Page 1

All‐in‐One
Foruniversalquantitativereal‐timePCR
Cat.No.AOPR‐0200(200qPCRreactions)
Cat.No.AOPR‐0600(600qPCRreactions)
Cat.No.AOPR‐1200(1200qPCRreactions)
PerformanceoptimizedwithAll‐In‐One™qPCRPrimersandAll‐in‐OneFirst‐StrandcDNA
SynthesisKit
™
qPCRMix
UserManualI
GeneCopoeia,Inc.
9620MedicalCenterDrive,#101
Rockville,MD20850
USA
301‐762‐0888
866‐360‐9531
inquiry@genecopoeia.com
www.genecopoeia.com
© 2009 GeneCopoeia, Inc.
Page 2
All-in-One™ qPCR Mix Manual
USER MANUAL I
™
All-in-One
I. Description
II. Related Products
III. Contents and Storage
IV. Preparation
V. Procedure
VI. Example
VII. Trouble Shooting Guide
VIII. Limited Use License and Warranty
I. Description
The All-in-One™ qPCR Mix provides fast and efficient SYBR
qPCR Mix uses a high-fidelity hot-start DNA polymerase, optimized reaction buffer and high-quality dNTPs
to enable specific and sensitive amplification of even low-copy genes. The All-in-One qPCR Mix reduces
experimental design time by providing a universal reaction condition that can be used with almost all primers
and most real-time PCR instruments.
II. Related Products
GeneCopoeia offers comprehensive solutions for studying gene expression. A careful process of co
develop-ment ensures that they work well together and provide robust and reproducible results.
qPCR Mix
®
Green-based real-time quantitative PCR. The
GeneCopoeia Description
All-in-One™ First-Strand cDNA
Synthesis Kit
All-in-One™ qPCR Primers
All-in-One™ qPCR Primer Array
All-in-One™ miRNA qRT-PCR
Detection Kits
All-in-One™ miRNA qPCR Primers
OmicsLink™ Expression-Ready
ORF cDNA Clones
Endofectin™ Transfection Reagents
Reverse transcription kit
Human, mouse and rat
primers
User specified, ready-to-use
primer arrays in 96-wellplate format
®
SYBR
Green-based Accurately quantify miRNA expression
Human, mouse and rat
primers
20,000 human
15,000 mouse
Optimized for specific cell
types
Produces first-strand cDNA using poly A
or total RNA as template
Validated, gene-specific primers ensure
specificity and sensitivity
Reliable tools ideal for analyzing the
expression of a focused panel of genes
such as pathways, diseases or
customized gene panels
Validated for robust, reproducible and
reliable quantitation of miRNA activity
Perform a variety of applications with
expression-ready clones
Transfect efficiently and with low toxicity
2
Page 3
All-in-One™ qPCR Mix Manual
III. Contents and Storage
Contents and storage recommendations for the All-in-One qPCR Mix
Cat. Nos. AOPR-0200, AOPR-0600
Contents Quantity Storage temperature/ conditions
2X All-in-One qPCR Mix
50X ROX Reference Dye
and AOPR-1200 are provided in the following table.
2 x 1 ml
3 x (2 x 1 ml)
6 x (2 x 1 ml)
1 x 80 µl
3 x 80 µl
6 x 80 µl
–20°C (Stable for at least 12 months)
Alternatively, the solution can also be
stored at -80°C in aliquots. Avoid
repeated freezing/ thawing.
–20°C (Stable for at least 12 months)
Alternatively, the solution can also be
stored at -80 °C in aliquots. Avoid
repeated freezing/ thawing.
IV. Preparation
Wearing a lab coat, disposable gloves and protective goggles are recommended when handling
chemicals.
IMPORTANT NOTES:
1. Store the kit at –20°C. Avoid storage or leaving reagents at 4°C or room temperature. Avoid light
exposure at all times.
2. Mix reagents thoroughly by gently inverting tubes several times avoiding bubbles and then briefly
centrifuge before use.
3. Prepare the reaction mix with PCR grade water.
4. Strictly follow standard procedures for PCR to avoid nucleic acid contamination and non-specific
amplification.
5. Read all procedures before setting up the PCR reaction
V. Procedure
1. Thaw the 2X All-in-One qPCR Mix and 50X ROX Reference Dye as needed.
2. Prepare the PCR reaction mix on ice. See the example below.
3
Page 4

All-in-One™ qPCR Mix Manual
Reagent Volume per reaction, Final concentration
2X All-in-One qPCR Mix a 10 μl 1X
PCR forward primer (2 µM)
b
2 µl 0.2 µM
PCR reverse primer (2 µM) 2 µl 0.2 µM
Template d 2 μl
50X Rox Reference Dye e 0.4 μl 1X
Water (double distilled)
■ Not using ROX Reference Dye 4 μl
■ Using ROX Reference Dye 3.6 μl
Final volume 20 μl
a.
