General Electric VIVID I N_VIVID Q N SERVICE MANUAL_SM_FQ091013_1 Breas PV101+ 102+ Ventilator - User Manual
Page 1

PRELIMINARY
1.NOV.2011
VIN-VQN_SVC_FRNT_CVR.FM
GE Healthcare
Vivid i n and Vivid q N
Service Manual
Vivid q N with software version SW 11.x.x (BT11)
Vivid i n with software version SW 9.x.x (BT’09)
Vivid i n with software version SW 6.x.x (BT’06)
Operating Documentation
Part Number: FQ091013
Revision: 1
Page 2

Page 3

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
FRONTMATTER.FM
Important Precautions
TRANSLATION POLICY
THIS SERVICE MANUAL IS AVAILABLE IN ENGLISH ONLY.
• IF A CUSTOMER’S SERVICE PROVIDER REQUIRES A LANGUAGE OTHER
THAN ENGLISH, IT IS THE CUSTOMER’S RESPONSIBILITY TO PROVIDE
TRANSLATION SERVICES.
WARNING
(EN)
• DO NOT ATTEMPT TO SERVICE THE EQUIPMENT UNLESS THIS SERVICE
MANUAL HAS BEEN CONSULTED AND IS UNDERSTOOD.
• FAILURE TO HEED THIS WARNING MAY RESULT IN INJURY TO THE SERVICE
PROVIDER, OPERATOR OR PATIENT FROM ELECTRIC SHOCK, MECHANICAL
OR OTHER HAZARDS.
AVERTISSEMENT
(FR)
WARNUNG
(DE)
CE MANUEL DE MAINTENANCE N’EST DISPONIBLE QU’EN ANGLAIS.
• SI LE TECHNICIEN DU CLIENT A BESOIN DE CE MANUEL DANS UNE AUTRE
LANGUE QUE L’ANGLAIS, C’EST AU CLIENT QU’IL INCOMBE DE LE FAIRE
TRADUIRE.
• NE PAS TENTER D’INTERVENTION SUR LES ÉQUIPEMENTS TANT QUE LE
MANUEL SERVICE N’A PAS ÉTÉ CONSULTÉ ET COMPRIS.
• LE NON-RESPECT DE CET AVERTISSEMENT PEUT ENTRAÎNER CHEZ LE
TECHNICIEN, L’OPÉRATEUR OU LE PATIENT DES BLESSURES DUES À DES
DANGERS ÉLECTRIQUES, MÉCANIQUES OU AUTRES.
DIESES KUNDENDIENST-HANDBUCH EXISTIERT NUR IN ENGLISCHER
SPRACHE.
• FALLS EIN FREMDER KUNDENDIENST EINE ANDERE SPRACHE BENÖTIGT,
IST ES AUFGABE DES KUNDEN FÜR EINE ENTSPRECHENDE ÜBERSETZUNG
ZU SORGEN.
• VERSUCHEN SIE NICHT, DAS GERÄT ZU REPARIEREN, BEVOR DIESES
KUNDENDIENST-HANDBUCH NICHT ZU RATE GEZOGEN UND VERSTANDEN
WURDE.
• WIRD DIESE WARNUNG NICHT BEACHTET, SO KANN ES ZU VERLETZUNGEN
DES KUNDENDIENSTTECHNIKERS, DES BEDIENERS ODER DES PATIENTEN
DURCH ELEKTRISCHE SCHLÄGE, MECHANISCHE ODER SONSTIGE
GEFAHREN KOMMEN.
-i
Page 4

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
ESTE MANUAL DE SERVICIO SÓLO EXISTE EN INGLÉS.
• SI ALGÚN PROVEEDOR DE SERVICIOS AJENO A GEHC SOLICITA UN IDIOMA
QUE NO SEA EL INGLÉS, ES RESPONSABILIDAD DEL CLIENTE OFRECER UN
SERVICIO DE TRADUCCIÓN.
AVISO
(ES)
ATENÇÃO
(PT-Br)
• NO SE DEBERÁ DAR SERVICIO TÉCNICO AL EQUIPO, SIN HABER
CONSULTADO Y COMPRENDIDO ESTE MANUAL DE SERVICIO.
• LA NO OBSERVANCIA DEL PRESENTE AVISO PUEDE DAR LUGAR A QUE EL
PROVEEDOR DE SERVICIOS, EL OPERADOR O EL PACIENTE SUFRAN
LESIONES PROVOCADAS POR CAUSAS ELÉCTRICAS, MECÁNICAS O DE
OTRA NATURALEZA.
ESTE MANUAL DE ASSISTÊNCIA TÉCNICA SÓ SE ENCONTRA DISPONÍVEL EM
INGLÊS.
• SE QUALQUER OUTRO SERVIÇO DE ASSISTÊNCIA TÉCNICA, QUE NÃO A
GEHC, SOLICITAR ESTES MANUAIS NOUTRO IDIOMA, É DA
RESPONSABILIDADE DO CLIENTE FORNECER OS SERVIÇOS DE TRADUÇÃO.
• NÃO TENTE REPARAR O EQUIPAMENTO SEM TER CONSULTADO E
COMPREENDIDO ESTE MANUAL DE ASSISTÊNCIA TÉCNICA.
• O NÃO CUMPRIMENTO DESTE AVISO PODE POR EM PERIGO A SEGURANÇA
DO TÉCNICO, OPERADOR OU PACIENTE DEVIDO A‘ CHOQUES ELÉTRICOS,
MECÂNICOS OU OUTROS.
AVISO
(PT-pt)
AVVERTENZA
(IT)
ESTE MANUAL DE ASSISTÊNCIA ESTÁ DISPONÍVEL APENAS EM INGLÊS.
• SE QUALQUER OUTRO SERVIÇO DE ASSISTÊNCIA TÉCNICA, QUE NÃO A
GEHC, SOLICITAR ESTES MANUAIS NOUTRO IDIOMA, É DA
RESPONSABILIDADE DO CLIENTE FORNECER OS SERVIÇOS DE TRADUÇÃO.
• NÃO TENTE EFECTUAR REPARAÇÕES NO EQUIPAMENTO SEM TER
CONSULTADO E COMPREENDIDO PREVIAMENTE ESTE MANUAL.
• A INOBSERVÂNCIA DESTE AVISO PODE RESULTAR EM FERIMENTOS NO
TÉCNICO DE ASSISTÊNCIA, OPERADOR OU PACIENTE EM CONSEQUÊNCIA
DE CHOQUE ELÉCTRICO, PERIGOS DE ORIGEM MECÂNICA, BEM COMO DE
OUTROS TIPOS.
IL PRESENTE MANUALE DI MANUTENZIONE È DISPONIBILE SOLTANTO IN
INGLESE.
• SE UN ADDETTO ALLA MANUTENZIONE ESTERNO ALLA GEHC RICHIEDE IL
MANUALE IN UNA LINGUA DIVERSA, IL CLIENTE È TENUTO A PROVVEDERE
DIRETTAMENTE ALLA TRADUZIONE.
• SI PROCEDA ALLA MANUTENZIONE DELL’APPARECCHIATURA SOLO DOPO
AVER CONSULTATO IL PRESENTE MANUALE ED AVERNE COMPRESO IL
CONTENUTO.
• NON TENERE CONTO DELLA PRESENTE AVVERTENZA POTREBBE FAR
COMPIERE OPERAZIONI DA CUI DERIVINO LESIONI ALL’ADDETTO ALLA
MANUTENZIONE, ALL’UTILIZZATORE ED AL PAZIENTE PER FOLGORAZIONE
ELETTRICA, PER URTI MECCANICI OD ALTRI RISCHI.
-ii
Page 5

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
FRONTMATTER.FM
KÄESOLEV TEENINDUSJUHEND ON SAADAVAL AINULT INGLISE KEELES.
• KUI KLIENDITEENINDUSE OSUTAJA NÕUAB JUHENDIT INGLISE KEELEST
ERINEVAS KEELES, VASTUTAB KLIENT TÕLKETEENUSE OSUTAMISE EEST.
HOIATUS
(ET)
• ÄRGE ÜRITAGE SEADMEID TEENINDADA ENNE EELNEVALT KÄESOLEVA
TEENINDUSJUHENDIGA TUTVUMIST JA SELLEST ARU SAAMIST.
• KÄESOLEVA HOIATUSE EIRAMINE VÕIB PÕHJUSTADA TEENUSEOSUTAJA,
OPERAATORI VÕI PATSIENDI VIGASTAMIST ELEKTRILÖÖGI, MEHAANILISE
VÕI MUU OHU TAGAJÄRJEL.
TÄMÄ HUOLTO-OHJE ON SAATAVILLA VAIN ENGLANNIKSI.
• JOS ASIAKKAAN PALVELUNTARJOAJA VAATII MUUTA KUIN
ENGLANNINKIELISTÄ MATERIAALIA, TARVITTAVAN KÄÄNNÖKSEN
HANKKIMINEN ON ASIAKKAAN VASTUULLA.
VAROITUS
(FI)
• ÄLÄ YRITÄ KORJATA LAITTEISTOA ENNEN KUIN OLET VARMASTI LUKENUT
JA YMMÄRTÄNYT TÄMÄN HUOLTO-OHJEEN.
• MIKÄLI TÄTÄ VAROITUSTA EI NOUDATETA, SEURAUKSENA VOI OLLA
PALVELUNTARJOAJAN, LAITTEISTON KÄYTTÄJÄN TAI POTILAAN
VAHINGOITTUMINEN SÄHKÖISKUN, MEKAANISEN VIAN TAI MUUN
VAARATILANTEEN VUOKSI.
ΠΡΟΕΙΔΟΠΟΙΗΣΗ
(EL)
FIGYELMEZTETÉS
(HU)
ΤΟ ΠΑΡΟΝ ΕΓΧΕΙΡΙΔΙΟ ΣΕΡΒΙΣ ΔΙΑΤΙΘΕΤΑΙ ΣΤΑ ΑΓΓΛΙΚΑ ΜΟΝΟ.
• ΕΑΝ ΤΟ ΑΤΟΜΟ ΠΑΡΟΧΗΣ ΣΕΡΒΙΣ ΕΝΟΣ ΠΕΛΑΤΗ ΑΠΑΙΤΕΙ ΤΟ ΠΑΡΟΝ
ΕΓΧΕΙΡΙΔΙΟ ΣΕ ΓΛΩΣΣΑ ΕΚΤΟΣ ΤΩΝ ΑΓΓΛΙΚΩΝ, ΑΠΟΤΕΛΕΙ ΕΥΘΥΝΗ ΤΟΥ
ΠΕΛΑΤΗ ΝΑ ΠΑΡΕΧΕΙ ΥΠΗΡΕΣΙΕΣ ΜΕΤΑΦΡΑΣΗΣ.
• ΜΗΝ ΕΠΙΧΕΙΡΗΣΕΤΕ ΤΗΝ ΕΚΤΕΛΕΣΗ ΕΡΓΑΣΙΩΝ ΣΕΡΒΙΣ ΣΤΟΝ ΕΞΟΠΛΙΣΜΟ
ΕΚΤΟΣ ΕΑΝ
ΕΧΕΤΕ ΣΥΜΒΟΥΛΕΥΤΕΙ ΚΑΙ ΕΧΕΤΕ ΚΑΤΑΝΟΗΣΕΙ ΤΟ ΠΑΡΟΝ
ΕΓΧΕΙΡΙΔΙΟ ΣΕΡΒΙΣ.
• ΕΑΝ ΔΕ ΛΑΒΕΤΕ ΥΠΟΨΗ ΤΗΝ ΠΡΟΕΙΔΟΠΟΙΗΣΗ ΑΥΤΗ, ΕΝΔΕΧΕΤΑΙ ΝΑ
ΠΡΟΚΛΗΘΕΙ ΤΡΑΥΜΑΤΙΣΜΟΣ ΣΤΟ ΑΤΟΜΟ ΠΑΡΟΧΗΣ ΣΕΡΒΙΣ, ΣΤΟ ΧΕΙΡΙΣΤΗ Ή
ΣΤΟΝ ΑΣΘΕΝΗ ΑΠΟ ΗΛΕΚΤΡΟΠΛΗΞΙΑ, ΜΗΧΑΝΙΚΟΥΣ Ή ΑΛΛΟΥΣ ΚΙΝΔΥΝΟΥΣ.
EZEN KARBANTARTÁSI KÉZIKÖNYV KIZÁRÓLAG ANGOL NYELVEN ÉRHETŐ EL.
• HA A VEVŐ SZOLGÁLTATÓJA ANGOLTÓL ELTÉRŐ NYELVRE TART IGÉNYT,
AKKOR A VEVŐ FELELŐSSÉGE A FORDÍTÁS ELKÉSZÍTTETÉSE.
• NE PRÓBÁLJA ELKEZDENI HASZNÁLNI A BERENDEZÉST, AMÍG A
KARBANTARTÁSI KÉZIKÖNYVBEN LEÍRTAKAT NEM ÉRTELMEZTÉK.
• EZEN FIGYELMEZTETÉS FIGYELMEN KÍVÜL HAGYÁSA A SZOLGÁLTATÓ,
MŰKÖDTETŐ VAGY A BETEG ÁRAMÜTÉS, MECHANIKAI VAGY EGYÉB
VESZÉLYHELYZET MIATTI SÉRÜLÉSÉT EREDMÉNYEZHETI.
-iii
Page 6

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
ÞESSI ÞJÓNUSTUHANDBÓK ER EINGÖNGU FÁANLEG Á ENSKU.
• EF ÞJÓNUSTUAÐILI VIÐSKIPTAMANNS ÞARFNAST ANNARS TUNGUMÁLS EN
ENSKU, ER ÞAÐ Á ÁBYRGÐ VIÐSKIPTAMANNS AÐ ÚTVEGA ÞÝÐINGU.
VIÐVÖRUN
(IS)
VÝSTRAHA
(CS)
• REYNIÐ EKKI AÐ ÞJÓNUSTA TÆKIÐ NEMA EFTIR AÐ HAFA SKOÐAÐ OG
SKILIÐ ÞESSA ÞJÓNUSTUHANDBÓK.
• EF EKKI ER FARIÐ AÐ ÞESSARI VIÐVÖRUN GETUR ÞAÐ VALDIÐ MEIÐSLUM
ÞJÓNUSTUVEITANDA, STJÓRNANDA EÐA SJÚKLINGS VEGNA RAFLOSTS,
VÉLRÆNNAR EÐA ANNARRAR HÆTTU.
TENTO SERVISNÍ NÁVOD EXISTUJE POUZE V ANGLICKÉM JAZYCE.
•VPŘÍPADĚ, ŽE POSKYTOVATEL SLUŽEB ZÁKAZNÍKŮM POTŘEBUJE NÁVOD
V JINÉM JAZYCE, JE ZAJIŠTĚNÍ PŘEKLADU DO ODPOVÍDAJÍCÍHO JAZYKA
ÚKOLEM ZÁKAZNÍKA.
• NEPROVÁDĚJTE ÚDRŽBU TOHOTO ZAŘÍZENÍ, ANIŽ BYSTE SI PŘEČETLI
TENTO SERVISNÍ NÁVOD A POCHOPILI JEHO OBSAH.
•VPŘÍPADĚ NEDODRŽOVÁNÍ TÉTO VÝSTRAHY MŮŽE DOJÍT ÚRAZU
ELEKTRICKÁM PROUDEM PRACOVNÍKA POSKYTOVATELE SLUŽEB,
OBSLUŽNÉHO PERSONÁLU NEBO PACIENTŮ VLIVEM ELEKTRICKÉHOP
PROUDU, RESPEKTIVE VLIVEM K RIZIKU MECHANICKÉHO POŠKOZENÍ NEBO
JINÉMU RIZIKU.
ADVARSEL
(DA)
WAARSCHUWING
(NL)
DENNE SERVICEMANUAL FINDES KUN PÅ ENGELSK.
• HVIS EN KUNDES TEKNIKER HAR BRUG FOR ET ANDET SPROG END
ENGELSK, ER DET KUNDENS ANSVAR AT SØRGE FOR OVERSÆTTELSE.
• FORSØG IKKE AT SERVICERE UDSTYRET MEDMINDRE
DENNE SERVICEMANUAL ER BLEVET LÆST OG FORSTÅET.
• MANGLENDE OVERHOLDELSE AF DENNE ADVARSEL KAN MEDFØRE SKADE
PÅ GRUND AF ELEKTRISK, MEKANISK ELLER ANDEN FARE FOR
TEKNIKEREN, OPERATØREN ELLER PATIENTEN.
DEZE ONDERHOUDSHANDLEIDING IS ENKEL IN HET ENGELS VERKRIJGBAAR.
• ALS HET ONDERHOUDSPERSONEEL EEN ANDERE TAAL VEREIST, DAN IS DE
KLANT VERANTWOORDELIJK VOOR DE VERTALING ERVAN.
• PROBEER DE APPARATUUR NIET TE ONDERHOUDEN VOORDAT DEZE
ONDERHOUDSHANDLEIDING WERD GERAADPLEEGD EN BEGREPEN IS.
• INDIEN DEZE WAARSCHUWING NIET WORDT OPGEVOLGD, ZOU HET
ONDERHOUDSPERSONEEL, DE OPERATOR OF EEN PATIËNT GEWOND
KUNNEN RAKEN ALS GEVOLG VAN EEN ELEKTRISCHE SCHOK,
MECHANISCHE OF ANDERE GEVAREN.
-iv
Page 7

