FFAs, whereas GPR41 and
GPR43 (FFA2) can be activated by short-chain FFAs. FFA1 is preferentially expressed in pancreatic beta-cells
and mediates the majority of the effects of FFAs on insulin secretion. Researches show that FFA1 is a potential
therapeutic target and plays an important role in the chain of events linking obesity and type2 diabetes.
-1-
Page 2
III. Representative Data
-11 -10 -9 -8 -7 -6 -5 -4 -3 -2
0
25
50
75
100
125
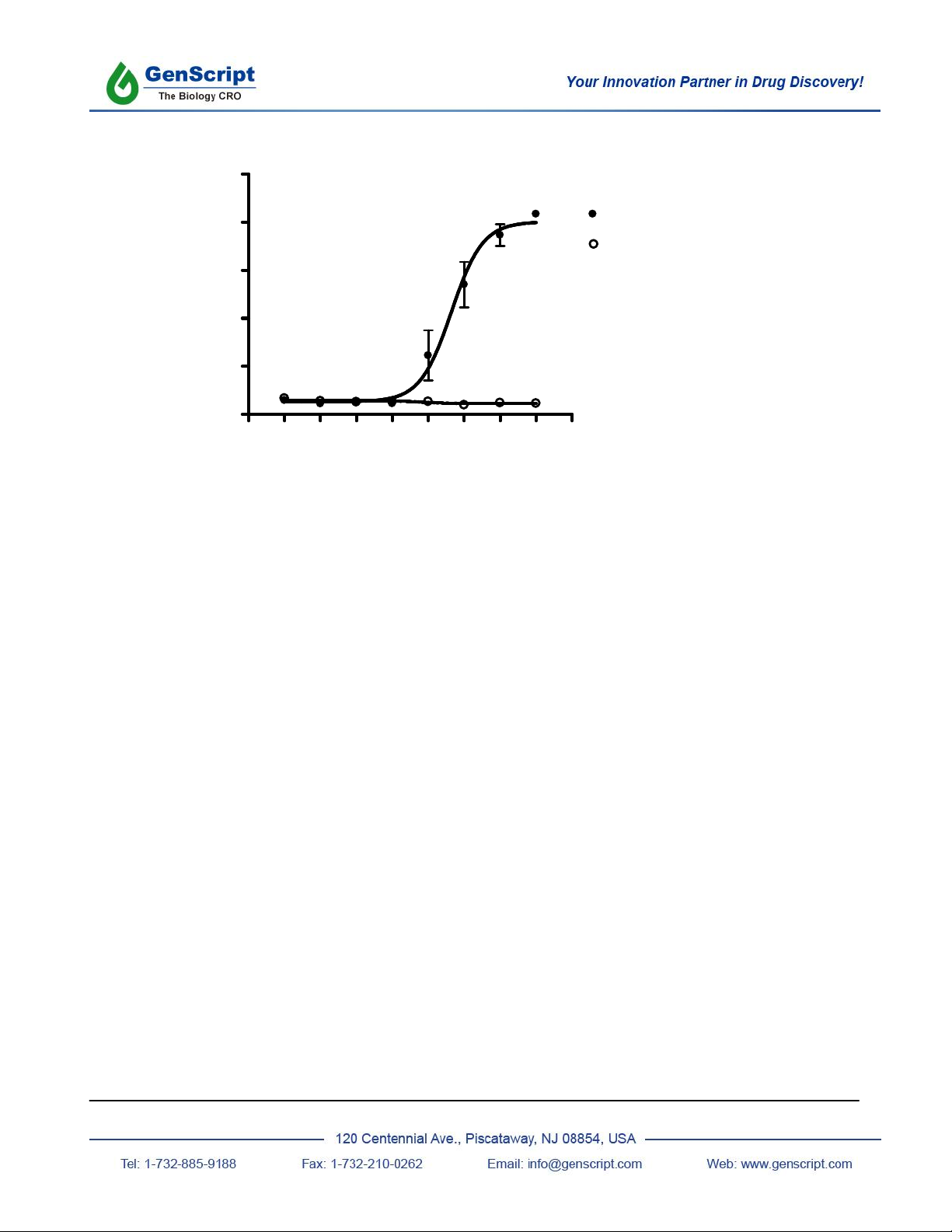
CHO-K1/FFA1/G15
CHO-K1
EC50= 4.4 M
Log[OA] M
RFU
Figure: Shown above are the intracellular calcium responses of CHO-K1 cells stably expressing Homo sapiens
free fatty acid receptor 1 (FFA1/GPR40) and of untransfected control cells. Cells were loaded with Calcium-4 and
then stimulated with the indicated concentrations of Oleic acid. Calcium responses were recorded on a FlexStation
plate reader. Data represent the average +/- standard deviation of triplicate determinations.