Use the 2X All-in-One qPCR Mix as half of the total reaction volume and adjust other reagents
accordingly. If the total reaction volume is changed, maintain each component in the proper
proportion.
b.
Primers are important considerations to ensure success with real-time PCR. All-in-One human,
mouse and rat primer sets from GeneCopoeia have been validated to provide specific and sensitive
amplification even with low copy number genes. For designing your own primers, you may wish to
use Oligo primer analysis software (Molecular Biology Insights) or Primer Premier software
(Premier Biosoft International).
c.
Primer concentration should be in the range of 0.2 to 0.6 µM. In general, a PCR reaction using 0.2
µM primers produces good results. If the PCR efficiency is low, consider increasing primer
concentration. However, keep in mind that non-specific PCR products may also increase with
increased primer concentration.
d.
Generally, the amount of DNA template should be less than 100 ng. Because different templates
contain varying copies of a target gene, it may be necessary to perform a gradient dilution to
determine the optimal amount of DNA template to use. If reverse transcript cDNA is used as
template, dilute before use. Do not add more than 5% of the original cDNA solution volume to the
total qPCR reaction solution.
e.
ROX Reference Dye is added only for qPCR instruments that require ROX for calibration
3. Mix the PCR reaction mix sufficiently and add to the PCR reaction tubes.
4. Briefly centrifuge to make sure all the reagents are at the bottom of the reaction tubes.
5. The following three-step method for programming the PCR reaction is recommended:
c
4
Page 5

All-in-One™ qPCR Mix Manual
Cycles Steps Temperature Time Detection
1 Initial denaturation
a
95°C 10 min No
Denaturation 95°C 10 sec No
40
Annealing b 55°C~60°C 20 sec No
Extension 72°C At least 15 sec C Yes
a.
The DNA polymerase used in the 2X All-in-One qPCR Mix is a special chemically modified hot-
start enzyme. The indicted initial denaturation is sufficient to activate the enzyme.
b.
The actual annealing temperature should be adjusted around the primer melting temperature
ranging from 55°C~60°C. However, the optimal annealing temperature may be outside of this
range. Adjust the temperature according to actual reaction conditions
c.
The extension time is specific for the instrument. See the documentation provided with your
instrument.
When using SYBR Green dye to monitor the qPCR reaction, a melting curve analysis should be
performed immediately after qPCR cycling. For instructions, consult the documentation for your qPCR
instrument. The following is an example adapted from the iQ5 real-time detection system from Bio-
Rad Laboratories. The conditions for your instrument may differ.
Temperature range
Heating rate Constant temperature Detection
72–95°C 0.5°C/unit time 10 sec/unit time Yes
25°C 30 sec No
VI. Example
Objective: The amplification efficiency and detection sensitivity of the 2X All-in-One qPCR Mix are
assessed by standard curves made by gradient dilution of plasmid DNA. The target fragment is 102 bp.
Equipment: iQ5 instrument (Bio-Rad Laboratories)
Procedure:
1. The plasmid is serially diluted to 6 concentrations ranging from 10
2. PCR reaction mix preparation (on ice )
5
to 1 molecule/μl.
5
Page 6
All-in-One™ qPCR Mix Manual
3. Mix the above reagents sufficiently. Aliquot to PCR tubes after a brief centrifugation.
4. Add 5 μl of the diluted plasmid template to each PCR tube. Use 5μl ddH
5. Program the PCR reaction and corresponding reading conditions of the melting curve:
Reagent components
2X All-in-One qPCR Mix 10 µl
PCR forward primer (2 µM) 2 µl
PCR reverse primer (2 µM) 2 µl
ddH20 1 µl
Total 15 µl
Cycles Steps Temperature Time Detection
Volume
O as a negative control.
