Page 1

G Cn po eiaee o
TM
Expressway to Discovery
G Cn po eiaee o
TM
Expressway to Discovery
Lenti-Pac™ FIV Expression Packaging Kit
for optimized production of recombinant lentivirus
Cat. No. FPK-LvTR-20 (20 transfections)
Cat. No. FPK-LvTR-40 (40 transfections)
User Manual Version V
GeneCopoeia, Inc.
9620 Medical Center Drive, #101
Rockville, MD 20850
USA
301-762-0888
866-360-9531
inquiry@genecopoeia.com
www.genecopoeia.com
© 2009 GeneCopoeia, Inc.
Page 2

Lenti-Pac™ FIV Expression Packaging Kit User Manual
USER MANUAL
Lenti-Pac™ FIV Expression Packaging Kit
I. Introduction
II. Kit Contents and Storage
III. Additional Materials Required or Recommended
IV. Getting Started
V. Lentivirus Production
VI. Lentivirus Titer Estimation by Transduction
VII. Transduction of Target Cells with Lentiviruses
VIII. Limited Use License and Warranty
I. Introduction
GeneCopoeia has multiple sets of over 40,000 human and mouse lentiviral ORF expression clones as well as
multiple sets of expression vector-based small hairpin RNAi (shRNA) clones against genome-wide target genes
from human, mouse, rat, and other species in FIV-based lentiviral vector systems. In addition, GeneCopoeia offers
precursor microRNA (miRNA) expression clones for all known human, mouse and rat miRNAs in a FIV-based
lentiviral vector system. The FIV- (feline immunodeficiency virus) based vectors are considered biologically safe and
have been shown to be as effective as HIV based vectors at transduction of genes into a wide variety of dividing and
non-dividing mammalian cells, both in vitro and in vivo. The lentiviral expression vectors can integrate into the
genome of the target cells, resulting in the stable expression of transgenes. The GeneCopoeia second generation
FIV-based lentivector systems meet Biosafety Level 2 (BSL-2) requirements based on the criteria published by the
Centers for Disease Control.
The GeneCopoeia Lenti-Pac™ FIV Expression Packaging System includes an optimized lentiviral packaging plasmid
mix, an eGFP positive control plasmid, a new transfection reagent, EndoFectin™ optimized for virus production and