IV. Thawing and Subculturing
Thawing: Protocol
1. Remove the vial from liquid nitrogen tank and thaw cells quickly in a 37°C water-bath.
2. Just before the cells are completely thawed, decontaminate the outside of the vial with 70% ethanol and
transfer the cells to a 15 ml centrifuge tube containing 9 ml of complete growth medium.
3. Pellet cells by centrifugation at 200 x g force for 5 min, and discard the medium.
4. Resuspend the cells in complete growth medium.
5. Add 2 ml of the cell suspension per well in a 6 well-plate.
6. Add Hygromycin B and Zeocin to concentrations of 100 μg/ml and 200 μg/ml respectively the following day.
Subculturing: Protocol
1. Remove and discard culture medium.
2. Wash cells with PBS (pH=7.4) to remove all traces of serum that contains trypsin inhibitor.
3. Add 2.0 ml of 0.05% (w/v) Trypsin- EDTA (GIBCO, Cat No. 25300) solution to 10 cm dish and observe the
cells under an inverted microscope until cell layer is dispersed (usually within 3 to 5 minutes).
Note: To avoid clumping, do not agitate the cells by hitting or shaking the dish while waiting for the cells to
detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
4. Add 6.0 to 8.0 ml of complete growth medium and aspirate cells by gently pipetting, centrifuge the cells 200 x
g force for 5min, and discard the medium.
5. Resuspend the cells in complete growth medium with Hygromycin B and Zeocin and add appropriate aliquots
of the cell suspension to new culture vessels.
6. Incubate cultures at 37°C.
-2-
Page 3

Subcultivation Ratio: 1:3 to 1:8 weekly.
Medium Renewal: Every 2 to 3 days
V. References
1. Sawzdargo, M., George, S. R., Nguyen, T., Xu, S. J., Kolakowski, L. F., and Odowd, B. F. (1997) Biochem.
Biophys. Res. Commun. 239, 543–547
2. Brown, J. A., Goldsworthy, S. M., Barnes, A. A., Eilert, M. M., Tcheang, L., Daniels, D., Muir, A. I.,
Wigglesworth, M. J., Kinghorn, I., Fraser, N. J., Pike, N. B., Strum, J. C., Steplewski, K. M., Murdock, P. R.,
Holder, J. C., Marshall, F. H., Szekeres, P. G., Wilson, S., Ignar, D. M., Foord, M., S., Wise, A., and Dowell, S.
J (2003) J. Biol. Chem. 278, 11312–11319
3. Thompson, A. L., Lim-Fraser, M. Y. C., Kraegen, E. W., and Cooney, G. J. (2000) Am. J. Physiol. 279, E577–
E584
GenScript USA Inc.
120 Centennial Ave., Piscataway, NJ 08854
Tel: 732-885-9188, 732-885-9688
Fax: 732-210-0262, 732-885-5878
Email: info@genscript.com
Web: http://www.genscript.com
For Research Use Only.
-3-
Page 4

Limited Use License Agreement
This is a legal agreement between you (Licensee) and GenScript USA Inc. governing use of GenScript's stable cell
line products and protocols provided to licensee. By purchasing and using the stable cell line, the buyer agrees to
comply with the following terms and conditions of this label license and recognizes and agrees to such restrictions:
1) The products are not transferable and will be used at the site where they were purchased. Transfer to another
site owned by buyer will be permitted only upon written request by buyer followed by subsequent written
approval by GenScript.
2) The purchaser cannot sell or otherwise transfer (a) this product (b) its components or (c) materials made using
this product or its components to a third party.
3) The products sold by GenScript are for laboratory and animal research purposes only. The products are not to
be used on humans, for consumption, or for any unlawful uses.
GenScript USA Inc. will not assert against the buyer a claim of infringement of patents owned or controlled by
GenScript USA Inc. and claiming this product based upon the manufacture, use or sale of a clinical diagnostic,
therapeutic and vaccine, or prophylactic product developed in research by the buyer in which this product or its
components has been employed, provided that neither this product nor any of its components was used in the
manufacture of such product. For information on the use of this product for other purposes, contact Marketing
Department, GenScript USA Inc., 120 Centennial Avenue, Piscataway, New Jersey 08840, U.S.A. Phone: 1-732885-9188. Fax: 1-732-210-0262. Email: marketing@genscript.com.
-4-
 Loading...
Loading...