2
1 Initial denaturation 95°C 10 min No
Denaturation 95°C 10 sec No
45
Melting curve reading 72°C~95°C
Cooling 25°C 30 sec No
Annealing 60°C 20 sec No
Extension 72°C 15 sec Yes
Heating Rate
0.5°C / 10 sec
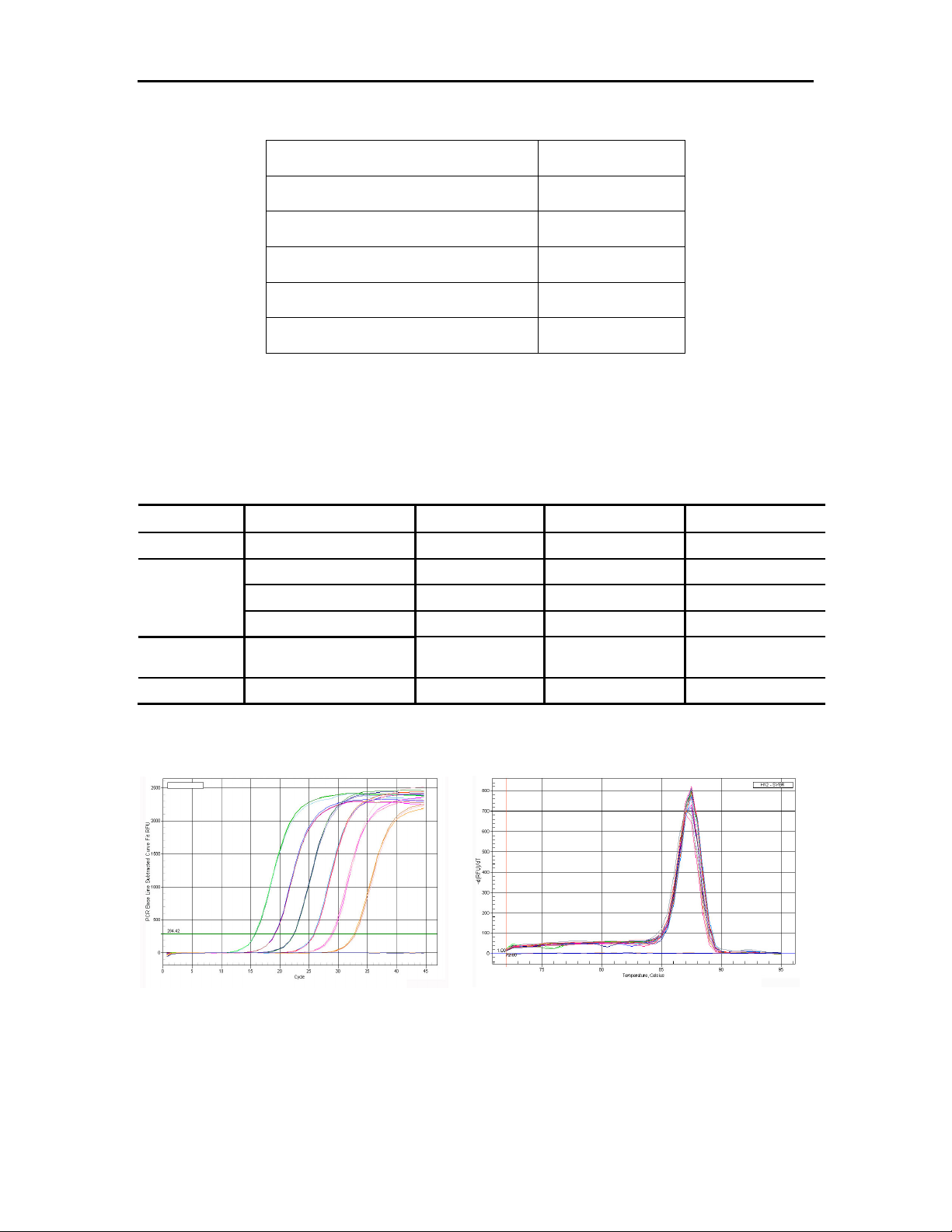
Analyze the amplification and corresponding melting curves after the qPCR experiment:
6.
Amplification curves of serially diluted plasmid DNA Peak values of amplified products in melting curves.
Yes
6
Page 7

All-in-One™ qPCR Mix Manual
7. Construct a standard curve using the Ct values from each amplification curve:
8. Conclusion: The peak values from the amplification and melting curves show that as low as 5
molecules can be detected when using plasmid DNA as a template and that there is only a single
amplified product, showing that very high sensitivity can be attained using the All-in-One qPCR Mix.
At the same time, high amplification efficiency is also shown by the good linear relationship among
each concentration of serially diluted plasmid.
Picture of a standard curve
VII. Trouble Shooting Guide
• The fluorescence detection temperature may not be appropriate.
Adjust accordingly.
• The set up position for reaction samples in the real-time PCR
instrument may not be right. Adjust accordingly.
• PCR cycle conditions, primer concentration and primer sequences
may not be appropriate. Adjust the primer concentration and
annealing temperature. If this does not work, redesign the primers.
Poor precision or failed
qPCR reactions
• The template sample purity may not be adequate. Purify the template
sample by phenol/chloroform extraction and ethanol precipitation. If
the samples are reverse transcribed cDNA, set up the qPCR reaction
with a diluted sample as other concentrated reagents in the RT
reaction mixture may be interfering with the qPCR.