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
FRONTMATTER.FM
ŠĪ APKALPES ROKASGRĀMATA IR PIEEJAMA TIKAI ANGĻU VALODĀ.
• JA KLIENTA APKALPES SNIEDZĒJAM NEPIECIEŠAMA INFORMĀCIJA CITĀ
VALODĀ, NEVIS ANGĻU, KLIENTA PIENĀKUMS IR NODROŠINĀT TULKOŠANU.
BRĪDINĀJUMS
(LV)
• NEVEICIET APRĪKOJUMA APKALPI BEZ APKALPES ROKASGRĀMATAS
IZLASĪŠANAS UN SAPRAŠANAS.
•ŠĪ BRĪDINĀJUMA NEIEVĒROŠANA VAR RADĪT ELEKTRISKĀS STRĀVAS
TRIECIENA, MEHĀNISKU VAI CITU RISKU IZRAISĪTU TRAUMU APKALPES
SNIEDZĒJAM, OPERATORAM VAI PACIENTAM.
ŠIS EKSPLOATAVIMO VADOVAS YRA IŠLEISTAS TIK ANGLŲ KALBA.
• JEI KLIENTO PASLAUGŲ TEIKĖJUI REIKIA VADOVO KITA KALBA – NE ANGLŲ,
VERTIMU PASIRŪPINTI TURI KLIENTAS.
ĮSPĖJIMAS
(LT)
•NEMĖGINKITE ATLIKTI ĮRANGOS TECHNINĖS PRIEŽIŪROS DARBŲ, NEBENT
VADOVAUTUMĖTĖS ŠIUO EKSPLOATAVIMO VADOVU IR JĮ SUPRASTUMĖTE
• NEPAISANT ŠIO PERSPĖJIMO, PASLAUGŲ TEIKĖJAS, OPERATORIUS AR
PACIENTAS GALI BŪTI SUŽEISTAS DĖL ELEKTROS SM
ŪGIO, MECHANINIŲ AR
KITŲ PAVOJŲ.
ADVARSEL
(NO)
OSTRZEŻENIE
(PL)
DENNE SERVICEHÅNDBOKEN FINNES BARE PÅ ENGELSK.
• HVIS KUNDENS SERVICELEVERANDØR TRENGER ET ANNET SPRÅK, ER DET
KUNDENS ANSVAR Å SØRGE FOR OVERSETTELSE.
• IKKE FORSØK Å REPARERE UTSTYRET UTEN AT DENNE
SERVICEHÅNDBOKEN ER LEST OG FORSTÅTT.
• MANGLENDE HENSYN TIL DENNE ADVARSELEN KAN FØRE TIL AT
SERVICELEVERANDØREN, OPERATØREN ELLER PASIENTEN SKADES PÅ
GRUNN AV ELEKTRISK STØT, MEKANISKE ELLER ANDRE FARER.
NINIEJSZY PODRĘCZNIK SERWISOWY DOSTĘPNY JEST JEDYNIE W JĘZYKU
ANGIELSKIM.
•JEŚLI FIRMA ŚWIADCZĄCA KLIENTOWI USłUGI SERWISOWE WYMAGA
UDOSTĘPNIENIA PODRĘCZNIKA W JĘZYKU INNYM NIŻ ANGIELSKI,
OBOWIĄZEK ZAPEWNIENIA STOSOWNEGO TłUMACZENIA SPOCZYWA NA
KLIENCIE.
• NIE PRÓBOWAĆ SERWISOWAĆ NINIEJSZEGO SPRZĘTU BEZ UPRZEDNIEGO
ZAPOZNANIA SIĘ Z PODRĘCZNIKIEM SERWISOWYM.
• NIEZASTOSOWANIE SIĘ DO TEGO OSTRZEŻENIA MOżE GROZIĆ
OBRAŻENIAMI CIAłA SERWISANTA, OPERATORA LUB PACJENTA W WYNIKU
PORAŻENIA PRĄDEM, URAZU MECHANICZNEGO LUB INNEGO RODZAJU
ZAGROŻEŃ.
-v
Page 8

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
ACEST MANUAL DE SERVICE ESTE DISPONIBIL NUMAI ÎN LIMBA ENGLEZĂ.
• DACĂ UN FURNIZOR DE SERVICII PENTRU CLIENŢI NECESITĂ O ALTĂ LIMBĂ
DECÂT CEA ENGLEZĂ, ESTE DE DATORIA CLIENTULUI SĂ FURNIZEZE O
TRADUCERE.
ATE NŢIE
(RO)
ОСТОРОЖНО!
(RU)
• NU ÎNCERCAŢI SĂ REPARAŢI ECHIPAMENTUL DECÂT ULTERIOR
CONSULTĂRII ŞI ÎNŢELEGERII ACESTUI MANUAL DE SERVICE.
• IGNORAREA ACESTUI AVERTISMENT AR PUTEA DUCE LA RĂNIREA
DEPANATORULUI, OPERATORULUI SAU PACIENTULUI ÎN URMA
PERICOLELOR DE ELECTROCUTARE, MECANICE SAU DE ALTĂ NATURĂ.
ДАННОЕ РУКОВОДСТВО ПО ОБСЛУЖИВАНИЮ ПРЕДОСТАВЛЯЕТСЯ ТОЛЬКО
НА АНГЛИЙСКОМ ЯЗЫКЕ.
• ЕСЛИ СЕРВИСНОМУ ПЕРСОНАЛУ КЛИЕНТА НЕОБХОДИМО РУКОВОДСТВО
НЕ НА АНГЛИЙСКОМ ЯЗЫКЕ, КЛИЕНТУ СЛЕДУЕТ САМОСТОЯТЕЛЬНО
ОБЕСПЕЧИТЬ ПЕРЕВОД.
• ПЕРЕД ОБСЛУЖИВАНИЕМ ОБОРУДОВАНИЯ ОБЯЗАТЕЛЬНО
ОБРАТИТЕСЬ
К ДАННОМУ РУКОВОДСТВУ И ПОЙМИТЕ ИЗЛОЖЕННЫЕ В НЕМ СВЕДЕНИЯ.
• НЕСОБЛЮДЕНИЕ УКАЗАННЫХ ТРЕБОВАНИЙ МОЖЕТ ПРИВЕСТИ К ТОМУ,
ЧТО СПЕЦИАЛИСТ ПО ТЕХОБСЛУЖИВАНИЮ, ОПЕРАТОР ИЛИ ПАЦИЕНТ
ПОЛУЧАТ УДАР ЗЛЕКТРИЧЕСКИМ ТОКОМ, МЕХАНИЧЕСКУЮ ТРАВМУ ИЛИ
ДРУГОЕ ПОВРЕЖДЕНИЕ.
ПРЕДУПРЕЖДЕНИЕ
(BG)
UPOZORENJE
(SR)
ТОВА СЕРВИЗНО РЪКОВОДСТВО Е НАЛИЧНО САМО НА АНГЛИЙСКИ ЕЗИК.
• АКО ДОСТАВЧИКЪТ НА СЕРВИЗНИ УСЛУГИ НА КЛИЕНТ
СЕ НУЖДАЕ ОТ
ЕЗИК, РАЗЛИЧЕН ОТ АНГЛИЙСКИ, ЗАДЪЛЖЕНИЕ НА КЛИЕНТА Е ДА
ПРЕДОСТАВИ ПРЕВОДАЧЕСКА УСЛУГА.
• НЕ СЕ ОПИТВАЙТЕ ДА ИЗВЪРШВАТЕ СЕРВИЗНО ОБСЛУЖВАНЕ НА ТОВА
ОБОРУДВАНЕ, ОСВЕН ВСЛУЧАЙ, ЧЕ СЕРВИЗНОТО РЪКОВОДСТВО Е
ПРОЧЕТЕНО И СЕ РАЗБИРА.
• НЕСПАЗВАНЕТО НА ТОВА ПРЕДУПРЕЖДЕНИЕ МОЖЕ ДА ДОВЕДЕ ДО
НАРАНЯВАНЕ НА ДОСТАВЧИКА НА СЕРВИЗНИ УСЛУГИ, НА
ОПЕРАТОРА
ИЛИ ПАЦИЕНТА ВСЛЕДСТВИЕНА ТОКОВ УДАР, МЕХАНИЧНИ ИЛИ ДРУГИ
РИСКОВЕ.
OVAJ PRIRUČNIK ZA SERVISIRANJE DOSTUPAN JE SAMO NA ENGLESKOM
JEZIKU.
• AKO KLIJENTOV SERVISER ZAHTEVA JEZIK KOJI NIJE ENGLESKI,
ODGOVORNOST JE NA KLIJENTU DA PRUŽI USLUGE PREVOĐENJA.
• NEMOJTE POKUŠAVATI DA SERVISIRATE OPREMU AKO NISTE PROČITALI I
RAZUMELI PRIRUČNIK ZA SERVISIRANJE.
• AKO NE POŠTUJETE OVO UPOZORENJE, MOŽE DOĆI DO POVREĐIVANJA
SERVISERA, OPERATERA ILI PACIJENTA UZROKOVANOG ELEKTRIČNIM
UDAROM, MEHANIČKIM I DRUGIM OPASNOSTIMA.
-vi
Page 9

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
FRONTMATTER.FM
TA SERVISNI PRIROČNIK JE NA VOLJO SAMO V ANGLEŠČINI.
• ČE PONUDNIK SERVISNIH STORITEV ZA STRANKO POTREBUJE NAVODILA V
DRUGEM JEZIKU, JE ZA PREVOD ODGOVORNA STRANKA SAMA.
OPOZORILO
(SL)
• NE POSKUŠAJTE SERVISIRATI OPREME, NE DA BI PREJ PREBRALI IN
RAZUMELI SERVISNI PRIROČNIK.
• ČE TEGA OPOZORILA NE UPOŠTEVATE, OBSTAJA NEVARNOST
ELEKTRIČNEGA UDARA, MEHANSKIH ALI DRUGIH NEVARNOSTI IN
POSLEDIČNIH POŠKODB PONUDNIKA SERVISNIH STORITEV, UPORABNIKA
OPREME ALI PACIENTA.
OVAJ SERVISNI PRIRUČNIK DOSTUPAN JE SAMO NA ENGLESKOM JEZIKU.
• AKO KLIJENTOV SERVISER ZAHTIJEVA JEZIK KOJI NIJE ENGLESKI,
ODGOVORNOST KLIJENTA JE PRUŽITI USLUGE PREVOĐENJA.
UPOZORENJE
(HR)
• NEMOJTE POKUŠAVATI SERVISIRATI OPREMU AKO NISTE PROČITALI I
RAZUMJELI SERVISNI PRIRUČNIK.
• AKO NE POŠTUJETE OVO UPOZORENJE, MOŽE DOĆI DO OZLJEDE
SERVISERA, OPERATERA ILI PACIJENTA PROUZROČENE STRUJNIM
UDAROM, MEHANIČKIM I DRUGIM OPASNOSTIMA.
UPOZORNENIE
(SK)
VARNING
(SV)
TÁTO SERVISNÁ PRÍRUČKA JE K DISPOZÍCII LEN V ANGLIČTINE.
• AK ZÁKAZNÍKOV POSKYTOVATEĽ SLUŽIEB VYŽADUJE INÝ JAZYK AKO
ANGLIČTINU, POSKYTNUTIE PREKLADATEĽSKÝCH SLUŽIEB JE
ZODPOVEDNOSŤOU ZÁKAZNÍKA.
• NEPOKÚŠAJTE SA VYKONÁVAŤ SERVIS ZARIADENIA SKÔR, AKO SI
NEPREČÍTATE SERVISNÚ PRÍRUČKU A NEPOROZUMIETE JEJ.
• ZANEDBANIE TOHTO UPOZORNENIA MÔŽE VYÚSTIŤ DO ZRANENIA
POSKYTOVATEĽA SLUŽIEB, OBSLUHUJÚCEJ OSOBY ALEBO PACIENTA
ELEKTRICKÝM PRÚDOM, PRÍPADNE DO MECHANICKÉHO ALEBO INÉHO
NEBEZPEČENSTVA.
DEN HÄR SERVICEHANDBOKEN FINNS BARA TILLGÄNGLIG PÅ ENGELSKA.
• OM EN KUNDS SERVICETEKNIKER HAR BEHOV AV ETT ANNAT SPRÅK ÄN
ENGELSKA ANSVARAR KUNDEN FÖR ATT TILLHANDAHÅLLA
ÖVERSÄTTNINGSTJÄNSTER.
• FÖRSÖK INTE UTFÖRA SERVICE PÅ UTRUSTNINGEN OM DU INTE HAR LÄST
OCH FÖRSTÅR DEN HÄR SERVICEHANDBOKEN.
• OM DU INTE TAR HÄNSYN TILL DEN HÄR VARNINGEN KAN DET RESULTERA I
SKADOR PÅ SERVICETEKNIKERN, OPERATÖREN ELLER PATIENTEN TILL
FÖLJD AV ELEKTRISKA STÖTAR, MEKANISKA FAROR ELLER ANDRA FAROR.
-vii
Page 10

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
BU SERVİS KILAVUZU YALNIZCA İNGİLİZCE OLARAK SAĞLANMIŞTIR.
•EĞER MÜŞTERİ TEKNİSYENİ KILAVUZUN İNGİLİZCE DIŞINDAKİ BİR DİLDE
OLMASINI İSTERSE, KILAVUZU TERCÜME ETTİRMEK MÜŞTERİNİN
SORUMLULUĞUNDADIR.
DİKKAT
(TR)
•SERVİS KILAVUZUNU OKUYUP ANLAMADAN EKİPMANLARA MÜDAHALE
ETMEYİNİZ.
• BU UYARININ GÖZ ARDI EDİLMESİ, ELEKTRİK ÇARPMASI YA DA MEKANİK
VEYA DİĞER TÜRDEN KAZALAR SONUCUNDA TEKNİSYENİN, OPERATÖRÜN
YA DA HASTANIN YARALANMASINA YOL AÇABİLİR.
(JA)
Traditional
Chinese
-viii
Page 11

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
(ZH-CN)
FRONTMATTER.FM
(KO)
-ix
Page 12

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
DAMAGE IN TRANSPORTATION
All packages should be closely examined at time of delivery. If damage is apparent write “Damage In
Shipment” on ALL copies of the freight or express bill BEFORE delivery is accepted or “signed for” by
a GE representative or hospital receiving agent. Whether noted or concealed, damage MUST be
reported to the carrier immediately upon discovery, or in any event, within 14 days after receipt, and the
contents and containers held for inspection by the carrier. A transportation company will not pay a claim
for damage if an inspection is not requested within this 14 day period.
CERTIFIED ELECTRICAL CONTRACTOR STATEMENT - FOR USA ONLY
All electrical Installations that are preliminary to position ing of the equipment at the site prepared for the
equipment shall be performed by licensed electrical contractors. Othe r conn ections between pieces o f
electrical equipment, calibrations and testing sha ll be performed by q ualified GE Healthcare person nel.
In performing all electrical work on these products, GE will use its own specially trained field engineers.
All of GE’s electrical work on these products will comply with the requirements of the applicable
electrical codes.
The purchaser of GE equipment shall only utilize qualified personnel (i.e., GE’s field engineers,
personnel of third-party service companies with equivalent training, or licensed electricians) to perform
electrical servicing on the equipment.
OMISSIONS & ERRORS
If there are any omissions, errors or suggestions for improving this documentation, please contact the
GE Healthcare Global Documentation Group with specific information listing the system type, manual
title, part number, revision number, page number and suggestion details.
Mail the information to:
Service Documentation,
GE Vingmed Ultrasound AS
P.O.Box: 141
NO 3191 HORTEN
NORWAY
GE Healthcare employees should use TrackWise to report service documentation issues. These issues
will then be in the internal problem reporting tool and communicated to the writer.
SERVICE SAFETY CONSIDERATIONS
DANGER
DANGEROUS VOLTAGES, CAPABLE OF CAUSING DEATH, ARE PRESENT IN
THIS EQUIPMENT. USE EXTREME CAUTION WHEN HANDLING, TESTING AND
ADJUSTING.
WARNINGWARNING
Use all Personal Protection Equipment (PPE) such as gloves, safety shoes, safety
glasses, and kneeling pad, to reduce the risk of injury.
-x
For a complete review of all safety requirements, see the Safety Considerations section in Chapter 1.
Page 13