TiterBoost™ reagent that further increases the titers 5-10 fold. When combined with GeneCopoeia FIV-based
lentiviral constructs the results are high titers and robust expression levels. The Lenti-Pac FIV Expression Packaging
System safely ensures efficient expression of recombinant transcripts in mammalian cells.
Advantages of OmicsLink™ Lentiviral ORF or shRNA Expression Clones and miExpress™ Precursor miRNA
Expression Clones:
High efficiency of gene delivery to virtually all cell types and whole model organisms
High expression levels of delivered genes (ORF expression clones)
High knockdown efficiency against target mRNA transcripts (shRNA clones)
High expression translation suppression of target genes and/or mRNA cleavage/degradation (miRNA clones)
Self-inactivation and no unwanted viral replication
2
Page 3
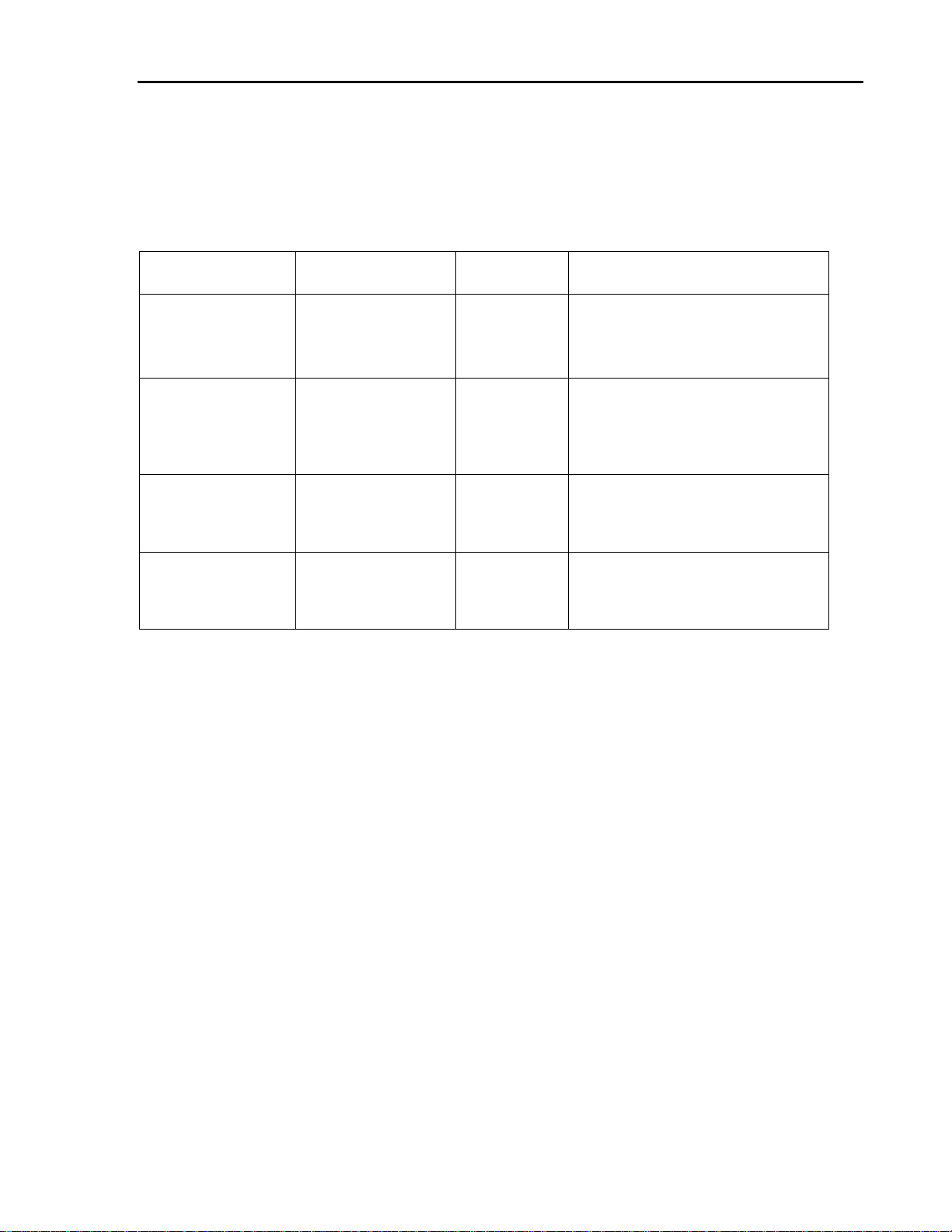
Lenti-Pac™ FIV Expression Packaging Kit User Manual
Contents
Quantity
Shipping
temperature
Storage temperature/ conditions
FIV packaging mix
100 μl (0.5 μg/μl)
200 μl (0.5 μg/μl)
Ambient
4–8°C (Stable for at least 6 months)
Alternatively, the packaging mix can
also be stored at -80 °C in aliquots.
Avoid repeated freezing/ thawing.
eGFP positive control
plasmid
25 μl (0.06 μg/μl)
25 μl (0.06 μg/μl)
Ambient
4–8°C (Stable for at least 6 months)
Alternatively, the packaging mix can
also be stored at -80 °C in aliquots.
Avoid repeated freezing/ thawing.
EndoFectin Lenti
transfection reagent
500 μl
1ml
Ambient
4–8°C (Stable for at least 12 months)
TiterBoost Viral Titer
Reagent (500x)
500 μl
1ml
Ambient
4–8°C (Stable for at least 12 months)
II. Contents and Shipping/ Storage
Contents and storage recommendations for the Lenti-Pac FIV Expression Packaging Kits
(Cat. Nos. FPK-LvTR-20 and FPK-LvTR-40) are provided in the following table.
III. Additional Materials Required or Recommended
1. GeneCopoeia GCI-L3 chemically competent cells (GeneCopoeia Cat No. STK300-10). Alternatively, Stbl3™
chemically competent cells (Invitrogen Cat No. C7373-03) may be used.