• Try to use 3.0% agarose gel electrophoresis to check the qPCR
products. Check the purity of the primers by electrophoresis or use
PAGE-purified primers if the bands are diffused. One may also use
phenol/chloroform extraction and ethanol precipitation methods to
treat the primers before the experiment.
7
Page 8
All-in-One™ qPCR Mix Manual
Abnormal melting
curves
Signal in the blank (No Template Control) sample
• There may be contamination of the positive samples in the qPCR
reaction system if the T
the same as the positive control. Eliminate sample application error
first. If the situation still persists, replace the PCR grade water and/or
primers and/or use a new 2X All-in-One qPCR Mix.
• If the T
positive control, the qPCR reaction may have produced nonspecific
amplification such as primer-dimers. Prepare the qPCR reaction mix
on ice and increase the temperature of fluorescence detection. If this
does not work, redesign the primers.
Double peaks and multiple peaks in the melting curve of the
positive control
• In the absence of other primers present in the reaction, double or
multiple peaks in the melting curve of the positive control indicate that
the qPCR reaction produced nonspecific amplification fragments.
Prepare the qPCR reaction mix on ice; optimize the qPCR reaction
conditions, for example, by increasing the annealing temperature,
decreasing the primer concentration or increasing the fluorescence
detection temperature (not more than the T
product). If this does not work, redesign the forward primer.
of the melting curve of the blank control is lower than the
m
of the melting curve of the blank control is
m
value of the expected
m
No signal (Ct) or late
appearing signal
• Not enough PCR cycles. For good sensitivity, one should generally
set up more than 35 PCR cycles, but more than 45 cycles may result
in too much background signal.
• The amount of template used may not be enough or the template may
be degraded. Use the highest concentration possible of diluted
template samples to set up the qPCR. At the same time, avoid
freezing and thawing the samples repeatedly.
• The amplification efficiency is low and the qPCR reaction conditions
are not optimal. Redesign the primers and optimize the reaction
conditions.
8
Page 9

All-in-One™ qPCR Mix Manual
VIII. Limited Use License and Warranty
Limited Use License
Following terms and conditions apply to use of all OmicsLink™ ORF Expression Clones in all lentiviral vectors and Packaging Kit (the
Product). If the terms and conditions are not acceptable, the Product in its entirety must be returned to GeneCopoeia within 5 calendar days.
A limited End-User license is granted to the purchaser of the Product. The Product shall be used by the purchaser for internal research
purposes only. The Product is expressly not designed, intended, or warranted for use in humans or for therapeutic or diagnostic use. The
Product must not be resold, repackaged or modified for resale, or used to manufacture commercial products without prior written consent
from GeneCopoeia. This Product should be used in accordance with the NIH guidelines developed for recombinant DNA and genetic
research. Use of any part of the Product constitutes acceptance of the above terms.
Limited Warranty
GeneCopoeia warrants that the Product meets the specifications described in the accompanying Product Datasheet. If it is proven to the
satisfaction of GeneCopoeia that the Product fails to meet these specifications, GeneCopoeia will replace the Product. In the event a
replacement cannot be provided, GeneCopoeia will provide the purchaser with a refund. This limited warranty shall not extend to anyone
other than the original purchaser of the Product. Notice of nonconforming products must be made to GeneCopoeia within 30 days of receipt
of the Product. GeneCopoeia’s liability is expressly limited to replacement of Product or a refund limited to the actual purchase price.
GeneCopoeia’s liability does not extend to any damages arising from use or improper use of the Product, or losses associated with the use
of additional materials or reagents. This limited warranty is the sole and exclusive warranty. GeneCopoeia does not provide any other
warranties of any kind, expressed or implied, including the merchantability or fitness of the Product for a particular purpose.
GeneCopoeia is committed to providing our customers with high-quality products. If you should have any questions or
concerns about any GeneCopoeia products, please contact us at 301-762-0888.
© 2009, GeneCopoeia, Inc.
GeneCopoeia, Inc.
9620 Medical Center Drive
Rockville, MD 20850
Tel: 301-762-0888 Fax: 301-762-3888
Email:
Web:
HUinquiry@genecopoeia.comU
HUwww.genecopoeia.comU
GeneCopoeia Products are for Research Use Only Copyright © 2010 GeneCopoeia, Inc.
Trademarks: GeneCopoeia™, OmicsLink™, All-in-One™, EndoFectin™ (GeneCopoeia Inc.);
®
SYBR
(Molecular Probes); iQ™5 (Bio-Rad); ROX® (Invitrogen). AOP2-0410
9
 Loading...
Loading...