2.NOV.2011
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
FRONTMATTER.FM
LEGAL NOTES
The contents of this publication may not be copied or duplicated in any form, in whole or in part, without
prior written permission of GE Healthcare.
GE Healthcare may revise this publication from time to time without written notice.
TRADEMARKS
All products and their name brands are trademarks of their respective holders.
COPYRIGHTS
All Material Copyright© 2011 by General Electric Company Inc. All Rights Reserved.
-xi
Page 14

2.NOV.2011
FRONTMATTER.FM
PRELIMINARY
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Revision History
REVISION DATE REASON FOR CHANGE
1 2011 NOV. 02
Initial release.
List of Effected Pages (LOEP)
Pages Revision Pages Revision Pages Revision
Title Page 1 1-1 to 1-26 1 7-1 to 7-176 1
Warnings i to -xii 1 2-1 to 2-12 1 8-1 to 8-154 1
TOC 1 3-1 to 3-180 1 9-1 to 9-20 1
4-1 to 4-40 1 10-1 to 10-36 1
5-1 to 5-48 1 Back Cover N/A
6-1 to 6-16 1
-xii
Page 15

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Table of Contents
CHAPTER 1
Introduction
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 1
Purpose of Chapter 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 1
Purpose of Service Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 2
Typical Users of the Service Manual (This Manual) . . . . . . . . . . . . . . . . . . . . . . 1 - 2
Vivid i n Models Covered in this Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 3
Vivid q N Models Covered in this Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 3
System History - Hardware and Software Versions . . . . . . . . . . . . . . . . . . . . . . 1 - 4
Purpose of Operator Manual(s) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 4
Important Conventions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 5
Conventions Used in this Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 5
Safety Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 7
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 7
Human Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 7
Mechanical Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 7
Electrical Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 9
Dangerous Procedure Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 11
Product Labels and Icons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 12
Universal Product Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 12
Label Descriptions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 14
Vivid i n/ Vivid q N Battery Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 17
Vivid i n and Vivid q N External Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 19
SafeLock Cart Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 20
EMC, EMI, and ESD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 21
Electromagnetic Compatibility (EMC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 21
CE Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 21
Electrostatic Discharge (ESD) Prevention . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 21
Standards Used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 22
Lockout/Tagout (LOTO) Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 23
Returning/Shipping Probes and Repair Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 24
Customer Assistance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 25
Table of Contents -xiii
Page 16

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Contact Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 25
System manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 - 26
-xiv
Page 17

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 2
Site Preparations
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 - 1
Purpose of Chapter 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 1
Console Requirements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 2
Unit Environmental Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 2
Cooling Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 2
Lighting Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 2
Time and Manpower Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 2
Electrical Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 3
EMI Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 4
Probe Environmental Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 5
Facility Needs. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 6
Purchaser Responsibilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 6
Mandatory Site Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 7
Site Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 7
Networking Pre-Installation Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 9
Connectivity Installation Worksheet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 10
Check List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 - 12
-xv
Page 18

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 3
System Setup
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 1
Purpose of Chapter 3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 1
Installation Reminders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 2
Average Installation Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 2
Installation Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 2
Safety Reminders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 3
The Tilt & Shock Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 4
Receiving the Vivid i n and Vivid q N. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 5
Examin All Packages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 5
Damage in Transportation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 6
Unpacking the Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 9
Examin All Packages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 10
Unpacking the Wooden Transportation box . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 11
Verifying the Transportation box Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 12
Physical Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 12
EMI Protection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 12
Preparing for Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 13
Confirming Customer Order . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 13
Verifying the Transportation Box Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 13
Component Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 14
Confirming SafeLock Cart Voltage Configuration . . . . . . . . . . . . . . . . . . . . . . 3 - 19
System Voltage Confirmation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 19
Ensuring Protection from EMI . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 20
Completing the Hardware Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 21
Connecting Printers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 21
Connecting Peripherals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 34
Charging the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 55
Connecting Probes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 58
Connecting the ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 61
Connecting the Unit to a Power Source . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 62
Switching the System ON/OFF . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 64
Mounting the Vivid i n/ Vivid q N on the SafeLock Cart (optional) . . . . . . . . . . . . . . . . 3 - 65
Mounting the Vivid i n and Vivid q N on the SafeLock Cart . . . . . . . . . . . . . . . 3 - 65
-xvi
Page 19

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Adjusting the SafeLock Cart Locking Mechanism . . . . . . . . . . . . . . . . . . . . . . 3 - 68
Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 69
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 69
Vivid i n/ Vivid q N Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 70
Service Screen Set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 75
Configuring Peripherals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 81
Software Options Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 84
Connectivity Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 85
Connectivity Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 85
Physical Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 88
Connectivity Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 98
Set Up Connection to a DICOM Server in a Network . . . . . . . . . . . . . . . . . . . 3 - 110
Setup Connection to HL7 Server in a Network . . . . . . . . . . . . . . . . . . . . . . . . 3 - 117
Query/Retrieve (Q/R) Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 120
MPEGVue Export Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 127
eVue Dataflow Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 132
Using MPEGVue/eVue on a Remote PC . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 136
Storing and Transporting the Unit. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 177
Safety Precautions for Moving the Vivid i n/ Vivid q N Unit . . . . . . . . . . . . . . 3 - 177
Transportation Box and Packaging Materials . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 177
Completing the Installation Paperwork. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 178
System Installation Details . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 178
Product Locator Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 178
Product Locator Installation Card. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 179
User Manual(s) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 - 180
-xvii
Page 20

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 4
General Procedures and Functional Checks
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 1
Purpose of Chapter 4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 1
Specific Equipment Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 2
General Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 3
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 3
Power ON/Boot-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 3
Power Shut Down . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 7
Log On to the System as ADM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 10
Using Removable Media . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 12
Labeling Removable Media . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 15
Formatting Removable Media . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 15
Verifying Removable Media . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 16
Archiving and Loading Presets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 17
Functional Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 19
Basic Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 19
Peripherals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 28
SafeLock Cart Functional Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 33
Back End Processor Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 34
System Turnover Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 37
Software Configuration Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 37
Site Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 - 38
-xviii
Page 21

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 5
Components and Function (Theory)
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 1
Purpose of Chapter 5 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 2
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 2
Signal Flow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 4
Vivid i n and Vivid q N Systems - Block Diagram . . . . . . . . . . . . . . . . . . . . . . . . 5 - 5
Front End Unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 6
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 6
Front End Unit - Location in the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 9
RFI Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 11
TR32 Boards (Transmitter/Receiver) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 15
Probe and MUX (P&M) Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 18
Back End Processor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 21
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 21
ETX SBC Central Processing Unit (CPU) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 23
ETX Base Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 23
Hard Disk . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 25
Fan Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 25
RTC (BIOS) Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 25
Back End Processor - Location of Components . . . . . . . . . . . . . . . . . . . . . . . . 5 - 26
Left/Right Speakers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 28
External Input/Output (I/O) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 29
Power Supply System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 30
Electrical Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 30
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 30
Power Supply Unit Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 31
DC Source Selector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 31
Rechargeable Battery Pack Assy (GPA) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 32
Monitor and Operator Panel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 34
Keyboard and Operator Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 34
Keyboard and Operator Panel Components . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 35
ECG Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 - 39
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 39
-xix
Page 22

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
ECG Patient I/O Module Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 40
Isolation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 40
ECG/Respiratory Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 41
Internal ECG/Respiratory Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 41
External ECG/Respiratory Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 43
Peripherals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 46
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 46
Cooling System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 48
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 - 48
-xx
Page 23

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 6
Service Adjustments
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 - 1
Purpose of Chapter 6 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 1
LCD Display Adjustments and Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Contrast Adjustment Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 2
Brightness Adjustment Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 3
Testing Your Contrast and Brightness Settings . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 3
Blue Tint Adjustment Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 3
Blue Tint "2" Adjustment Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 5
External Monitor Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 8
Keyboard Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 10
Configuring Print Orientation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 10
Video System Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 10
Beamformer Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 10
Battery Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 - 11
-xxi
Page 24

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 7
Diagnostics/Troubleshooting
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Purpose of Chapter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 1
Diagnostics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 2
Diagnostic Tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 2
Diagnostic Procedure Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 2
Accessing the Diagnostic Test Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 3
Diagnostic Test Window Menu Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 10
Performing Full System Diagnostics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 19
Accessing the Full System Diagnostic Options . . . . . . . . . . . . . . . . . . . . . . . . 7 - 20
Performing Front End (FE) Diagnostics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 24
Accessing the Front End Diagnostic Options . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 25
Calibration Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 25
Radio Frequency Interface (RFI) Diagnostic Tests . . . . . . . . . . . . . . . . . . . . . 7 - 31
TR 32 Diagnostic Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 46
Probe and MUX Diagnostic Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 61
Monitoring Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 86
Accessing the Monitoring Diagnostic Test Options . . . . . . . . . . . . . . . . . . . . . 7 - 86
Performing Back End Diagnostics on the System . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 108
Accessing the Back End Diagnostic Test Options . . . . . . . . . . . . . . . . . . . . . 7 - 108
InSite ExC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 137
Service Desktop . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 137
Error Logs Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 139
Diagnostics Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 144
Image Quality Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 151
Calibration Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 151
Configuration Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 152
*.ZipUtilities Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 153
Replacement Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 165
PM Page . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 165
Automatic Error Log. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 166
Adding Bookmarks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 166
Extracting Logs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 - 166
-xxii
Page 25

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 8
Replacement Procedures
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 - 1
Purpose of Chapter 8 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 1
External Component Replacement Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 3
Bearing Handle Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 3
Battery Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 5
Hard Disk Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 7
Control Panel and Keyboard Replacement Procedure . . . . . . . . . . . . . . . . . . . 8 - 9
Rear Cover & Latch Assembly Replacement Procedure . . . . . . . . . . . . . . . . . 8 - 19
LCD Display Cover Hinges Replacement Procedure . . . . . . . . . . . . . . . . . . . . 8 - 21
Bottom Assembly Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 25
LCD Display Frame Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 27
Internal Component Replacement Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 29
P&M (Probe and MUX) Board Replacement Procedure . . . . . . . . . . . . . . . . . . 8 - 29
TR32 and RFI Boards Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . 8 - 32
BEP Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 38
BIOS Battery Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 45
HVPS Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 46
Fan Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 49
Speaker Assembly Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 52
LCD Display Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 53
LCD Flex Cable Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 58
SafeLock Cart Components Replacement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 61
Overview of SafeLock Cart Replaceable Components . . . . . . . . . . . . . . . . . . . 8 - 62
SafeLock Cart Replacement Procedures - Quick Reference List . . . . . . . . . . . 8 - 63
Upper Cover (Rear) Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 64
AC Cable Hook Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 65
AC Power Cable Bumper Protector Replacement Procedure . . . . . . . . . . . . . 8 - 66
ECG Cable Protector Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . 8 - 68
Lower Cover (Rear) Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 71
AC Distribution Assembly Replacement Procedure . . . . . . . . . . . . . . . . . . . . . 8 - 72
Peripheral Power Cable Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . 8 - 74
Main Cable Harness Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 79
USB Board Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 80
Gas Spring Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 82
DVD Holder Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 85
LAN Isolation Box and Holder Replacement Procedure . . . . . . . . . . . . . . . . . . 8 - 86
-xxiii
Page 26

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Top Shelf Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 87
Handrest Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 89
Probe Shelf Assembly Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . 8 - 90
Probe Cable Hook Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 92
Front Wheel Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 93
Rear Wheel Replacement Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 95
Software Loading. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 97
Software Upgrade Procedure Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 97
Setting the BIOS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 108
BIOS Firmware Update . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 118
Formatting and Partitioning the Hard Disk - Automatic Procedure . . . . . . . . 8 - 121
Formatting and Partitioning the Hard Disk - Manual Procedure . . . . . . . . . . . 8 - 123
Full Re-ghost and Software Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 128
Installing Software only from DVD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 130
Installing Software only or Patch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 134
Installation of PMC Version . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 135
Software Roll-back Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 139
Performing a Complete System Back-up. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 141
Database Merge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 141
How to Restore User-defined Presets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 147
Peripherals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 148
Connecting and Removing Peripherals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 - 148
External USB Hard Disk Replacement Procedure . . . . . . . . . . . . . . . . . . . . . 8 - 150
-xxiv
Page 27

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 9
Renewal Parts
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 - 1
Purpose of Chapter 9 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 1
List of Abbreviations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 2
Mechanical Hardware Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 3
Electronic Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 4
Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 7
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 8
Probes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 9
Peripherals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 11
SafeLock Cart Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 12
Optional Modo Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 14
Vivid i n Spare Part Kits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 15
Product Manuals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 19
Product Manuals - BT’11 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 19
Product Manuals - BT’09 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 19
Product Manuals - BT’06 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 - 20
-xxv
Page 28

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
CHAPTER 10
Care and Maintenance
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 - 1
Periodic Maintenance Inspections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 1
Purpose of Chapter 10 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 1
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 2
Why Perform Maintenance Procedures?. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 3
Keeping Records . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 3
Quality Assurance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 3
Maintenance Task Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 4
How Often Should Care & Maintenance Procedures be Performed? . . . . . . . 10 - 4
Tools Required. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 7
Special Tools, Supplies and Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 7
System Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 9
Preliminary Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 9
Functional Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 10
Input Power Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 11
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 12
Physical Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 15
Optional Diagnostic Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 16
Probe Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 17
Probe Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 17
Probe Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 17
Basic Probe Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 18
Probe Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 18
Returning and Shipping of Defective Probes . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 20
Electrical Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 21
Safety Test Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 21
GEMS Current Leakage Limits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 22
Outlet Test Wiring Arrangement - USA & Canada . . . . . . . . . . . . . . . . . . . . . 10 - 23
SafeLock Cart - Grounding Continuity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 23
SafeLock Cart - Chassis Current Leakage Test . . . . . . . . . . . . . . . . . . . . . . . 10 - 25
Isolated Patient Lead (Source) Leakage – Lead-to-Ground . . . . . . . . . . . . . 10 - 27
Isolated Patient Lead (Source) Leakage – Lead-to-Lead . . . . . . . . . . . . . . . 10 - 28
Isolated Patient Lead (Sink) Leakage - Isolation Test . . . . . . . . . . . . . . . . . . 10 - 28
Probe Current Leakage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 30
-xxvi
Page 29

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Excessive Current Leakage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 33
Possible Causes of Excessive Current Leakage . . . . . . . . . . . . . . . . . . . . . . 10 - 33
Vivid i n/ Vivid q N Inspection Certificates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 - 34
-xxvii
Page 30

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
-xxviii
Page 31

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Chapter 1
Introduction
Section 1-1 Overview
1-1-1 Purpose of Chapter 1
This chapter describes important issues related to safely servicing the Vivid i n and Vivid q N portable
ultrasound scanner. The service provider mu st read and under stand all the information presented here
before installing or servicing a unit.
Table 1-1 Contents in Chapter 1
Section Description Page Number
1-1
1-2
1-3
1-4
1-5
1-6
1-7
1-8
Overview
Important Conventions
Safety Considerations
Product Labels and Icons
EMC, EMI, and ESD
Lockout/Tagout (LOTO) Requirements
Returning/Shipping Probes and Repair Parts
Customer Assistance
1-1
1-5
1-7
1-12
1-21
1-23
1-24
1-25
Chapter 1 - Introduction 1-1
Page 32

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-1-2 Purpose of Service Manual
This manual provides installation and service information for the Vivid i n and Vivid q N portable
ultrasound scanner, and contains the following chapters:
• Chapter 1 - Introduction
Contains a content summary and warnings.
• Chapter 2 - Site Preparations
Contains pre-installation requirements for the Vivid i n and Vivid q N portable ultrasound scanner.
• Chapter 3 - System Setup
Contains installation procedures and an installation checklist.
• Chapter 4 - General Procedures and Functional Checks
Contains functional checks that are reco m men de d as par t of the inst alla tio n procedure, or as
required during servicing and periodic maintenance.
• Chapter 5 - Components and Function (Theory)
Contains block diagrams and functional explanations of the electronic circuits.
• Chapter 6 - Service Adjustments
Contains instructions for performing service adjustments to the Vivid i n and Vivid q N portable
ultrasound scanner.
• Chapter 7 - Diagnostics/Troubleshooting
Provides instructions for setting up and running diagnostic, troubleshooting and other related
routines for the Vivid i n and Vivid q N portable ultrasound scanner.
• Chapter 8 - Replacement Procedures
Provides disassembly and reassembly procedures for all Field Replaceable Units (FRUs).
• Chapter 9 - Renewal Parts
Contains a complete list of field replaceable parts for the Vivid i n and Vivid q N portable ultrasound
scanner.
• Chapter 10 - Care and Maintenance
Provides periodic maintenance procedures for the Vivid i n and Vivid q N portable ultrasound
scanner.
1-1-3 Typical Users of the Service Manual (This Manual)
This manual is intended for the following categories of users:
• Hospital service personnel.
• Contractors (some parts of Chapter 2 - Site Preparations).
1-2 -
Page 33