2. GeneCopoeia 293Ta Lentiviral packaging cell line (GeneCopoeia Cat No. Clv-PK-01). Alternatively, the HEK
293T/17 cell line (ATCC Cat No. CRL-11268) can also be used.
3. H1299 cell line (ATCC Cat No. CRL-5803), or HT-1080 cell line (ATCC Cat No. CCL-121) for lentivirus titer
estimation. H1299 cells are preferred over HT-1080 cells for lentivirus titration.
4. DMEM with glucose, L-glutamine and sodium pyruvate (Mediatech Cat No. 10-013-CV)
5. Fetal bovine serum (Thermo Scientific Cat No. SH300700.02)
6. Opti-MEM® I Reduced-Serum Medium (Invitrogen Cat No. 31985-062/31985-070).
7. Polybrene® (Sigma-Aldrich Cat No. H9268): 10 mg/ml solution dissolved in 150 mM NaCl and sterile-filtered.
Store usable aliquots at -20oC. Working stock can be stored at 4oC for up to two months.
8. Crystal Violet (Sigma-Aldrich Cat No. C3886): 0.5% (W/V) solution dissolved in 25% methanol.
9. Penicillin-Streptomycin for mammalian cell culture (Sigma-Aldrich Cat No. P4333)
10. Antibiotics for selecting stably transduced cells: puromycin (Invivogen Cat No. ant-pr-1/ant-pr-5), hygromycin B
(Invivogen Cat No. ant-hm-1/ant-hm-5), Neomycin (G-418) (Invivogen Cat No. ant-gn-1/ant-gn-5)
11. BD Falcon® 5-ml or 14-ml Tubes (BD Falcon Cat No. 352053/352059).
3
Page 4

Lenti-Pac™ FIV Expression Packaging Kit User Manual
IV. Getting Started
Upon receipt of a new lentiviral expression plasmid, it is recommended to transform the plasmid into GeneCopoeia
GCI-L3 Chemically Competent E. coli Cells (GeneCopoeia Cat No. STK300-10), or any Stbl3™-equivalent
competent cell strain, and to prepare plasmid DNA with a proven plasmid DNA purification method. GeneCopoeia
lentiviral ORF expression, shRNA or miRNA constructs contain the ampicillin resistance gene.
Quality of plasmid
It is critical to use plasmid of the highest quality. Determine the DNA concentration by reading the absorption at 260
nm. DNA purity is measured by using the 260 nm / 280 nm ratio (the ratio should be in the range of 1.8 to 2.0).
Check the integrity of the plasmid by agarose gel electrophoresis.
Condition of cells
Always use healthy cells that are well maintained and passaged regularly. Make sure the culture is free from
bacteria, fungi, or Mycoplasma contamination. If the cells are from a recent liquid nitrogen stock, passage the cells
at least 2 times before transfection.
V. Lentivirus Production
The GeneCopoeia FIV-Based Lentiviral Expression System is a modified version of the second generation selfinactivating (SIN) lentiviral vector system which incorporates enhanced bio-safety features and is optimized for
production of high viral titers. In this system, recombinant lentiviral particles are generated by co-transfecting an
FIV-based lentiviral ORF expression plasmid together with the GeneCopoeia Lenti-Pac FIV Expression Packaging
Kit into GeneCopoeia 293Ta lentiviral packaging cells (GeneCopoeia Cat No. CLv-PK-01).
The lentiviral ORF/shRNA/miRNA expression plasmid (lentiviral transfer vector) contains the elements required for
packaging, transduction and stable integration of the viral expression construct into genomic DNA leading to
expression of the ORF or shRNA hairpin. However, it lacks the elements essential for transcription and packaging of
an RNA copy of the ORF/shRNA/miRNA construct into recombinant pseudoviral particles. These elements are
provided by the Lenti-Pac FIV packaging mix, an optimized mixture of plasmids that express the structural,
regulatory, and replication genes required to produce lentivirus. See figure 1 below for schematics of this process.