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-1-4 Vivid i n Models Covered in this Manual
The Vivid i n models documented in this manual is shown in Table 1-2 below:
Table 1-2 Vivid i n Models
Model H-Cat No. Part Number Description Comments
Vivid i n Portable
Ultrasound Scanner
H45561AC
H45551DE
FL000110 Laptop-style system BT’09
FL000090 Laptop-style system BT’06
1-1-5 Vivid q N Models Covered in this Manual
The Vivid q N models documented in this manual is shown in Table 1-3 below.
Table 1-3 Vivid q N Model
Model H-Cat No. Part Number Description Comments
Vivid q N Portable
Ultrasound Scanner
H45571BK
FQ000001 Laptop-style system BT’11
Chapter 1 - Introduction 1-3
Page 34

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-1-6 System History - Hardware and Software Versions
The Vivid i n is a compact, fully portable, phased, linear array ultrasound imaging scanner.
Weighing only 5 Kgs (11 lbs), this laptop-style system is extremely versatile and, depending upon the
installed software, can be used for a variety of applications.
The Vivid q N ultrasound imaging scanner is similar to the Vivid-i™ - however, newly d esigned to offer
additional features including the ability to support use of M4S-RS probes. Also weighing only 5 Kgs
(11 lbs), this laptop-style system is extremely versatile and, depending upon th e installed software, can
be used for a variety of applications.
Note: Upgrade options may be available with future versions.
1-1-7 Purpose of Operator Manual(s)
The Operator Manual(s) should be fully read and understood before operating the Vivid i n or Vivid q N
system, and also kept near the unit for quick reference.
1-4 -
Page 35

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-2 Important Conventions
1-2-1 Conventions Used in this Manual
1-2-1-1 Model Designations
This manual covers the Vivid i n and Vivid q N ultr asound units listed in Table 1-2 on page 1-3.
1-2-1-2 Icons
Pictures, or icons, are used wherever they will reinforce the printed message. The icons, labels and
conventions used on the product and in the service information are described in this chapter.
1-2-1-3 Safety Precaution Messages
Various levels of safety precautions are found on the equip ment and throughout this service manual.
Different levels of severity are identified by one of the following icons which precede precautionary
statements in the text.
DANGER: Indicates the presence of a hazard that will cause severe personal injury or death if the
instructions are ignored.
WARNING: Indicates the presence of a hazard that can cause severe personal injury and property
damage if the instructions are ignored.
CAUTION: Indicates the presence of a hazard that can cause property da mag e but ha s absolu te ly n o
personal injury risk.
Note: Notes are used to provide important information about an item or a procedure. Be sure to read
the notes as the information they contain can often save you time or effort.
Chapter 1 - Introduction 1-5
Page 36

GE HEALTHCARE
N
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-2-1-4 Standard Hazard Icons
Important information will always be preceded by the exclamation point contained within
a triangle, as seen throughout this chapter. In addition to text, several different graphical
icons (symbols) may be used to make you aware of specific types of hazards that could
cause harm.
Table 1-4 Standard Hazard Icons
ELECTRICAL MECHANICAL RADIATION
LASER HEAT PINCH
Other hazard icons make you aware of specific procedures that should be followed.
Table 1-5 Standard Icons Indicating a Special Procedure Be Used
AVOID STATIC ELECTRICITY TAG AND LOCK OUT WEAR EYE PROTECTION
TAG
&
EYE
EYE
PROTECTIO
PROTECTION
1-6 Section 1-2 - Important Conventions
Page 37

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-3 Safety Considerations
1-3-1 Introduction
The following safety precautions must be observed duri ng all phases of operation, service and repair of
this equipment. Failure to comply with these precautions or with specific warnings elsewhere in this
manual, violates safety standards of design, manufacture and intended use of the equipment.
1-3-2 Human Safety
Operating personnel must not remove the system covers.
Servicing should be performed by authorized personnel only.
Only personnel who have participated in Vivid i n/ Vivid q N Training are authorized to service the
equipment.
Local laws may restrict this device for sale or use by or on the order of a physician.
1-3-3 Mechanical Safety
DANGER: WHEN THE VIVID I n or Vivid q N SCANNER IS USED WITH THE SAFELOCK
CART, MAKE SURE THE UNIT IS FIRMLY SECURED IN THE CORRECT POSITION ON
THE CART SINCE IT MAY BECOME UNSTABLE AND TIP OVER.
DANGER: WHENENVER THE SAFELOCK CART IS TO BE MOVED ALONG ANY INCLINE,
USE EXTREME CAUTION. MAKE SURE THAT THE VIVID I
n or Vivid q N SCANNER AND
ALL PERIPHERALS ARE SECURELY MOUNTED ON THE CART BEFORE ATTEMPTING
TO MOVE IT.
DANGER: ULTRASOUND PROBES ARE HIGHLY SENSITIVE MEDICAL INSTRUMENTS
THAT CAN EASILY BE DAMAGED BY IMPROPER HANDLING. USE CARE WHEN
HANDLING AND PROTECT FROM DAMAGE WHEN NOT IN USE. DO NOT USE A
DAMAGED OR DEFECTIVE PROBE. FAILURE TO FOLLOW THESE PRECAUTIONS CAN
RESULT IN SERIOUS INJURY AND EQUIPMENT DAMAGE.
DANGER: NEVER USE A PROBE THAT HAS BEEN SUBJECTED TO MECHANICAL
SHOCK OR IMPACT. EVEN IF THE PROBE APPEARS TO BE UNBROKEN, IT MAY IN
FACT BE DAMAGED.
CAUTION: The Vivid i n and Vivid q N portable ultrasound scanner weighs 5kg (11 lbs.) or more,
depending on carry-on peripherals when ready for use. In addition, the SafeLock cart weighs
approximately 40kg (88 lbs.), excluding peripherals. Care must be used when moving the unit or
replacing its parts. Failure to follow the precautions listed could result in injury, uncontrolled
motion and costly damage.
ALWAYS:
• Use two people when moving or lifting more then 16 kg (35 lbs).
• Use the handle to move the system
• Be sure the pathway is clear.
• Use slow, careful motions.
• Do not let the system strike walls or door frames.
Chapter 1 - Introduction 1-7
Page 38

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
• Whenever the Vivid i n or Vivid q N sca nner is mounted on the SafeLock Cart and b eing moved on
inclines, make sure that the scanner and all peripherals are securely mounted on the SafeLock cart
before attempting to move it.
WARNING: Always lock the SafeLock cart in its parked (locked) position after moving the
system. Failure to do so could result in personal injury or equipment damage.
WARNING: Equipment damage could result if special care is not taken when transporting the
Vivid i n or Vivid q N system (and SafeLock cart if applicable) in a vehicle.
ALWAYS:
• Eject any disks from the MOD (if installed).
• Ensure that the Vivid i n or Vivid q N system is well prepared and packed in its original
packaging before transporting. Special care must be taken to correctly position the packing
material.
For further information, refer to Chapter 3 - System Setup.
• Place the probes in their carrying case.
• Secure the SafeLock cart in an upright position and lock the wheels (brake).
• Ensure that the Vivid i n or Vivid q N system (and SafeLock cart if applicable) is firmly
secured while inside the vehicle.
• Secure the system with straps or as directed otherwise to prevent motion during transport.
• Prevent vibration damage by driving cautiously. Avoid unpaved roads, excessive speeds,
and erratic stops or starts.
1-8 Section 1-3 - Safety Considerations
Page 39

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-3-4 Electrical Safety
To minimize shock hazard, the equipment must be connected to a well grounded power source. The
system is equipped with a three-conductor AC power cable. This must be plugged into an approved
electrical outlet with safety grounding.
To ensure proper grounding, connect this equipment to a receptacle marked "HOSPITAL ONLY" OR
"HOSPITAL GRADE".
The power outlet used for this equipment should not be shared with other types of eq uipment. Both the
system power cable and the power connector must meet international electrical standards.
1-3-4-1 Probes
All the probes for the Vivid i n and Vivid q N ultrasound unit are designed and manufactured to provide
trouble-free, reliable service. To ensure this, correct handling of probes is important and the following
points should be noted:
• Do not drop a probe or strike it against a hard surface, as this may damage the transducer elements,
acoustic lens, or housing.
• Do not use a cracked or damaged probe. In this event, call your field service representative
immediately to obtain a replacement.
• Avoid pulling, pinching or kinking the probe cable, since a damaged cable may compromise the
electrical safety of the probe.
• To avoid the risk of a probe accidentally falling, do not allow the probe cables to become entangled,
or to be caught in the SafeLock Cart wheels.
NOTE: For detailed information on handling endocavity probes and invasive intracardiac probes, refer to the
appropriate supplementary instructions for each probe. In addition, refer to the Vivid i n/Vivid q N User
Manual for detailed probe handling instructions.
1-3-4-2 Peripherals
1-3-4-2-1 Safety and Environmental Guidelines
WARNING: Environmental Dangers
ALL DEVICES MEETING IEC60950 MUST BE KEPT OUTSIDE THE PATIENT ENVIRONMENT AS
DEFINED IN IEC60601-1-1, UNLESS THE DEVICES, ACCORDING TO IEC60601-1-1, ARE
EQUIPPED WITH:
A) ADDITIONAL EARTH PROTECTION
OR:
B) AN EXTRA ISOLATING TRANSFORMER
WARNING:
Commercial devices such as laser cameras, printers, VCRs and external monitors, usually
exceed allowable leakage current limits and, when plugged into separate AC outlets, are in
violation of patient safety standards. Suitable electrical isolation of such external AC outlets, or
the provision of extra protective earth for the device, is required in order to meet UL60601-1 and
IEC60601-1-1 standards for electrical leakage.
• Patient Vicinity UL 60601-1
Sub clause 2.12.20DV - D2 Addition
An area in which patients are normally cared for, the patient vicinity is the space with surf aces likely
to be in contact with the patient or attendant who can touch the patient. This encloses a space within
the room of 1.83 m (6 ft.) beyond the perimeter of the bed (examination table, dental chair,
treatment booth, and the like) in its intended location, and extending vertically 2.29 m (7.5 ft.) ab ove
the floor.
Chapter 1 - Introduction 1-9
Page 40

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
• Patient Environment IEC 60601-1-1
Sub clause 2.204
Such an area is an environment in which medical diagnosis, monitoring o r treatm ent is carr ied out.
It is very difficult to attach unique dimensions to the PATIENT ENVIRONMENT. In practice a
distance of 2,5 m (8.2 ft.) above the floor on which the medical personnel stand and a horizontal
distance of 1,5 m (4.9 ft.) have justified themselves as indicative of the dimensions of the Patient
Environment. The patient environment/vicinity is depicted as a dashed line in this procedure - see
the example in Figure 1-1.
Figure 1-1 Patient Safety Environment
1-10 Section 1-3 - Safety Considerations
Page 41

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-3-5 Dangerous Procedure Warnings
Warnings, such as the warnings below, precede potentially dangerous procedures throughout this
manual. Instructions contained in the warnings must be followed.
DANGEROUS VOLTAGES, CAPABLE OF CAUSING DEATH, ARE PRESENT IN THIS
EQUIPMENT. USE EXTREME CAUTION WHEN HANDLING, TESTING AND ADJUSTING.
EXPLOSION WARNING
DO NOT OPERATE THE EQUIPMENT IN AN EXPLOSIVE ATMOSPHERE.
OPERATION OF ANY ELECTRICAL EQUIPMENT IN SUCH AN ENVIRONMENT CONSTITUTES A
DEFINITE SAFETY HAZARD.
EQUIPMENT IS NOT SUITABLE FOR USE IN THE PRESENCE OF A FLAMMABLE ANAESTHETIC
MIXTURE WITH AIR OR WITH OXYGEN OR NITROUS OXIDE.
DO NOT SUBSTITUTE PARTS OR MODIFY EQUIPMENT
BECAUSE OF THE DANGER OF INTRODUCING ADDITIONAL HAZARDS, DO NOT INSTALL
SUBSTITUTE PARTS OR PERFORM ANY UNAUTHORIZED MODIFICATION OF THE
EQUIPMENT.
Chapter 1 - Introduction 1-11
Page 42

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-4 Product Labels and Icons
The Vivid i n and Vivid q N portable u ltrasound scanner comes eq uipped with product labels and icons.
These represent pertinent information regarding the operation of the unit.
1-4-1 Universal Product Labels
The following diagrams illustrate the labels found on the Vivid i n and Vivid q N ultrasound units. For an
explanation of label icons and symbols, refer to Tab l e 1- 8 on page 1-1 4.
Table 1-6 Main Label for Vivid q N
DESCRIPTION ILLUSTRATION
First version of Main Label
Table 1-7 Main Label for Vivid i n 1 of 2
DESCRIPTION ILLUSTRATION
Main Label Rev.2
1-12 Section 1-4 - Product Labels and Icons
Page 43

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Table 1-7 Main Label for Vivid i n (cont’d) 2 of 2
DESCRIPTION ILLUSTRATION
First version of Main Label
Chapter 1 - Introduction 1-13
Page 44

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-4-2 Label Descriptions
The following table shows label icons and symbols that may be found on the Vivid i n and Vivid q N
ultrasound unit and/or SafeLock Cart, and provides a description of each label’s purpose and location.
Table 1-8 Label Icons and Symbols - Description and Location 1 of 3
Label Name Description Location
Manufacturer’s name and location.
Identification and Rating
Plate
Type/Class Label Used to indicate the degree of safety or protection. Bottom panel of the adapter
Date of Manufacture.
Model and Serial numbers.
Electrical ratings (Volts and Amps)
"CAUTION" The equilateral triangle is usually used
in combination with other symbols to advise or warn
the user.
“ATTENTION - Consult accompanying documents”
is intended to alert the user to refer to the User
Manual or other instructions when complete
information cannot be provided on the label.
On the base of the unit
Various.
Various.
Device Listing/Certification
Labels
"CAUTION - Dangerous voltage" (the lightning
flash with arrowhead in equilateral triangle) is used
to indicate electric shock hazards.
Laboratory logos or labels that denote conformity
with industry safety standards, such as UL or IEC.
Indicates Equipment Type BF applied part for
medical equipment. Identifies a BF type applied
part complying with IEC 60601-1.
CE certification mark. On the base of the unit
Equipment Type CF IEC 60601-1 indicates
equipment having a floating applied part that
provides a degree of protection suitable for direct
cardiac contact.
Indicates compliance with UL safety standard UL
60601-1 Medical Electrical Equipment, Part 1
General Requirements for Safety.
Various.
On the base of the unit
Probe connectors.
On the base of the unit
Above the ECG inlet, ECG connector
and surgical probes.
On the base of the unit
1-14 Section 1-4 - Product Labels and Icons
Page 45
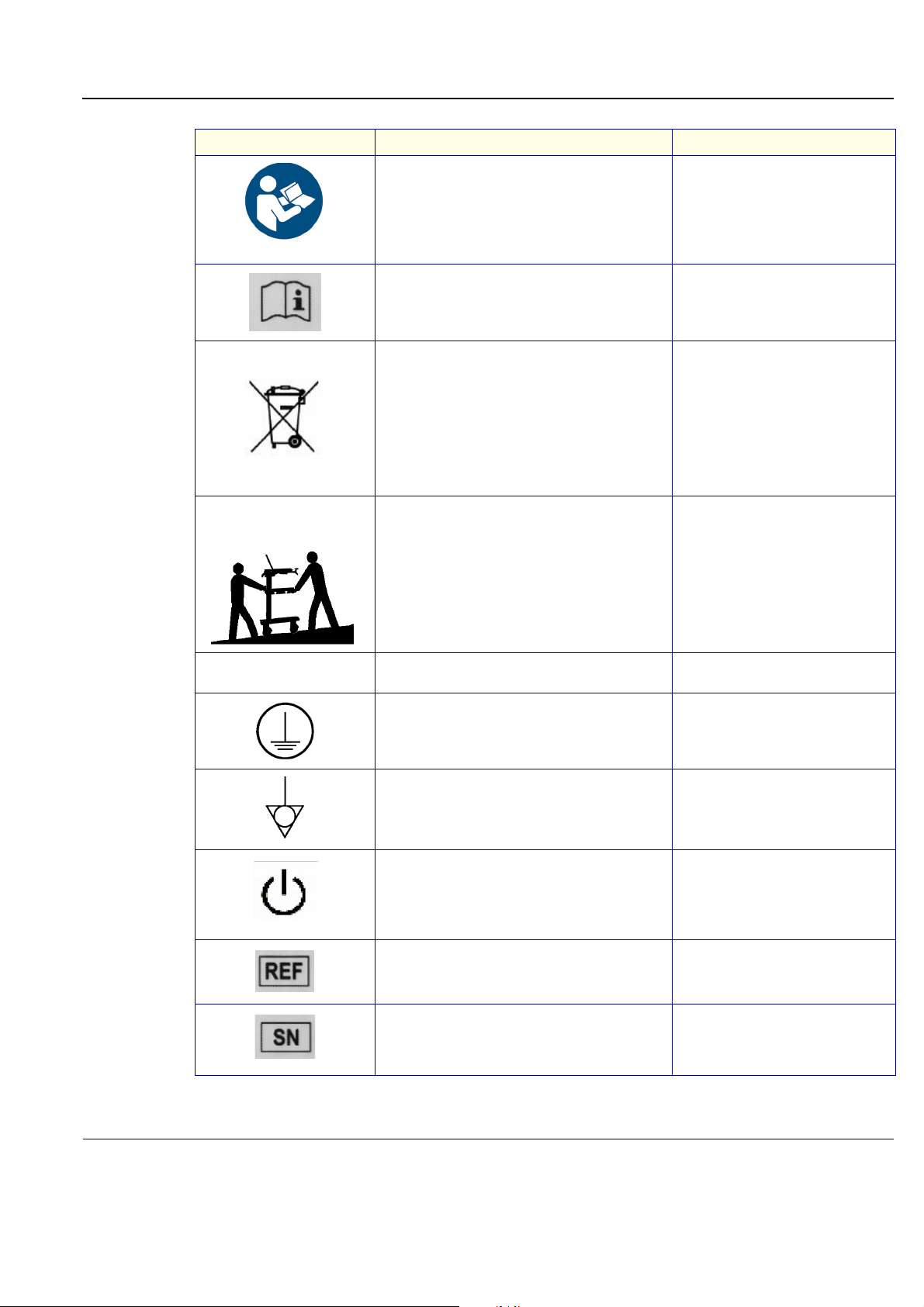
GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Table 1-8 Label Icons and Symbols - Description and Location (cont’d) 2 of 3
Label Name Description Location
Follow instructions for use.
CAUTION - This machine
weighs...Special care must
be used to avoid..."
Read and understand all instructions in the User's
Manual before attempting to use the ultrasound
unit.
Consult instructions for use. On the base of the unit
Waste Electrical and Electronic Equipment (WEEE)
Disposal
This symbol indicates that waste electrical and
electronic equipment must not be disposed of as
unsorted municipal waste and must be collected
separately.
Please contact an authorized representative of the
manufacturer for information concerning the
decommissioning of your equipment.
This precaution is intended to prevent injury that
may be caused by the weight of the machine if one
person attempts to move it considerable distances
or on an incline.
Main Label
On the base of the unit.
On the rear of the SafeLock cart.
Used in the Service and User Manual
which should be adjacent to
equipment at all times for quick
reference.
"DANGER - Risk of explosion
used in..."
The system is not designed for use with flammable
anesthetic gases.
"Protective Earth" Indicates the protective earth
(grounding) terminal.
"Equipotentiality" Indicates the terminal to be used
for connecting equipotential conductors when
interconnecting (grounding) with other equipment.
“ON” indicates the power ON position of the power
switch. “Standby” indicates the power stand by
position of the power switch.
CAUTION This Power Switch DOES NOT
ISOLATE Mains Supply.
Model number On the base of the unit
Serial number On the base of the unit
See EXPLOSION WARNING on
page 1 - 11.
Rear of the SafeLock cart.
Peripherals
Adjacent to ON/Standby Switch
Chapter 1 - Introduction 1-15
Page 46

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Table 1-8 Label Icons and Symbols - Description and Location (cont’d) 3 of 3
Label Name Description Location
Date of manufacture On the base of the unit
1-16 Section 1-4 - Product Labels and Icons
Page 47

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-4-3 Vivid i n/ Vivid q N Battery Safety
The lithium ion battery provides power when an AC power so urce is not available. L ithium ion batteries
last longer than conventional batteries and do not require replacement as often. You can expect one
hour of battery life with a single, fully-charged battery.
The lithium ion technology used in the system’s battery is significantly less hazardous to the
environment than the lithium metal technology used in some other ba tteries (such a watch batteries).
Used batteries should not be placed with common household waste products. Contact local authorities
for the location of a chemical waste collection program nearest you.
NOTE: Regulations vary for different countries. Dispose of a used battery in accord ance with local regulations.
CAUTION:
SUITABLE FOR USE WITH THE VIVID I
USE ONLY BATTERIES APPROVED BY GE MEDICAL SYSTEMS AS
n and Vivid q N portable ultrasound scanner
WARNING: The Vivid i n/ Vivid q N battery has a safety device.
DO NOT ATTEMPT TO DIS-ASSEMBLE OR ALTER THE BATTERY!
Always observe the following precautions:
• Charge batteries only when the ambient temperature is between 0
o
and discharge the batteries between -10
and 55o C (14o and 131o F)
o
and 65o C (32o and 149o F).
• Do not short-circuit the battery by directly connecting the negative terminals with metal objects.
• Do not heat the battery or discard it in a fire.
• Do not expose the battery to temperatures over 60
o
C (140o F). Keep the battery away from fire and
other heat sources.
• Do not charge the battery near a heat source, such as, a fire or heater.
• Do not leave the battery in direct sunlight.
• Do not pierce the battery with a sharp object, hit it, or step on it.
• Do not use a damaged battery.
• Do not apply solder to a battery.
• Do not connect the battery to an electrical power outlet.
WARNING: In the event that the Vivid i n/Vivid q N portable ultrasound scanner will not be used for
a long period, it is necessary to remove the battery from the system while not in use.
CAUTION: To prevent the battery bursting, igniting, or fumes from the battery causing
equipment damage, always observe the following precautions:
• Do not immerse the battery in water or allow it to get wet.
• Do not place the battery into a microwave oven or pressurized container.
• If the battery leaks or emits an odor, remove it from all possible flammable sources.
• If the battery emits an odor or heat, is deformed or discolored, or in a way appea rs abnormal during
use, recharging or storage, immediately remove it and stop using it.
• If you have any questions about the battery, consult your local GE representative.
• Storage of the battery pack:
Short term (less than 1 month): between 0
Long-term (more than 3 months):between 10
o
C (32o F) and 50o C (122o F).
o
C (50o F) and 35o C (95o F)
Note: When storing a battery pack for more than 6 months, it is necessary to charge the battery
pack at least once every 6 months in order to prevent leakage and deterioration in
performance (as a result of self-discharging).
Chapter 1 - Introduction 1-17
Page 48

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Note: When charging the battery for the first time after long-term storage, recover the battery
pack to original performance through repeating several cycles of full charging and
discharging.
NOTE:
When shipped, the Vivid i n/ Vivid q N battery is in a state of being approximately 30% charged. Before use, it is
necessary to fully charge and discharge the battery pack up to 3 times, in order to utilize Li-lon smart packs.
Important:
• For Vivid i n and Vivid q N portable ultrasound scanners supplied from the factory with a dummy
battery, when unpacking the unit, do not discard the dummy battery.
• When charging the Vivid i n or Vivid q N lithium ion battery on an external charger, since the battery
in fact forms one of the four legs of the scanner, removing it will leave the unit standing unbalanced.
For this reason, the dummy battery should be kept, so it may be inserted in position to provide
stability to the scanner while the lithium ion battery is being charged.
• In addition, use of the dummy battery is recommended during transportation or long-term storage
of the Vivid i n and Vivid q N portable ultrasound scanner.
1-18 Section 1-4 - Product Labels and Icons
Page 49

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-4-4 Vivid i n and Vivid q N External Labels
In addition to the labels described in the previous section, additional labels may be found on the
Vivid i n and Vivid q N ultrasound unit, as described in the following section.
• Rating Labels section, see below.
1-4-4-1 Rating Labels
Indicates the ultrasound unit’s basic power compliance and regulatory compliance information
For illustrations, see: Table 1-6 on page 1-12 and Table 1-7 on page 1-12
Chapter 1 - Introduction 1-19
Page 50

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-4-5 SafeLock Cart Labels
The labels shown in the example below are located on the rear side of the cart.
Figure 1-2 Vivid i n and Vivid q N SafeLock Cart Labels (Example)
1-20 Section 1-4 - Product Labels and Icons
Page 51

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-5 EMC, EMI, and ESD
1-5-1 Electromagnetic Compatibility (EMC)
Electromagnetic compatibility describes a level of performance of a device within its electromagnetic
environment. This environment consists of the device itself and its surroundings, including other
equipment, power sources and persons with which the device must interface. Inadequate compatibility
results when a susceptible device fails to perform as intended due to interference from its environment,
or when the device produces unacceptable levels of emission. This interference is often refe rred to as
radio–frequency or electromagnetic interference (RFI/EMI) and can be radiated through space or
conducted over interconnecting power or signal cables. In addition to electromagnetic energy, EMC
also includes possible effects from electrical fields, magnetic fields, electrostatic discharge and
disturbances in the electrical power supply.
The Vivid i n/ Vivid q N system and the SafeL ock Cart sho uld be oper ated at a dis tance of more
than 15 feet from any magnetic field.
1-5-2 CE Compliance
The Vivid i n and Vivid q N portable ultrasound scanner conforms to all applicable conducted and
radiated emission limits and to immunity from electrostatic discharge, radiated and conducted RF fields ,
magnetic fields and power line transient requirements.
For applicable standards refer to the Safety Chapter in the Vivid i n and Vivid q N User Manual.
NOTE: For CE Compliance, it is critical that all covers, screws, shielding, gaskets, mesh and clamps are in good
condition and installed tightly without skew or stress. Proper installation following all comments noted
in this service manual is required in order to achieve full EMC performance.
1-5-3 Electrostatic Discharge (ESD) Prevention
CAUTION: DO NOT TOUCH ANY BOARDS WITH INTEGRATED CIRCUITS PRIOR TO
TAKING THE NECESSARY ESD PRECAUTIONS:
1.ALWAYS CONNECT YOURSELF, VIA AN ARM-WRIST STRAP CONNECTED TO THE
BOTTOM COVER WHENEVER YOU OPEN THE SYSTEM FOR MAINTENANCE.
2.FOLLOW GENERAL GUIDELINES FOR HANDLING OF ELECTROSTATIC SENSITIVE
EQUIPMENT.
Chapter 1 - Introduction 1-21
Page 52

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-5-4 Standards Used
To fulfill the requirements of relevant EC directives and/or European Harmonized/International
standards, the following documents/standards have been used:
Table 1-9 Standards Used
Scope Standard/Directive
IEC 60601-1
Product safety requirements for Electrical Medical devices
Class I, Type B with BF and CF applied parts
(ICE catheter and ECG leads)
EN 60601-1
UL 60601-1
C 22.2 601-1 M90
Product safety requirements for Electrical Medical devices
- safety requirements for medical electrical systems
Product safety requirements for Electrical Medical devices
- programmable electrical medical systems
Product safety requirements for Ultrasound systems
Product EMC requirements
Group 1. Class B
Biological evaluation of Medical devices ISO 10993-1
General requirements for basic safety and essential performance
- usability
Patient privacy HIPPA
IEC 60601-1-1
EN 60601-1-1
IEC 60601-1-4
EN 60601-1-4
IEC 60601-2-37
EN 60601-2-37
IEC 60601-1-2
EN 60601-1-2
IEC60601-1-6
EN60601-1-6
NOTE: For CE Compliance, it is critical that all covers, screws, shielding, gaskets, mesh and clamps are in good
condition and installed tightly without skew or stress. Proper installation following all comments noted
in this service manual is required in order to achieve full EMC performance.
1-22 Section 1-5 - EMC, EMI, and ESD
Page 53

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-6 Lockout/Tagout (LOTO) Requirements
Follow local Lockout/Tagout (LOTO) requirements by ensuring yo u are in total control of th e AC power
plug at all times during the service process.
To apply Lockout/Tagout:
1.) Plan and prepare for shutdown.
2.) Shutdown the equipment.
3.) Isolate the equipment.
4.) Apply Lockout/Tagout Devices.
5.) Control all stored and residual energy.
6.) Verify isolation.
All potentially hazardous stored or residual energy is relieved.
NOTICE
Energy Control and Power Lockout for Vivid i n and Vivid q N.
When servicing parts of the system where there is exposure to voltage greater than 30 Volts:
1.) Turn off the breaker.
2.) Unplug the system.
3.) Maintain control of the system power plug.
4.) Wait for at least 20 seconds for capacitors to discharge as there are no test points to verify isolation.
Beware that parts inside the unit may be energized even if the power is turned off when the cord is still
plugged into the AC Outlet.
Chapter 1 - Introduction 1-23
Page 54

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-7 Returning/Shipping Probes and Repair Parts
Equipment being returned must be clean and free of blood and other in fectious substances.
GE Healthcare policy states that body fluids must be properly removed from any part or equipment prior
to shipment. GE Healthcare employees, as well as customers, are responsible for ensuring that parts/
equipment have been properly decontaminated prior to shipment. Under no circumstance should a part
or equipment with visible body fluids be taken or shipped from a clinic or site (for example, body coils
or an ultrasound probe). The purpose of the regulation is to protect employees in the transportation
industry, as well as the people who will receive or open this package.
1-24 Section 1-7 - Returning/Shipping Probes and Repair Parts
Page 55

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 1-8 Customer Assistance
1-8-1 Contact Information
If this equipment does not work as indicated in this service ma nual or in the Vivid i n and Vivid q N User
Manual, or if you require additional assistance, please contact the local distributor or appropriate
support resource, as listed below.
Prepare the following information before you call:
• System ID and/or serial number.
• Software version.
• Date and time of occurrence.
• Sequence of events leading to issue.
• Is the issue reproduceable?
• Imaging mode, probe, preset/application.
• Media brand, speed, capacity, type.
• Detailed description of any problem encountered.
• Save secondary image capture, cine loop.
• Where applicable, save the appropriate log files (by pressing <Ctrl F> [or <Alt D>]).
Remember to save the log files for each day on a separate floppy disk, labelled accordingly.
Note: Restart the application before resuming clinical scanning.
Table 1-10 Phone numbers for Customer Assistance
LOCATION PHONE NUMBER
USA
GE Medical Systems
Ultrasound Service Engineering
9900 Innovation Drive
Wauwatosa, WI 53226
Canada 1-800-668-0732
Latin America
Europe (OLC- EMEA)
GE Ultraschall Deutschland GmbH
Beethovenstraße 239
Postfach 11 05 60, D-42655 Solingen
Germany
Service: On-site
Service Parts
Application Support
Service
Application Support
OLC - EMEA
Phone: +49 (0)212 2802 - 652
+33 1 3083 1300
Fax: +49 (0) 212 2802 - 431
1-800-437-1171
1-800-558-2040
1-800-682-5327 or 1-262-524-5698
1-800-321-7937
1-262-524-5698
Online Services Ultrasound Asia
Australia
China
India
Japan
Korea
Singapore
Phone: +(61) 1-800-647-855
+(86) 800-810-8188
+(91) 1800-425-8025
+(81) 42-648-2940
+(82) 2620 13585
+(95) 6277-3444
Chapter 1 - Introduction 1-25
Page 56

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
1-8-2 System manufacturer
Table 1-11 System manufacturer
MANUFACTURER PHONE NUMBER FAX NUMBER
GE VINGMED ULTRASOUND A/S
STRANDPROMENADEN 45
P.O. BOX 141
NO-3191 HORTEN
NORWAY
+47 3302 1100 +47 3302 1350
1-26 Section 1-8 - Customer Assistance
Page 57

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Chapter 2
Site Preparations
Section 2-1 Overview
2-1-1 Purpose of Chapter 2
This chapter provides the information required to plan and prepare for the installation of a
Vivid i n or Vivid q N ultrasound unit. Included are descriptions of the electrical and facility requirements
that must be met by the purchaser. A worksheet is provided at the end of this chapter (see Figure 2-2 on
page 2-10) to help ensure that all the required network information is available, prior to installation.
Table 2-1 Contents in Chapter 2
Section Description Page Number
2-1
2-2
2-3
2-4
Overview
Console Requirements
Facility Needs
Connectivity Installation Worksheet
2-1
2-2
2-6
2-10
Chapter 2 - Site Preparations 2-1
Page 58

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 2-2 Console Requirements
2-2-1 Unit Environmental Requirements
Table 2-2 Environmental Requirements
Relative Humidity
Requirement Temperature
(non-condensing)
Air Pressure
Operational
Storage
Transport
+10 — +40
-10 — +60
-10 — +60
o
C (50 — 104oF)
o
C (14 — 140oF)
o
C (14 — 140oF)
30 — 85% 700 — 1060 hPa
30 — 95% 700 — 1060 hPa
30 — 95% 700— 1060 hPa
CAUTION: If the system has been in storage or has been transported, please see the acclimation
requirements before powering
ON and/or using the system. Refer to the Installation Warnings section
on page 3-2.
2-2-2 Cooling Requirements
The cooling requirement for the Vivid i n and Vivid q N ultrasound unit environment is
3500 BTU/hr. This figure does not include the cooling requ ired for l ights, people , or oth er equipmen t in
the room.
NOTE: Each person in the room places an additional 300 BTU/hr demand on the environmental
cooling.
2-2-3 Lighting Requirements
For system installation, updates and repairs, bright lighting is required. However, operator and patient
comfort may be optimized if the room lighting is subdued and indirect when a scan is being per formed.
Therefore, a combination lighting system (dim/bright) is recommended. Keep in mind that lighting
controls and dimmers can be a source of EMI which could degrade image quality. These controls should
be selected to minimize possible interference.
2-2-4 Time and Manpower Requirements
Site preparation takes time. Begin pre-installation checks as soon as possible to allow sufficient time to
make any required changes. If possible, begin these checks as many as six weeks before system
delivery.
CAUTION: Only one person is required to unpack the Vivid i n or Vivid q N ultrasound unit; at least
two people must be available to roll the system down the wheeling ramp. Attempts to move the
system considerable distances (or on an incline) by one person alone, could result in personal
injury, and/or damage to the system.
2-2 Section 2-2 - Console Requirements
Page 59