The lentivirus generated with this system is pseudotyped with vesicular stomatitis virus-G protein (VSV-G) which
exhibits wide cell tropism and generates high titers.
In addition to Lenti-Pac FIV packaging mix, this kit also includes a positive control lentiviral transfer vector that
expresses the eGFP protein, EndoFectin Lenti Reagent (Cat No. EFL1001-01), and TiterBoost reagent. The
EndoFectin Lenti transfection reagent is optimized for transferring plasmids into packaging cells. It guarantees
higher transfection efficiency and lower cell toxicity compared to other commercially available transfection reagents.
The unique TiterBoost reagent from GeneCopoeia enhances the production of lentiviral particles.
4
Page 5

Lenti-Pac™ FIV Expression Packaging Kit User Manual
Figure 1. Schematics of Lentivirus production and infection of target cells
The following procedure provides optimized steps for lentivirus production in 293Ta packaging cells. The yield of
recombinant lentiviral particles typically produced under these optimized conditions is 10 ml of 1–3 x 10
units (ifu) per ml of un-concentrated supernatant from one 10-cm culture dish for eGFP or mCherry positive controls
when measured by transduction of HT-1080 or H1299 cells. This amount of pseudoviral particles is generally
sufficient to infect 5–10x10
as the size of insert increases. Actual lentivirus titers for your gene of interest will vary accordingly.
Caution: Following this protocol results in the production of pseudoviral particles capable of infecting mammalian
cells. The recommended guidelines for working with BSL-2 safety class must be adhered to.
1. Plate packaging cells
6
target cells at a MOI (multiplicity of infection) equal to 1. The titers of lentivirus decrease
6
infection
Two days before transfection, plate 1.3–1.5 x 106 of the GeneCopoeia 293Ta lentiviral packaging cells or
comparable cells in a 10-cm dish in 10 ml of D-MEM supplemented with 10% heat-inactivated fetal bovine
serum so that the cells are 70–80% confluent at the moment of transfection. Incubate the cells at 37°C with 5%
CO2.
Note: Plating the packaging cells 2 days prior to transfection significantly increases the titer of lentivirus.
Use heat-inactivated fetal bovine serum for lentivirus production. Heat-inactivated serum can be purchased
from other vendors or prepared by incubating thawed serum for 30 minutes at 56°C with gentle shaking.
2. Prepare DNA/EndoFectin Lenti complex
In a sterile polypropylene tube, dilute 2.5 µg of the lentiviral ORF/shRNA/miRNA expression plasmid and 5.0 µl
(0.5 µg/µl) of Lenti-Pac FIV mix into 200 µl of Opti-MEM® I (Invitrogen). In a separate tube, dilute 15 µl of
EndoFectin Lenti into 200 µl of Opti-MEM I. Add diluted EndoFectin Lenti reagent drop-wise to the DNA solution
while gently vortexing the DNA-containing tube. Do not reverse the addition sequence. Use round-bottom
5
Page 6

Lenti-Pac™ FIV Expression Packaging Kit User Manual
polypropylene tubes such as Falcon® 5-ml or 14-ml tubes (BD) for larger volumes. Incubate the mixture for 10–
25 minutes at room temperature to allow the DNA-EndoFectin complex to form.
Note: The DNA-EndoFectin complex must be formed in the absence of proteins even though the complex is
able to transfect cells in the presence of proteins such as 10% serum. Opti-MEM I is recommended for diluting
both DNA and EndoFectin Lenti reagent. Serum-free D-MEM can be used in place of Opti-MEM I but the
transfection efficiency will be compromised. The ratio of 3.0 µl of EndoFectin Lenti per 1 µg of plasmid has been
found to be optimal. Increasing the ratio does not further improve transfection efficiency.
3. Transfect packaging cells
Add the DNA-EndoFectin Lenti complex directly to each dish and gently swirl the dish to distribute the complex.
Incubate the cells in a CO2 incubator at 37°C overnight (8–14 hours). Replace the overnight culture medium
with fresh D-MEM medium supplemented with 2–5% heat-inactivated fetal bovine serum and penicillinstreptomycin. Add 1/500 volume of the TiterBoost reagent to the culture medium and continue incubation
in the CO2 incubator at 37°C.