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-2-5 Electrical Requirements
NOTE: GE Medical Systems requires a dedicated mains power line and Ground for the proper
operation of its Ultrasound equipment. This dedicated power line shall originate at the last
distribution panel before the system.
Sites with a mains power system with defined Neutral and Live:
The dedicated line shall consist of one phase, a neutral (not shared with any other circuit), and a full
size Ground wire from the distribution panel to the Ultrasound outlet.
Sites with a mains power system without a defined Neutral:
The dedicated line shall consist of one phase (two lines), not shared with any other circuit, and a full
size Ground wire from the distribution panel to the Ultrasound outlet.
NOTE: Please note that image artifacts can occur, if at any time within the facility, the Ground from the
main facility's incoming power source to the Ultrasound unit is only a conduit.
2-2-5-1 Vivid i n/ Vivid q N Power Requirements
Electrical specifications for the Vivid i n/ Vivid q N system are as follows:
Table 2-3 Electrical Requirements
Adaptor
AC DC converter
2-2-5-2 Inrush Current
Inrush current is not a factor for consideration, due to the inrush current limiting prop erties of the power
supplies.
Table 2-4 Inrush Current
Voltage
100 V 4.5 A 9 A
240 V 2.3 A 4.5 A
2-2-5-3 Site Power Outlets
A dedicated AC power outlet must be within reach of the unit without requiring the use of extension
cords. Other outlets adequate for the external peripherals, medical and test equipment required to
support this unit must also be present and located within 1 m (3.2 ft) of the unit. Electrical installation
must meet all current local, state, and national electrical codes.
Input
Voltage
100V AC to
240V AC
Console Only Console with all Peripherals
Output
Voltage Tolerances Op. Current Frequency
20V ±10% 0.5 to 1A 50-60 Hz
Inrush Current
2-2-5-4 Mains Power Plug
The Vivid i n and Vivid q N portable ultrasound scanner are supplied with a mains power plug, as
standard. In the event that the unit arrives withou t a power plug, or with the wrong plug, conta ct your
GE dealer. When necessary, the installation engineer will supply the appropriate power plug to meet
the applicable local regulations.
Chapter 2 - Site Preparations 2-3
Page 60

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-2-5-5 Power Stability Requirements
• Voltage drop-out
Max 10 ms.
Note: Only relevant if system is operating without a battery.
• Power Transients
(All applications)
Less than 25% of nominal peak voltage for less than 1 millisecond for any type of transient, including
line frequency, synchronous, asynchronous, or aperiodic transients.
2-2-6 EMI Limitations
Ultrasound machines are susceptible to Electromagnetic Interference (EMI) from radio frequencies,
magnetic fields, and transients in the air or wiring. They also gener ate EMI. The Vivid i n and Vivid q N
ultrasound units comply with limits as stated on the EMC label. However, there is no guarantee that
interference will not occur in a particular installation.
NOTE: Possible EMI sources should be identified before the unit is installed, and should not be on the
same line as the ultrasound system. A dedicated line sh ould be used for the ultrasound system.
Electrical and electronic equipment may produce EMI unintentionally as the result of a defect. Sources
of EMI include the following:
• Medical lasers.
• Scanners.
• Cauterizing guns.
•Computers.
•Monitors.
•Fans.
• Gel warmers.
• Microwave ovens.
• Portable phones.
• Broadcast stations and mobile broadcasting machines.
2-4 Section 2-2 - Console Requirements
Page 61

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-2-6 EMI Limitations (cont’d)
The following table lists recommendations for preventing EMI:
Table 2-5 EMI Prevention/ Abatement
EMI Rule Details
Ground the unit.
Be aware of RF sources.
Replace and/or reassemble
all screws, RF gaskets,
covers and cores.
Replace broken RF gaskets.
Do not place labels where
RF gaskets touch metal.
Use GE-specified harnesses
and peripherals.
Take care with cellular
phones.
Properly address peripheral
cables.
Poor grounding is the most likely reason an ultrasound unit will have noisy images.
Check the grounding of the power cord and power outlet.
Keep the unit at least 5m (16.4 ft) away from other EMI sources. Special shielding may
be required to eliminate interference problems caused by high frequency, high powered
radio or video broadcast signals.
After you finish repairing or updating the system, replace all covers and tighten all
screws. Any cable with an external connection requires a magnet wrap at each end.
Install the shield over the front of the card cage. Loose or missing covers or RF gaskets
allow radio frequencies to interfere with the ultrasound signals.
If more than 20% or a pair of the fingers on an RF gasket are broken, replace the gasket.
Do not turn ON the unit until any loose metallic part is removed and replaced, if required.
Never place a label where RF gaskets meet the unit. Otherwise, the gap created will
permit RF leakage. In case a label has been found in such a location, move the label to
a different, appropriate location.
The interconnect cables are grounded and require ferrite beads and other shielding.
Cable length, material, and routing are all important; do not make any changes that do
not meet all specifications.
Cellular phones may transmit a 5 V/m signal that causes image artifacts.
Do not allow cables to lie across the top of the system. Loop any peripheral cable excess
length into one bundle.
2-2-7 Probe Environmental Requirements
Table 2-6 Probe Operation and Storage Temperatures
Electronics
Operation
Storage
Note: System and electronic probes are designed for storage temperatures of -20o to +50o C. When
exposed to large temperature variations, the probes should be kept at room temperature for a
minimum of 10 hours before use.
10 — 40oC (50 — 104oF)
-10 — 60oC (14 — 140oF)
Chapter 2 - Site Preparations 2-5
Page 62

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 2-3 Facility Needs
2-3-1 Purchaser Responsibilities
The work and materials required to prepare the site are the responsibility of the purchaser. To avoid
delay, complete all pre-installation work before delivery. Use the Pre-installation Check List (provided
in Table 2-7 on page 2-12) to verify that all the required steps have been completed.
Purchaser responsibilities include:
• Procuring the required materials.
• Completing the preparations prior to de livery of the ult ra so un d sys te m.
• Paying the costs of any alterations and modifications not specifically provided for in the sales
contract.
Note: All relevant preliminary electrical installations at the prepared site must be performed by
licensed electrical contractors. Other connections between electrical equipment, and
calibration and testing, must also be performed by qualified personnel. The products involved
(and the accompanying electrical installations) are h ighly sophisticated and special engineering
competence is required. All electrical work on these products must comply with the
requirements of applicable electrical codes. The purchaser of GE equipment must utilize only
qualified personnel to perform electrical servicing of the equipment.
To avoid delays during installation, the individual or team who will perform the installation should be
notified at the earliest possible date (preferably prior to installation), of the existence of any of the
following variances:
• Use of any non-listed product(s).
• Use of any customer provided product(s).
• Placement of an approved product further from the system than the interface kit allows.
The prepared site must be clean prior to delivery of the system. Carpeting is not recomme nded because
it collects dust and creates static. Potential sources of EMI should also be investigated before delivery.
Dirt, static, and EMI can negatively impact system reliability.
2-6 Section 2-3 - Facility Needs
Page 63

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-3-2 Mandatory Site Requirements
The following are mandatory site requirements. Additional (optional) recommendations, as well as a
recommended ultrasound room layout, are provided in section 2-3-3 - Site Recomme ndations (see
below).
• A dedicated single branch power outlet of adequate amperage (see Table 2-3 on page 2-3.) that
meets all local and national codes and is located less than 2.5 m (8.2 ft) from the unit’s propo sed
location. Refer to the Electrical Requirements section on page 2-3.
• A door opening of at least 76 cm (2.5 ft) in width (if using the SafeLock Cart).
• The proposed location for the unit is at least 0.2 m (0.67 ft) from the walls, to enable cooling.
• Power outlet and place for any external peripheral are within 2 m (6.5 ft.) of each other with
peripheral within 1 m of the unit to connect cables.
• Power outlets for other medical equipment and gel warmer.
• Power outlets for test equipment within 1 m (3.3 ft) of the ultrasound unit.
• Clean and protected space for storage of probes (either in their case or on a rack).
• Material to safely clean probes (perform ed usin g a pla stic container, never metal).
• In the case of a network option:
• An active network outlet in the vicinity of the ultrasound unit.
• A network cable of appropriate length (regular Pin-to-Pin network cable).
• An IT administrator who will assist in configuring the unit to work with your local network. A fixed
IP address is required. Refer to the form provided in Figure 2-2 on page 2-10 for network details
that are required.
NOTE: All relevant preliminary network outlets installations at the prepared site must be performed by
authorized contractors. The purchaser of GE equipment must utilize only qualified personnel to
perform servicing of the equipment.
2-3-3 Site Recommendations
The following are (optional) site recommendations. Mandatory site requirements are provided in the
Mandatory Site Requirements section, above.
• Door opening of 92 cm (3 ft) in width (if using the SafeLock Cart).
• Accessible circuit breaker for a dedicated power outlet.
• Sink with hot and cold running water.
• Receptacle for bio–hazardous waste, for example, used probe sheaths.
• Emergency oxygen supply.
• Storage area for linens and equipment.
• Nearby waiting room, lavatory, and dressing room.
• Dual level lighting (bright and dim).
• Lockable cabinet for software and manuals.
Chapter 2 - Site Preparations 2-7
Page 64

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-3-3-1 Recommended Ultrasound Room Layout
Figure 2-1 below shows a floor plan illustrating the recommended layout of the Ultrasound Room and
depicting the minimal room layout requirements.
Vivid i n or
Dedicated Power Outlets
Vivid q N
Dedicated Analog Telephone Line
for Connection to InSite
GE Cabinet for
Software and Manuals
36 IN.
(optional)
(92 CM)
Figure 2-1 Recommended Floor Plan 4.3m x 5.2m (14 ft x 17 ft)
Hospital Network
2-8 Section 2-3 - Facility Needs
Page 65

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-3-4 Networking Pre-Installation Requirements
2-3-4-1 Stand-alone Unit (without Network Connection)
None.
2-3-4-2 Unit Connected to Hospital’s Network
Supported networks:
•Wired LAN
• Wireless LAN
2-3-4-3 Purpose of the DICOM Network Function
DICOM services provide the operator with clinically useful features for moving images and patient
information over a hospital network. Examples of DICOM services include the transfer of images to
workstations for viewing or transferring images to remote printers. As an added benefit, transferring
images in this manner frees up the on-board moni tor and peripherals, enabling viewing to be don e while
scanning continues. With DICOM, images can be archived, stored, and retrieved faste r, easier, a nd at
a lower cost.
2-3-4-4 DICOM Option Pre-Installation Requirements
To configure the Vivid i n/ Vivid q N ultrasound unit to work with other network connections, the network
administrator must provide the required information, which should include the following:
• Vivid i n/ Vivid q N Details: DICOM network details for the Vivid i n/ Vivid q N unit, including
the host name, local port, IP address, AE title and net mask.
• Routing Information: IP addresses for the default gateway and other routers in use at
the site.
• DICOM Application Information: Details of the DICOM devices in use at the site, including the
DICOM host name, AE title and IP addresses.
Chapter 2 - Site Preparations 2-9
Page 66

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 2-4 Connectivity Installation Worksheet
Site System Information
Site:
Dept:
Vivid i n SN:
(or Vivid q N SN:)
CONTACT INFORMATION
Name
TCP/IP Settings
Scanner IP Settings
Name - AE Title:
IP Address:
Subnet Mask:
Default Gateway:
Type:
Title
Floor:
Room:
REV:
Phone
Comments:
E-Mail Address
Remote Archive Setup
(Echo Server/GEMNet Server/EchoPac PC)
Name - AE Title:
IP Address:
Subnet Mask:
Default Gateway:
Server Name:
Remote DB User Name:
Services (Destination Devices)
Device Type
1
2
3
4
5
6
7
8
9
10
11
12
Manufacturer
Name
Figure 2-2 Connectivity Installation Worksheet
2-10 Section 2-4 - Connectivity Installation Worksheet
IP Address
Port
AE Title
Page 67

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Vivid i n or Vivid q N
Host Name
AE Title
ROUTING INFORMATION
ROUTER1
ROUTER2
ROUTER3
DICOM APPLICATION INFORMATION
NAME
Store 1
Store 2
Store 3
Local Port
Destination
IP Addresses
...
...
...
MAKE/REVISION IP ADDRESSES PORTAE TITLE
IP Address
Net Mask
Default
...
...
GATEWAY IP Addresses
...
...
...
...
...
...
...
Store 4
Store 5
Store 6
Work list
Storage
Commit
MPPS
...
...
...
...
...
...
Figure 2-3 Worksheet for DICOM Network Information
Chapter 2 - Site Preparations 2-11
Page 68

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
2-4-1 Check List
Table 2-7 Vivid i n/ Vivid q N Pre-Installation Check List
Action Yes No
Schedule at least 3 hours for installation of the system.
Notify installation team of the existence of any variances from the basic installation.
Make sure system and probes have been subject to acclimation period.
Environmental cooling is sufficient.
Lighting is adjustable to adapt to varying operational conditions of the scanner.
Electrical facilities meet system requirements.
EMI precautions have been taken and all possible sources of interference have been
removed.
Mandatory site requirements have been met.
If a network is used, IP address has been set for the system and a dedicated network
outlet is available.
2-12 Section 2-4 - Connectivity Installation Worksheet
Page 69

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Chapter 3
System Setup
Section 3-1 Overview
3-1-1 Purpose of Chapter 3
This chapter provides instructions for installing the Vivid i n and Vivid q N ultrasound unit. Before
beginning the installation process, an appropriate site must be prepared, as described in Chapter 2 -
Site Preparations. Once the site has been prepared, installation can proceed as described in this
chapter.
Included in this chapter are guidelines for transporting the unit to a new site, as well as procedures tha t
describe how to receive and unpack the equipment, and (if necessary) how to file a damage or loss
claim. Instructions for checking and testing the unit, probes, and external peripherals for electrical safety
are also provided.
NOTE: Depending on the customer’s specific requirements, the Vivid i/Vivid q N portable ultrasound
scanner may have been supplied with or without the SafeLock Cart (optional). Where
applicable, the installation procedures for each scenario are clearly identified in this chapter.
Table 3-1 Contents in Chapter 3
Section Description Page Number
3-1
3-2
3-3
3-4
3-5
3-6
3-7
3-8
3-9
3-10
3-11
3-12
Overview
Installation Reminders
Receiving the Vivid i n and Vivid q N
Unpacking the Equipment
Preparing for Installation
Ensuring Protection from EMI
Completing the Hardware Installation
Mounting the Vivid i n/ Vivid q N on the SafeLock Cart (optional)
Configuration
Connectivity Setup
Storing and Transporting the Unit
Completing the Installation Paperwork
3-1
3-2
3-5
3-9
3-13
3-20
3-21
3-65
3-69
3-85
3-177
3-178
Chapter 3 - System Setup 3-1
Page 70

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-2 Installation Reminders
3-2-1 Average Installation Time
The Vivid i n/ Vivid q N installation and functional checkout will take approximately one hour; Vivid i n
or Vivid q Nconsoles with optional equipment may take slightly longer.
Once the site has been prepared, the average installation time required is shown in Table 3-2 below.
Table 3-2 Average Installation Time
Average
Description
Unpacking the scanner 20 minutes
Installing the scanner 30 minutes Time may vary, according to the required configuration
DICOM Option
(connectivity)
InSite Option 30 minutes
Installation Time
30 minutes Time may vary, according to the required configuration
Comments
3-2-2 Installation Warnings
1.) The Vivid i n/Vivid q N portable ultrasound scanner weighs only 5 Kgs (11 lbs). (This is the weight
of the scanner without any optional accessories). However since the SafeLock Cart weighs
approximately 40 kg (88 lbs), two persons are always required to unpack it.
2.) There are no operator-serviceable components. To prevent shock, do not remove any covers or
panels. Should problems or malfunctions occur, unplug the power cord. Only qualified service
personnel should carry out servicing and troubleshooting.
3-2-2-1 System Acclimation Time
Following transport, the Vivid i n/ Vivid q N system may be very cold, or hot. Allow time for the system
to acclimate before being switched ON. Acclimation requires 1 hour for each 2.5
o
temperature of the system is below 10
C or above 35oC.
CAUTION
Turning the system ON after arrival at the site - without allowing time for acclimation - may cause
system damage!
Table 3-3 Vivid i n and Vivid q N System Acclimation Time
ALLOWED TRANSPORTATION AND STORAGE TEMPERATURES OUT OF SPEC!
60 55 50 45 40 35 30 25 20 15 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40
°C
140 131 122 113 104 96 86 77 68 59 50 41 32 23 14 5 -4 -13 -22 -31 -40
°F
o
C increment, when the
864200000002468101214161820
Hrs
3-2 Section 3-2 - Installation Reminders
Page 71