Note:
a. Replace the culture medium that contains the DNA-EndoFectin Lenti complex within 16 hours post-
transfection.
b. TiterBoost reagent at working concentration (1×) typically boosts the titer of lentivirus products 5-10 fold.
This reagent is readily removed during commonly used lentivirus concentration/purification procedures
such as ultracentrifugation, ultrafiltration and other chromatographic methods. If crude lentiviral particles
are used directly to transduce target cells, the volume of lentiviral particles should not exceed 1/10 of that
of culture medium; otherwise some adverse effects may occur. TiterBoost at 1/20× or lower concentrations
has negligible effects on commonly used mammalian cell lines. It's advised to test the effects of TiterBoost
on your target cells beforehand if large volumes of crude lentiviral particles are used.
4. Harvest lentivirus
Collect the pseudovirus-containing culture medium in sterile capped tubes 48 hours post transfection and
centrifuge the tubes at 500 x g for 10 minutes to get rid of cell debris. Following centrifugation, filter the
supernatant through 0.45 µm polyethersulfone (PES) low protein-binding filters.
Note:
a. Peak virus production is normally achieved 24–48 hours post transfection. Alternatively, lentivirus-
containing medium may be collected multiple times at 36, 48 and 60 hours post-transfection. Lentiviral
containing supernatants should be replaced with fresh DMEM supplemented with 2–5% heat-inactivated
fetal bovine serum, penicillin-streptomycin and TiterBoost reagent.
b. Do not use nitrocellulose filters as nitrocellulose is known to bind lentivirus and reduce titers.
The supernatant containing lentiviral particles can be used directly to determine the titer and to transduce target
cells in vitro as long as the target cells can survive in conditioned medium. Lentiviral stocks should be aliquoted and
stored at -80°C. Expect significant loss of viral titer with each freeze/thaw cycle.
VI. Lentivirus Titer Estimation by Transduction
At this point it is recommended that the pseudoviral stock is titered to ensure it is viable and to test what fraction of
target cells can be transduced. This enables the number of copies of viral construct per target cell to be controlled.
There are several methods to determine the titer of pseudovirus stock. The procedure below is based on the
transduction of H1299 or HT-1080 cells. Different cells can also be used but titers may vary up to several orders of
magnitude.
6
Page 7

Lenti-Pac™ FIV Expression Packaging Kit User Manual
Day 1: Plate H1299 or HT-1080 cells
1. Plate ~5 x 104 of the H1299 or HT-1080 cells per well in a 24-well plate 24 hours prior to viral infection. Use 0.5 ml
of DMEM supplemented with 10% heat-inactivated fetal bovine serum and penicillin-streptomycin for each well.
Incubate the cells at 37°C with 5% CO2 overnight.
Day 2: Transduce H1299 or HT-1080 cells
2. Dilute Polybrene to 10 µg/ml with DMEM containing 5% heat-inactivated fetal bovine serum and penicillin-
streptomycin.
3. Remove old culture medium from each well. Add 0.25 ml of Polybrene (from Step 2). The final concentration of
Polybrene will be 5 µg/ml after adding diluted lentivirus. For each pseudoviral stock, use five wells.
3a. For lentivirus containing a fluorescent marker and to be analyzed with flow cytometry (step 5a): Infect H1299 or
HT-1080 cells by adding 0.1 μl of lentivirus (10 μl of 10 0-fold diluted viral stock) into the first well, 0.5 μl of lentivirus
(50 μl of 100-fold diluted viral stock) into the second well, 2.0 μl into the third well, 10 μl into the fourth well, and 50 μl
into the fifth well. Add appropriate amount of DMEM containing 5% heat-inactivated serum and penicillinstreptomycin so that the final volume reaches 0.5 ml per well.
3b. For lentivirus to be analyzed by drug selection and colony counting (step 5b-10): Infect H1299 or HT-1080 cells
by adding 0.001 μl of lentivirus (10 μl of 10,000-fold diluted viral stock) into the first well, 0.01 μl of lentivirus (10 μl of
1,000-fold diluted viral stock) into the second well, 0.1 μl of lentivirus (10 μl of 100 -fold diluted viral stock) into the
third well, 0.5 μl of lentivirus (50 μl of 100-fold diluted viral stock) into the fourth well, and 2.0 μl into the fifth well.
Add appropriate amount of DMEM containing 5% heat-inactivated serum and penicillin-streptomycin so that the final
volume reaches 0.5 ml per well.
Place the plates for 2 hours at 4-8°C; then transfer the plates to a 37°C incubator with 5% CO2 and incubate cells
overnight.
Note:
a. Dilute lentivirus with only culture medium. Do not use water or other buffers.
b. The optimal concentration of Polybrene depends on cell type and may need to be empirically determined,
but is usually in the range of 2–10 µg/ml. Prepare enough for an extra well as a negative control.
c. Excessive exposure to Polybrene (>12 hr) can be toxic to some cells.
Day 3: Replace medium/split cell culture
4. Trypsinize and transfer the cells to 6-well plates (each 6-well plate will be used for one lentivirus with one control
well of non-transduced cells). Incubate in DMEM supplemented with 10% fetal bovine serum and penicillinstreptomycin for additional 48 hours.
Day 5: Determine titer by fluorescence analysis (step 5a) or drug selection (5b)
5a. The fraction of eGFP fluorescent cells can be counted by FACS (fluorescent activated cell sorting). Alternatively
the eGFP fluorescence may be visualized under a fluorescent microscope. Normally 10 random fields of view are
used to estimate the overall fraction of fluorescing cells in each well. The cells are then trypsinized, suspended with
complete DMEM, and the total number of cells in each well is determined by using a hemocytometer. The averaged
fraction of fluorescent cells is multiplied by the corresponding total cell numbers, then divided by the actual volume
of added lentivirus supernatant (in ml, e.g. 0.1 μl equals 10–4 ml) to determine the titer of the pseudovirus in the
supernatant.
5b. For HT-1080 or H1299 cells transduced with lentiviral stocks lacking a fluorescent marker, replace the old
medium with fresh complete DMEM containing an appropriate selection drug. (Note: The concentration of the
selection drug should be determined empirically beforehand.) Then follow steps 6–10 below:
Days 6-14: Select stably transduced cells and count colonies (continued from step 5b)
6. Replace medium with fresh complete medium containing the appropriate selection drug every 3–4 days until drug-
resistant colonies become visible (generally 7–14 days after selection). There should not be any colonies in the
mock well control.
7
Page 8

Lenti-Pac™ FIV Expression Packaging Kit User Manual
7. Remove medium and wash dishes twice with cold PBS.
8. Add enough 10% formalin to cover each dish. Incubate for 5 minutes at room temperature to fix the cells.
9. Decant 10% formalin, add 0.5% crystal violet and incubate for 10 minutes at room temperature.
10. Remove the crystal violet solution and wash the dishes with tap water until there is no background staining.
Count the blue colonies and calculate the titer of lentiviral stock.
Note: Viral titers determined by drug selection/colony counting are significantly lower than those determined by
FACS.
VII. Transduction of Target Cells with Lentiviruses
The transduction efficiency depends upon the target cells and experimental procedure. It is recommended that the
titrated pseudoviral stock containing the positive control eGFP construct is used to determine the concentration of
pseudoviral particles required for the desired MOI of the target cells. After these test transductions are performed, it
should be possible to determine the optimum concentration of pseudoviral particles for transduction based on eGFP
fluorescence.
Day 1: Plate cells
1. Plate 2–10 x 104 of the target cells per well in a 24-well plate 24 hours prior to viral infection. Use 0.5 ml of DMEM
supplemented with 5% heat-inactivated serum and penicillin-streptomycin for each well. Incubate the cells at 37°C
with 5% CO2 overnight.
Day 2: Transduce target cells
2. For each well, prepare 0.5 ml of virus suspension diluted in complete medium with Polybrene at a final
concentration of 5–8 µg/ml.
Note:
Use several dilutions of pseudoviral stock (0.1 μl to 100 μl). In addition, we recommend including a transduction with
the eGFP control and other appropriate positive and negative controls. Mix the virus with the medium gently by
rotation or inversion. Do not vortex.