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-2-3 Safety Reminders
DANGER
WARNINGWARNING
CAUTION
CAUTION
CAUTION
CAUTION
CAUTION
WHEN USING ANY TEST INSTRUMENT THAT IS CAPABLE OF OPENING THE
AC GROUND LINE (I.E., METER’S GROUND SWITCH IS OPEN), DO NOT TOUCH
THE UNIT!
Two people are required to unpack the SafeLock Cart, as it is heavy. Two people are always
required whenever a part weighing 19kg (35 lb.) or more must be lifted.
If the unit is very cold or hot, do NOT turn ON power to the unit until it has had sufficient time to
acclimate to its operating environment.
To prevent electrical shock, connect the unit to a properly grounded power outlet.
Do NOT use a three-prong to two-prong adapter, as this defeats safety grounding.
To ensure proper grounding, connect this equipment to a receptacle marked "HOSPITAL ONLY"
OR "HOSPITAL GRADE".
Do NOT wear the ESD wrist strap when you work on live circuits where more than
30 V peak is present.
Do NOT operate the unit unless all board covers and frame panels are securely in place, to
ensure optimal system performance and cooling. (When covers are removed, EMI may be
present).
CAUTION
Note: The Vivid i n/Vivid q N User Manual should be fully read and understood before operating the
ACOUSTIC OUTPUT HAZARD
ALTHOUGH THE ULTRASOUND ENERGY TRANSMITTED FROM THE VIVID I N AND VIVID Q N
PROBE IS WITHIN AIUM/NEMA STANDARDS, AVOID UNNECESSARY EXPOSURE.
ULTRASOUND ENERGY CAN PRODUCE HEAT AND MECHANICAL DAMAGE.
Important: When the SafeLock Cart is connected to the wall outlet and the main circuit breaker is in the
ON position, the AC Box Fan is operated, even if the system is turned OFF.
unit. Keep the manual near the unit for reference.
Chapter 3 - System Setup 3-3
Page 72

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-2-4 The Tilt & Shock Indicators
3-2-4-1 Overview
Unproper handeling during transportation may harm the equepment inside the package even if the
package itself is undamaged.
To make it easier to detection if the handeling during transportation has been unproper, a set of Tilt &
Shock indicators have been attached to the transportation box.
3-2-4-2 Position of the Tilt & Shock Indicators
The Tilt & Shock indicators have been attached to the right side of the transportation box as illustrated
in the figure below.
Figure 3-1 Tiltwatch and Shockwatch positions on Transportation Box
3-4 Section 3-2 - Installation Reminders
Page 73

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-3 Receiving the Vivid i n and Vivid q N
3-3-1 Examin All Packages
Examin all packages closely at time of delivery, as described in the procedure below.
Table 3-4 Examin All Packages
STEP TASK ILLUSTRATIONS
1
2 Is the Shock Indicator red colored inside the middle of the
3 Is the Tilt Indicator red colored inside the middle of the
Is dammage apparent?
• If yes; continue with the instructions in subsection
3-3-2 - Damage in Transportation.
• If no; continue with step 2.
indicator?
• If yes: The Shock Indicator has been trigged.
Make a remark on the Post Delivery Checklist about the
trigged indicator before you continue with step 2.
• If no: Continue with step 2.
indicator?
• If yes: The Tilt Indicator has been trigged.
Make a remark on the Post Delivery Checklist about the
trigged indicator and then follow the rest of the
instructions in subsection 3-3-2 - Damage in
Transportation.
• If no: Continue with the instructions in Section 3-3 -
Receiving the Vivid i n and Vivid q N.
RED COLOR
RED COLOR
WARNINGWARNING
For Vivid i n and Vivid q N portable ultrasound scanners supplied from the factory with
a dummy battery, when unpacking the unit, do not discard the dummy battery.
When charging the Vivid i n or Vivid q N lithium ion battery on an external charger, since
the battery in fact forms one of the four legs of the scanner, removing it will leave the
unit standing unbalanced. For this reason, the dummy battery should be kept, so it may
be inserted in position to provide stability to the scanner while the lithium ion battery
is being charged.
In addition, use of the dummy battery is recommended during transportation or
long-term storage of the Vivid i n and Vivid q N portable ultrasound scanner.
Chapter 3 - System Setup 3-5
Page 74

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-3-2 Damage in Transportation
Follow this procedure if damage is apparent, or if any of the Tilt & Drop Indicators show failure:
Table 3-5 Damage in Transportation
STEP TASK
1
2
3
Write “Damage In Shipment” on ALL copies of the freight or express bill BEFORE delivery is accepted or “signed
for” by a GE representative or hospital receiving agent.
Report the damage to the carrier.
• Whether noted or concealed, damage MUST be reported to the carrier immediately upon discovery, or in
any event, within 14 days after receipt, and the contents and containers held for inspection by the carrier.
• A transportation company will not pay a claim for damage if an inspection is not requested within this 14 day
period.
Report the damage on the Post Delivery Checklist.
Specify if the tilt & drop indicators show failure in the “Packing” field on the Post Delivery Checklist.
3-6 Section 3-3 - Receiving the Vivid i n and Vivid q N
Page 75

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-3-2-1 Transportation Box Label
The Transportation Box Label is located at the front of the transportation box.
Figure 3-2 Label mounted (Vivid i n label is sshown in this example)
3-3-2-2 The Vivid q N Transportation Box Label details
KEEP AIR PRESSURE
BETWEEN
70 kPa and 106 kPa
MANUFACTURER
RECYCLING.
RECYCLABLE WOOD
RELATIVE HUMIDITY
BETWEEN 30 and 95%
TOP.
UPRIGHT
TRANSPORTATION
& STORAGE
FRAGILE,
HANDLE WITH CARE
KEEP
TRANSPORTATION
TEMPERATURE
BETWEEN -10°C and
+60°C.
HANDLE WITH CARE
KEEP DRY,
PROTECT FROM MOISTURE
Figure 3-3 Vivid q N Transportation Box Label details
Chapter 3 - System Setup 3-7
Page 76

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-3-2-3 The Vivid i n Transportation Box Label details
RELATIVE HUMIDITY
BETWEEN 30 and 95%
TOP.
UPRIGHT
TRANSPORTATION
& STORAGE
FRAGILE,
HANDLE WITH CARE
KEEP TRANSPORTATION
TEMPERATURE BETWEEN
-20°C and +60°C
RECYCLING.
RECYCLABLE WOOD
HANDLE WITH CARE
KEEP DRY,
PROTECT FROM MOISTURE
Figure 3-4 Vivid i n Transportation Box Label details
3-8 Section 3-3 - Receiving the Vivid i n and Vivid q N
Page 77

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-4 Unpacking the Equipment
CAUTION
Please read this section fully before unpacking the Vivid i n and Vivid q N ultrasound unit.
Figure 3-5 Shipping Box for the Vivid i n only
Figure 3-6 To remove the Lid of the Box
Open the Lock
Break the seal
Chapter 3 - System Setup 3-9
Page 78

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-4 Unpacking the Equipment (cont’d)
Figure 3-7 Transportation box - lid removed
Table 3-6 Shipping Carton Dimensions and Weights
Description Height Width Depth
Scanner with
probes, peripherals and accessories)
a.Weight is approximate and will vary depending on supplied periphera l s
3-4-1 Examin All Packages
Examin the Transportation box closely at time of delivery, as described in the procedure that follow.
Weight
85 cm 87 cm 66cm 35 kgs. Empty
a
3-10 Section 3-4 - Unpacking the Equipment
Page 79

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-4-2 Unpacking the Wooden Transportation box
After completing a visual inspection of the Transportation box and Drop/Tilt indicators, proceed
unpacking as follows:
1.) Remove sealers and unlock two locking levers at two sides of the Box as shown in Figure 3-6 on
page 3-9
2.) Remove the packing slip (shipping consignment note) detailing the contents of the Transportation
Box, keep close to hand and be ready to mark the check list.
3.) Remove the surface packing material (paper pad, silica gel, etc.). Continue to remove additional
packing material as applicable during the following procedure steps.
4.) Carefully remove the box containing the probes.
5.) Take out the package containing the following accessories: cables (AD/DC; ECG; Network),
Isolation Box, and software CD.
6.) Verify the content against the Delivery Note.
7.) Remove each of the boxes (one, two, or more, depending on options ordered) containing the
peripherals and Vivid i n and Vivid q N.
8.) Carefully remove the Vivid i n and Vivid q N ultrasound unit from the box and place at a flat and
stable location- refer to Figure 3-8 on page 3-11.
Figure 3-8 The Vivid i n/ Vivid q N
Chapter 3 - System Setup 3-11
Page 80

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-4-3 Verifying the Transportation box Contents
After unpacking, it is important to verify that all items ordered by the customer have been received (refer
to the Delivery Note). Compare all items listed on the packing slip (shipping consignment note) with
those received and report any items that are missing, back-o rdered, or damaged, to your GE Healthcare
sales representative.
NOTICE
It is recommended to keep and store the transportation box and all other packing materials in
case the unit has to be moved to a different location in the future.
For warranty purposes, storage of the above is required for one year from date of purchase.
3-4-4 Physical Inspection
3-4-4-1 System Voltage Settings
Verify that the Vivid i n/ Vivid q N AC adapter is set to the correct voltage. The Voltage settings are
220-240V AC.
WARNINGWARNING
Setting the ultrasound components to the wrong voltage setting will most likely destroy the
equipment.
3-4-5 EMI Protection
The Vivid i n/ Vivid q N Ultrasound Unit has been designed to minim ize the e ffect s of Electro Magn etic
Interference (EMI). Many of the covers, shields, and screws are provided primarily to protect the system
from image artifacts caused by this interference. For this reason, it is imperative that all covers and
hardware are installed and secured before the unit is put into operation.
3-12 Section 3-4 - Unpacking the Equipment
Page 81

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-5 Preparing for Installation
3-5-1 Confirming Customer Order
When preparing for installation of a Vivid i n or Vivid q N system, it is important to verify that all items
ordered by the customer have been received. Compare all items listed on the packing slip (shipping
consignment note) with those received and report any items that are missing, back-ordered, or
damaged, to your GE Healthcare sales representative.
3-5-2 Verifying the Transportation Box Contents
The following sections list the contents of the shipping carton that are additional to the
Vivid i n and Vivid q N. These includ e extern al accessor ies and probes, as well as a region al language
support kit, and optional peripherals (as ordered). Ensure that all relevant components are present
before completing the installation.
NOTE: The transportation box contains an External Accessory Kit and Optional Peripherals Check
List. When checking the contents of the carton, make sure the Check List is completed. In the
event that any items are missing, contact your local GE Healthcare representative.
3-5-2-1 Probes
The transportation box will contain the probes that have been ordered with the system.
For a list of probes available for use with the Vivid i n/Vivid q N portable ultrasound scanner, refer to
Section 9-7 "Probes" on page 9-9.
Chapter 3 - System Setup 3-13
Page 82

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-3 Component Inspection
After verifying that all the required parts are included in the shipping crate, inspect the system
components using the checklist supplied below. In addition, ensure that all the labels described in
Chapter 1 - Introduction are present, accurate and in good condition, and enter the serial number printed
on the main label into the system installation details card, as descr ibed in System Installation Details on
page 3-178.
3-5-3-1 Damage Inspection Checklist
Visually inspect the contents of the shipping carton for damage. If any parts are damaged or missing,
contact an authorized GE Service Representative.
•A Damage Inspection Checklist for the Vivid i n/Vivid q N portable ultrasound scanner is provided
in Table 3-7 below.
•A Damage Inspection Checklist for the SafeLock Cart (optional) is provided in Table 3-8 on
page 3-15.
Table 3-7 Damage Inspection Checklist - Vivid i n/ Vivid q N System
b
Step Item Recommended Procedure
1
Console
2
Control Console
3
Probes
4
LCD Display
5
Fans Verify that the system’s cooling fans and peripheral fans are operating.
6
Rear Panel
7
Covers
8
Peripherals
9
AC DC System
Verify that the system is switched OFF and unplugged. Clean the console and
control panel.
Physically inspect the control console for missing or damaged items. Verify the
proper illumination of all the control panel buttons.
Check all probes for wear and tear on the lens, cable, and connector. Look for bent
or damaged pins on the connector and in the connector socket on the unit. Verify
that the EMI fingers around the probe connector socket housing are intact. Check
the probe locking mechanism and probe switch.
Clean the LCD display by gently wiping with a dry, soft, lint-free non-abrasive
folded cloth. Inspect the monitor for scratches and raster burn.
Check the rear panel connectors for bent pins, loose connections and loose or
missing hardware. Screw all the cable connectors tightly to the connector sockets
on the panel. Verify that the labeling is in good condition.
Check that all screws are tightly secured in place, that there are no dents or
scratches and that no internal parts are exposed.
Check and clean the peripherals in accordance with the manufacturer’s directions.
To prevent EMI or system overheating, dress the peripheral cables inside the
peripheral cover.
Check the AC DC unit is not damaged or cracked board and verify that the output
cable is properly secured.
10
Power Cord
Check the power cord for cuts, loose hardware, tire marks, exposed insulation, or
any deterioration. Verify continuity. Replace the power cord, as required.
3-14 Section 3-5 - Preparing for Installation
Page 83

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-3-1 Damage Inspection Checklist (cont’d)
Table 3-8 Damage Inspection Checklist - SafeLock Cart (optional)
b
Step Item Recommended Procedure
1
Probe Holders Clean the gel wells with warm water and a damp cloth to remove all traces of gel.
Peripheral
2
Power and USB
Audio Panels
3
Covers Check that all screws are in place, all chassis and internal covers are installed.
4
Peripherals
5
AC System
6
Power Cord
Check the panel connectors for bent pins, loose connections and loose or missing
hardware. Screw all the cable connectors tightly to the connector sockets on the
panel. Verify that the labeling is in good condition.
Check and clean the peripherals in accordance with the manufacturer’s directions.
To prevent EMI or system overheating, dress the peripheral cables inside the
peripheral cover.
Check the AC board connectors and the associated cabling for good connection
and proper insulation. Verify that the connections are secured.
Check the power cord for cuts, loose hardware, tire marks, exposed insulation, or
any deterioration. Verify continuity.
Tighten the clamps that secure the power cord to the unit and the outlet plug to the
cord. Replace the power cord and/or clamp, as required.
Clamp securing power cord
7
Front Castors
8
Rear Castors Check that the rear castors can roll and swivel.
Check that the front castors can swivel, and can be placed in the locked position
by pressing the foot brake lever (lower) down on each castor. Check that the upper
lever releases the SafeLock Cart wheel lock on each castor wheel.
Chapter 3 - System Setup 3-15
Page 84

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-3-2 Front and Side View of the Vivid i n/ Vivid q N Ultrasound Unit
Figure 3-9 below shows the Vivid i n/ Vivid q N ultrasound unit components that are visible from the front
and side of the ultrasound unit.
Figure 3-9 Front and Side View of the Vivid i n
1
Display Monitor: tilts up and down.
2
Speakers: Two loudspeakers for Doppler sound
3
Control Panel: Contains the alphanumeric keyboard and the buttons used to operate the
ultrasound unit.
4
Rear Handle
5 Air Vents
6 Probe Ports: Two active probe connectors (one for a pencil probe).
7
ECG Connector
8 ON/OFF Switch
9 Alphanumeric Keyboard and Operation Button
10 Release Latch
3-16 Section 3-5 - Preparing for Installation
Page 85

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-3-3 Rear View of the Vivid i n and Vivid q N Ultrasound Unit
Figure 3-10 shows a view of the Vivid i n/ Vivid q N ultrasound unit rear panel and external
peripheral/accessory connectors
Figure 3-10 View of the Vivid i n/ Vivid q N Rear Panel
1 Two interchangeable USB ports (digital printer, CD-RW and other peripherals)
2 Docking connector (currently not in use)
3 Port for DC IN (AC Adapter)
4 SVGA Output (VCR option or CRT monitor option)
5 LAN 10/100 Base-TX Ethernet network connector
6 PCMCIA port for PC card.
7 Ejection lever for PCMCIA device
Chapter 3 - System Setup 3-17
Page 86