3. Infect the target cells by removing the old culture medium and replacing it with 0.5 ml of diluted viral supernatant.
For one well (mock well control), add 0.5 ml of complete DMEM with Polybrene. Place the plates in a 37°C incubator
with 5% CO2 and incubate cells overnight. (Optional: Place the plates for 2 hours at 4-8°C; then transfer the plates
to a 37°C incubator with 5% CO2 and incubate cells overnight.)
Note:
Incubating cells with lentivirus for 2 hours at low temperatures can significantly increase the transduction efficiency.
But it may be omitted if the cells cannot tolerate low temperatures.
Day 3: Replace medium/Split cell culture
4. Remove the culture medium and replace with 0.5 ml of complete medium (without Polybrene). Or split the cells
1:3 to 1:5 depending on the type of cells, and continue incubating for 48 hours in DMEM.
Day 5: Analyze transduced cells or start drug selection of stably transduced cells
5a. The infected target cells can be analyzed for transient expression of transgenes using an appropriate biological
assay. If you have used an internal eGFP control, determine the percentage of infected cells by counting fluorescing
cells by flow cytometry or with a fluorescent microscope.
5b. To select stably transduced cells, replace old medium with fresh complete medium containing the appropriate
selection drug every 3–4 days until drug-resistant colonies become visible (generally 7–14 days after selection).
8
Page 9

Lenti-Pac™ FIV Expression Packaging Kit User Manual
GeneCopoeia Products are for Research Use Only Copyright © 2009 GeneCopoeia, Inc.
Trademarks: GeneCopoeia™, Lenti-Pac™, EndoFectin™, TiterBoost™, miExpress™ (GeneCopoeia Inc.);
Opti-MEM®, Stb13™ (Invitrogen); Falcon® (Bectin, Dickinson & Company); Polybrene® (Abbott) LPF5-0710
X. Limited Use License and Warranty
Limited Use License
Following terms and conditions apply to use of all Om icsLink™ ORF Expression Clo nes in all lentiviral vectors and Packaging Kit (the Prod uct). If the
terms and conditions are not acceptable, the Product in its entirety must be returned to GeneCopoeia within 5 calendar days. A limited End-User
license is granted to the purchaser of the Product. The Product shall be used by the purchaser for internal research purposes only. The Product is
expressly not designed, intended, or warranted for use in humans or for therapeutic or diagnostic use. The Product must not be resold, repackaged or
modified for resale, or used to manufacture commercial products without prior written consent from GeneCopoeia. This Product should be used in
accordance with the NIH guidelines developed for recombinant DNA and genetic research. Use of any part of the Product constitutes acceptance of
the above terms.
Limited Warranty
GeneCopoeia warrants that the Product meets the specifications described in the accompanying Product Datasheet. If it is proven to the satisfaction
of GeneCopoeia that the Product fails to meet these specifications, GeneCopoeia will replace the Product. In the event a repl acement cannot be
provided, GeneCopoeia will provide the purchaser with a refund. This limited warranty shall not extend to anyone other than the original purchaser of
the Product. Notice of nonconforming products must be made to GeneCopoeia within 30 days of receipt of the Product. GeneCopoeia’s liability is
expressly limited to replacement of Product or a refund limited to the actual pu rchase price. GeneCopoeia’s liability d oes not extend to any damages
arising from use or improper use of the Product, or losses associated with the use of additional materials or reagents. This limited warranty is the sole
and exclusive warranty. GeneCopoeia does not provide any other warranties of any kind, expressed or implied, including the merchantability or
fitness of the Product for a particular purpose.
GeneCopoeia is committed to providing our customers with high-quality products. If you should have any questions or concerns about any
GeneCopoeia products, please contact us at 301-762-0888.
© 2009, GeneCopoeia, Inc.
GeneCopoeia, Inc.
9620 Medical Center Drive, #101
Rockville, Maryland 20850
Tel: 301-762-0888 Fax: 301-762-8333
Email: inquiry@genecopoeia.com
Web: www.genecopoeia.com
9
 Loading...
Loading...