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-3-4 Vivid i n/ Vivid q N SafeLock Cart
1
4
3
10
2
13
5
9
8
6
11
Figure 3-11 Vivid i n/ Vivid q N SafeLock Cart Components
7
12
Table 3-9 Vivid i n/ Vivid q N SafeLock Cart Components
Label Item Label Item
1
Top Shelf Assembly (with handle)
2
Probe Shelf (with probe holders)
3
LAN Isolation Box
4
Peripheral Power Outlet
5
Rear Upper Cover
6
AC Distribution Assembly (behind cover)
7
Bottom Cover - Front
10
11
12
13
8
Bottom Cover - Rear (AC Assembly Cover)
9
Rear Lower Cover
SafeLock Cart Bottom Assembly
Front Wheels (with locking lever)
Rear Wheels
AC Cable Hook
3-18 Section 3-5 - Preparing for Installation
Page 87

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-5-4 Confirming SafeLock Cart Voltage Configuration
1.) Check the safety rating plate located at the rear of the SafeLock Cart, above AC input connector.
2.) Remove the bottom cover (rear) from the SafeLock Ca rt to provide access to the jumper on the AC
unit. See Figure 3-12 below.
Figure 3-12 Jumper on AC Unit
3.) Make sure that the jumper is set to either 100 to 120 V AC or to 220 - 240 V AC, in accordance with
the voltage rating shown on the safety rating plate (step 1).
3-5-4-1 Connection and Usage of the optional Modo Cart
Please refer to the “Vivid i n Cart, User Manual – Supplement to Vivid i n User manual”,
Direction Number FL092096.
3-5-5 System Voltage Confirmation
3-5-5-1 System Voltage Settings
Verify that the Vivid i n and Vivid q N is set to the correct voltage.
The Vivid i n and Vivid q N voltage settings are found on the base (underside) of the system.
For illustrations of the label with the voltage setting, see:
• Table 1-6 "Main Label for Vivid q N" on page 1-12
• Table 1-7 "Main Label for Vivid i n" on page 1-12
WARNINGWARNING
CONNECTING THE VIVID I n/ Vivid q N TO THE WRONG VOLTAGE LEVEL WILL MOST
LIKELY DESTROY THE SCANNER.
Chapter 3 - System Setup 3-19
Page 88

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-6 Ensuring Protection from EMI
The Vivid i n/ Vivid q N unit has been designe d to minimize the effects of Electro-Mag netic Interference
(EMI). Many of the covers, shields, and screws are provided primarily to protect the system from image
artifacts caused by this interference. For this reason, it is imperative that all covers and hardware are
installed and secured before the unit is put into operation.
Ensure that the system is protected from electromagnetic interference (EMI), as follows:
• Operate the system at least 15 feet away from equipment that emits strong electromagnetic
radiation.
• Operate the system in an area enclosed by walls, floors and ceilings comprised of wood, plaster or
concrete, which help prevent EMI.
• Shield the system when operating it in the vicinity of radio broadcast equipment, if necessary.
• Do not operate mobile phones or other EMI emitting devices in the ultrasound room.
• Verify that all EMI rules listed in the following table are followe d :
Note: The Vivid i n/ Vivid q N
environmentally qualified facilities, in terms of the prevention of radio wave interference.
Operation of the ultrasound unit
interference to radios and television sets situated near the medical equipment.
Table 3-10 EMI Prevention/ Abatement
EMI Rule Details
Ground the unit.
Be aware of RF sources.
Replace and/or reassemble
all screws, RF gaskets,
covers and cores.
Replace broken RF gaskets.
Do not place labels where
RF gaskets touch metal.
ultrasound unit is approved for use in hospitals, clinics and other
in an inappropriate environment can cause electronic
Poor grounding is the most likely reason an ultrasound unit will have noisy images. Check
the grounding of the power cord and power outlet.
Keep the unit at least 5m (16.4 ft) away from other EMI sources. Special shielding may be
required to eliminate interference problems caused by high frequency, high powered radio
or video broadcast signals.
After you finish repairing or updating the system, replace all covers and tighten all screws.
Any cable with an external connection requires a magnet wrap at each end. Install the shield
over the front of the card cage. Loose or missing covers or RF gaskets allow radio
frequencies to interfere with the ultrasound signals.
If more than 20% or a pair of the fingers on an RF gasket are broken, replace the gasket.
Do not turn on the unit until any loose metallic part is removed and replaced if needed.
Never place a label where RF gaskets meet the unit. Otherwise, the gap created will permit
RF leakage. In case a label has been found in such a location, move the label to a different
appropriate location.
Use GE specified harnesses
and peripherals.
Take care with cellular
phones.
Properly address peripheral
cables.
The interconnect cables are grounded and require ferrite beads and other shielding. Cable
length, material, and routing are all important; do not make any changes that do not meet
all specifications.
Cellular phones may transmit a 5 V/m signal that causes image artifacts.
Do not allow cables to lie across the top of the card cage or hang out of the peripheral bays.
Loop any peripheral cable excess length inside the peripheral bays or hang on the hooks
provided below the console. Attach the monitor cables to the frame.
3-20 Section 3-6 - Ensuring Protection from EMI
Page 89

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
Section 3-7 Completing the Hardware Installation
The hardware installation procedures described in this section provide instructions and information for
the possible scenarios, as follows:
• Vivid i n/ Vivid q N scanner but no SafeLock Cart - connections direct to the system
• Vivid i n/ Vivid q N scanner with SafeLock Cart
• Vivid i n/ Vivid q N scanner but no SafeLock Cart - connections via USB hub
3-7-1 Connecting Printers
3-7-1-1 Printer Connection and Configuration - Overview
NOTE: Before connecting a printer to the Vivid i n/ Vivid q N scanner, refer to the Safety and Environme ntal
Guidelines on page 1-9.
This section provides information common to all printers approved for use with the Vivid i n/ Vivid q N
ultrasound scanner, as follows:
• Printer Categories and Outline of Procedure Instructions, below.
• Configuring Hot Keys to Activate Printing Direct from the Control Panel on page 3-22.
• Configuring Printing Orientation (Portrait or Landscape) and Paper Size on page 3-23.
3-7-1-1-1 Printer Categories and Outline of Procedure Instructions
NOTE: The printers approved for use with the Vivid i n / Vivid q N system ar e categorize d in Table 3-11 below.
Refer to the appropriate instructions for connecting a specific printer, as applicable.
Table 3-11 Printer Categories and Connection Procedures
Printer Category Connection Procedure
Report Printer (Color)
Report Printer (Color) with Network Capabilities
Video Printer (B&W)
Video Printer (Color)
• HP470 Color Printer on page 3-26
• HP Officejet Pro 8000 Color Printer on page 3-28
• Sony UP-D897 B/W Video Printer on page 3-29
• Sony UP-D25MD Color Video Printer on page 3-33
• Sony UP-D23MD Color Video Printer
Refer to:
Sony UP-D25MD Color Video Printer on page 3-33
Chapter 3 - System Setup 3-21
Page 90

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-1-2 Configuring Hot Keys to Activate Printing Direct from the Control Panel
1.) Press Config (or F2).
2.) Select Connectivity (in the lower part of window).
3.) Select the Additional Outputs tab.
Figure 3-13 Configuring Control Panel Hot Keys for Printing
4.) From the Button drop-down menu (see Figure 3-13), select to configure either the Print button or
Alt+Print to activate a variety of outputs.
5.) Use the left or right arrows below to add any of the available options (listed on the left) as Outputs
(listed on right).
6.) From the listed Outputs, select (highlight) the required output, right-click and select Properties to
configure the output device accordingly.
Printing may now be activated direct from the Vivid i n/ Vivid q N Control Panel, using the hot key
you have configured for this purpose.
7.) Proceed to perform the B/W and Color Printer Test on page 4-29.
3-22 Section 3-7 - Completing the Hardware Installation
Page 91

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-1-3 Configuring Printing Orientation (Portrait or Landscape) and Paper Size
To change the printing orientation and printing paper size, configure the printer as described below.
To configure printers for portrait/landscape printing, the change must be performed at the Windows
operating system level. The user first accesses the system at the Application s level from the Additional
Outputs screen. By selecting the Advanced option under Selected Devices, the user accesses the
Printer Properties dialog box and proceeds with the configuration procedur e as explained below.
An additional procedure is required to configure the Sony UP-D897 Printer. This is described in
Configuring the Sony UP-D897 Printer on page 3-30.
1.) On the system console, press Config and select Connectivity.
2.) From the Additional Outputs screen under Selected Devices, select Advanced...
Figure 3-14 Advanced option at Windows level
Selected Devices
Select Advanced
Chapter 3 - System Setup 3-23
Page 92

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3.) Click Configure in the Printer Properties dialog to enter Print Setup.
Figure 3-15 Printer Properties Dialog Box
4.) In the Orientation area, select Portrait or Landscape as needed.
Figure 3-16 Printer Setup
5.) From the Paper Size drop-down list, select the required paper size.
3-24 Section 3-7 - Completing the Hardware Installation
Page 93

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
6.) Click Properties. The printer properties screen opens - Figure 3-17.
Orientation:
From scroll-down list,
select Portrait
Figure 3-17 Printing Properties - Selecting Orientation
7.) Verify that the selected orientation and paper size are correct.
8.) Click OK twice and then once more to return to the Advanced Outputs tab.
Chapter 3 - System Setup 3-25
Page 94

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-2 HP470 Color Printer
NOTICE
NOTICE
IMPORTANT Do not connect the DeskJet Color Printer to the Vivid i n/ Vivid q N system while
scanning is in progress!
IMPORTANT During Stand-by mode, it is NOT recommended to introduce or remove USB
devices; this may cause the system to lock-up during the boot-up procedure.
Figure 3-18 DeskJet Color Printer
The DeskJet Color Printer connection in the different scenarios is explaine d below.
• Connection via LAN
There are a few DeskJet Color Printers that can be connected to the system via LAN - r efer to Add
Printer on page 3-80. The DeskJet Color Printer can be connected to an external, non-isolated
power source.
WARNING Do not attempt to use a different type of DeskJet Color Printer (brand or model) other
than the DeskJet Color Printer provided by GE Medical Systems. The ultrasound system is an
extremely sensitive and complex medical system. Any unauthorized peripherals may cause
system failure or damage!
3-26 Section 3-7 - Completing the Hardware Installation
Page 95

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
• No SafeLock Cart - connection directly to the Vivid i n/ Vivid q N system
This only applies to HP 460 models which are supplied p re-installe d with the app ropr iate softwa re
drivers. The DeskJet Color Printer can be connected to either of the USB ports (upper or lower) on
the rear connectors panel.
When connecting directly to the system it is necessary to use an additional power source. As a
safety precaution, this must be done via an isolation transformer.
The DeskJet Color Printer can be connected once the system is powered ON, or after shutdown.
WARNING Do not attempt to use a different type of DeskJet Color Printer (brand or model) other
than the DeskJet Color Printer provided by GE Medical Systems. The ultrasound system is an
extremely sensitive and complex medical system. Any unauthorized peripherals may cause
system failure or damage!
• No SafeLock Cart - connection via USB hub
As stated above. Safety considerations must be taken into account. The DeskJet Color
Printer must be powered via an isolation transformer. Either USB port may be used to
communicate with the device.
• Vivid i n/ Vivid q N mounted on SafeLock Cart
The DeskJet Color Printer may be connected to any of the USB outlets provided on the SafeLock
Cart. One must use the additional power source to activate the DeskJet Color Printer. However, this
must be powered from the SafeLock Cart itself, via one of the isolated peripheral power outlets
provided on the cart.
Chapter 3 - System Setup 3-27
Page 96

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-3 HP Officejet Pro 8000 Color Printer
NOTE: The HP8000 Color Printer is a Non-Medical device.
NOTE: The HP8000 Color Printer can be connected to the Vivid i n/ Vivid q N LAN only.
When using the USB connection, the printer should be connected through an isolated transformer,
since the Vivid i n/ Vivid q NUSB is not isolated.
NOTE: When powering the HP8000 Color Printer from the SafeLock Cart, the printer may be used via a USB
or LAN connection.
Figure 3-19 HP Officejet Pro 8000 Color Printer
3-7-1-3-1 Connecting the HP Officejet Pro 8000 to the Vivid i n and Vivid q N Scanner Mounted on the SafeLock Cart
NOTE: Follow the installation procedure for mounting the Vivid i/q system on the SafeLock cart, as
described in Mounting the Vivid i n/ Vivid q N on the SafeLock Cart (optional) on page 3-65.
NOTE: When using the Vivid i/q system mounted on the SafeLockcart, the HP Officejet Pro 8000
printer must be connected to the AC outlet at the rear of the SafeLock cart - see Figure 3-20.
1.) Connect the printer to the AC power outlet located at the rear of the SafeLock cart.
2.) Plug the SafeLock cart into the mains power supply.
3.) Using the USB communications cable, connect the HP Officejet Pro 8000 printer to the Vivid i/q
system.
USB Cable
AC Power Cable
Figure 3-20 AC Power Cable and USB Cable Connected to SafeLock Cart
4.) Proceed to perform the following functionality tests:
- SafeLock Cart - Groundin g Co ntinu i ty on page 10-2 3
- SafeLock Cart - Chassis Current Leakage Test on page 10-25
- Color Printer Test on page 4-29
3-28 Section 3-7 - Completing the Hardware Installation
Page 97

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-4 Sony UP-D897 B/W Video Printer
NOTICE
IMPORTANT During Stand-by mode, it is NOT recommended to introduce or remove USB
devices; this may cause the system to lock-up during the boot-up procedure.
Figure 3-21 Black and White Printer
The B/W Printer connection in the different scenarios is explained below.
NOTE: After physical connection of the printer to the Vivid i n/ Vivid q Nsystem, to configure the appropriate hot
keys to activate printing direct from the control panel, follow the instructions in Configuring Hot Keys to
Activate Printing Direct from the Control Panel on page 3-22.
• No SafeLock Cart - connection directly to the Vivid i n/ Vivid q N system
The B/W Printer can be connected to either of the USB ports (upper or lower) on the rear connectors
panel.
When connecting the B/W Printer directly to the system, it is necessary to use an additional power
source. As a safety precaution, this must be done via an isolation transformer.
The B/W Printer can be connected once the system is powered ON, or after shutdown. All software
drivers for the B/W Printer are pre-configured and installed designated to be used with the specific
B/W Printer supplied by GE Medical Systems.
WARNING Do not attempt to use a different type of B/W Printer (brand or model) other tha n t he
B/W Printer provided by GE Medical Systems. The ultrasound system is an extremely sensitive
and complex medical system. Any unauthorized peripherals may cause system failu r e or
damage!
• No SafeLock Cart - connection via USB hub
As stated above. Safety considerations must be taken into account. The B/W Printer must be
powered via an isolation transformer. Either USB port may be used to communicate with the
device.
• Vivid i n/ Vivid q N mounted on SafeLock Cart
The B/W Printer may be connected to any of the USB outlets provided on the SafeLock Cart. You
must use the additional power source to activate the B/W Printer. However, this must be powered
from the SafeLock Cart itself, via one of the isolated peripheral power outlets provided on the cart.
NOTE: Once the printer is connected, perform the following functionality check:
• B/W and Color Printer Test on page 4-29 should be performed
Chapter 3 - System Setup 3-29
Page 98

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
3-7-1-4-1 Configuring the Sony UP-D897 Printer
To configure the Sony UP-D897 Printer, the following steps are required:
1.) Click the Tcpip tab on the Connectivity screen (see Figure 3-14 on page 3-23).
2.) Select Advanced Settings - see Figure 3-22.
Click Advanced
Settings
Figure 3-22 Tcpip screen
3.) The Network Connection screen opens (Figure 3-23). Click the "Up" button to open the Windows
Control Panel.
Figure 3-23 Network Connections showing highlighted "Up One Level" button
4.) Navigate to Printers and Faxes and double-click Sony UP-D897 to open the Sony UP-D897
Printer Configuration dialog.
3-30 Section 3-7 - Completing the Hardware Installation
Page 99

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
5.) From the menu, select Printer > Properties.
Figure 3-24 Sony UP-D897 printer configuration dialog box
6.) The Sony UP-D897 Properties screen opens. Select Printing Preferences...,
Figure 3-25 Sony UP-D897 Properties Screen
Chapter 3 - System Setup 3-31
Page 100

GE HEALTHCARE
DIRECTION FQ091013, REVISION 1 VIVID I N AND VIVID Q N SERVICE MANUAL
7.) From the Printing Preferences dialog, under Orientation:
A.) Under Paper, click the scroll-down arrow and select the required paper size.
B.) Under Orientation, choose th e required printing orientation.
Figure 3-26 Printing Preferences screen
8.) Click OK and return to the Connectivity screen.
3-32 Section 3-7 - Completing the Hardware Installation
 Loading...
Loading...