Page 1
GE Healthcare
Instructions 71-5017-88 AC Ion Exchange Columns
Mono Q 5/50 GL and
Mono S 5/50 GL
Quick information
Mono Q™ 5/50 GL and Mono STM 5/50 GL are TricornTM high per formance columns.
The columns are pre-packed glass columns for high performance ion exchange
chromatography of proteins, peptides, polynucleotides and other biomolecules.
The columns are supplied with two union M6 female/1/16” male for connection
TM
to FPLC
System, two fingertight connector 1/16” for connecting 1/16” tubing
to column and ÄKTA, two stop plugs 1/16” male to seal the column (attached to
column when delivered) and instruction.
Column data
Matrix Polystyrene/divinyl benzene
Bead form Rigid, s pherical, po rous monodis perse
Particle size 10 μm
Column dimensions 5 × 50 mm
Bed volume 1 ml
Average loading capacity 50 mg
(will var y depending on s ample and loa ding conditi ons)
pH stability
regula r use 2–12
cleani ng 1–14
Tem pera tur e
operating 4 to 40 ºC
Flow rate (water at room temperature)
recommended 0.5–3.0 ml /min
maximum 3 ml/m in
Pressure over column
maximum 4 MPa, 40 bar, 580 psi
Mono Q Mono S
Type of exchanger Strong anion Strong cation
Charge d group -CH
Ionic capacity 0.27–0.37 mmol 0.12–0.15 mmol
Cl
Note: Before co nnecting th e column to a chrom atography sys tem, start t he pump and remo ve all air
and debr is in the syste m, particul arly in the tubi ng and valves.
-N+(CH3)3 -CH2-SO
2
-
/ml mediu m H+/ml mediu m
-
3
First-time use
Equilibrate the column for first-time use or after long-term storage as follows:
a) 5 column volumes (CV) distilled water at 1 ml/min at room temperature.
b) 5 CV star t buffer at 2 ml/min at room temperature.
c) 5 CV elution buffer at 2 ml/min at room temperature.
d) 5 CV star t buffer at 2 ml/min at room temperature.
Try these conditions fi rst
Start buffer (Mono Q)*: 20 mM Tris-HCl, pH 8.0
Elution buffer (Mono Q)*: 20 mM Tris-HCl + 1.0 M NaCl, pH 8.0
Star t buffer ( Mono S) *: 20 mM 2-[N-morpholino] ethanesulphonic acid ( MES), pH 6.0
Elution buffer (Mono S)*: 20 mM MES + 1.0 M NaCl, pH 6.0
* Users of Ä KTATM design s ystem may sele ct one of the buf fer recipes re commended fo r anion
exchange chromatography at pH 8 or cation exchange chromatography at pH 6.
Separation by gradient elution
Flow: 2 ml/min at room temperature
1. Equilibrate column with 5–10 column volumes (CV) of start buffer or until
baseline, eluent pH and conductivity are stable.
2. Adjust the sample to the chosen starting pH and ionic strength and apply to
the column (see sample recommendations).
3. Wash with 5–10 CV of start buffer or until the baseline, the eluent pH and the
conductivity are stable i.e. when all unbound material has washed through
the column.
4. Begin elution using a gradient volume of 10–20 CV and an increasing ionic
strengt up to 0.5 M NaCl (50% elution buffer).
5. Wash with 2–5 CV of 1 M NaCl (100% elution buffer) to elute any remaining
ionically-bound material.
6. Requilibrate with at least 5–10 CV of start buff er or until eluent pH and
conductivity reach the required values.
Read the section ”Optimization” for information about how to optimize a
separation.
Buff ers and solvent resistance
Recommended to have an on-line filter upstream of the injection valve. Buffers
and solvents with increased viscosity will affect the back-pressure and flow rate.
▼
De-gas and filter all solutions through a 0.22 μm filter.
Daily use
All commonly used aqueous buffers, pH 2–12
Urea, up to 8 M
Guanidine hydrochloride, up to 6 M
Acetonitrile, up to 30% in aqueous buffers
Non-ionic detergents
Cationic detergents (Mono Q)
Anionic detergents (Mono S)
Cleaning
Acetonitrile, up to 100%
Sodium hydroxide, up to 2 M
Ethanol, up to 100%
Methanol, up to 100%
Acetic acid, up to 75%
Isopropanol, up to 100%
Hydrochloric acid, up to 1 M
1% Trifluoroacetic a cid
Avoid:
Oxidizing agents
Anionic detergents (Mono Q)
Cationic detergents (Mono S)
Sample recommendations
Net charge of target molecule negative (Mono Q), positive (Mono S)
Recomm ended initial sample load ≤ 45 mg
Prepar ation Disso lve the sample i n start buf fer,
filter throu gh a 0.22 μm filte r or
centri fuge at 10 000 × g for 10 min
In-depth information
Delivery/storage
The column is delivered in degassed 20% ethanol sealed with two stop plugs to
prevent the column from drying out. For column storage, wash with 5 column
volumes of distilled water followed by 5 column volumes of 20% ethanol. Degas
the ethanol/water mixture thoroughly and apply at a low flow rate to avoid overpressuring the column. Store at room temperature or, for long periods, store at +4°
C to +8 °C. Ensure that the column is sealed well to avoid drying out. Do not freeze.
Choice of eluent
To avoid local disturbances in pH caused by buf fering ions par ticipating in the ion
exchange process, select an eluent with buffering ions of the same charge as the
substituent groups on the ion exchanger.
Choose the start buf fer pH so that substances to be bound to the ion exchanger
are charged, e.g. at least 1 pH unit above the isoelectric point for anion exchangers
and at least 1 pH unit below the isoelectric point for cation exchangers. Figure 2
and Figure 3 list a selection of standard aqueous buffers.
Piperidine
BICINE
(25 ˚C)
pKa
(25 °C)
3.13
3.86
4.21
4.75
5.76
6.27
7.20
7.56
8.33
pKa
4.75
5.33
6.48
6.65; 9.10
7.76
8.07
8.52
8.88
9.50
9.73
10.55
11.12
5
467891011pH
N-methyl piperazine
Piperazine
bis-Tris
Fig 2. Recommen ded buffer s for anion excha nge chromatog raphy.
Triethhanolamine
Tris
N-methyldiethanolamine
bis-Trispropane
Propane-1,3-diamino
Ethanolamine
Piperazine
Propane-1,3-diamino
▼
pH 2.5 3 4 5 6 7 8 9
Citric acid
Lactic acid
Butanedioic acid
Acetic acid
Methyl Malonic acid
MES
Phosphate
HEPES
Fig 3. Recommen ded buffers f or cation excha nge chromato graphy.
Table 1 lists suggested volatile buffers that can be used in cases where the purified
substance has to be freeze-dried.
Table 1. Volatile buffer systems.
pH Substance
3.3–4.3; 4.8–5.8 Pyridine/formic acid
3.3–4.3; 9.3–10.3 Trimethylamine/formic acid
4.3–5.8 Pyridine/acetic acid
3.3–4 .3; 8.8–9.8 A mmonia/formic acid
4.3–5. 3; 8.8–9.8 Ammonia /acetic acid
5.9–6.9; 9.3–10.3 Trimethylamine/carbonate
5.9–6.9; 8.8–9.8 A mmonium carb onate/ammonia
4.3–5.3; 7.2–8.2 N-ethylmorpholine/acetate
▼
Fig 1. Illustration of how to lock the upper adapter. The locking ring (black) must be
in down position to prevent uncontrolled adjustment of the column’s bed height.
Tricorn
™
Page 2
Optimization
Perform a first run as described in the section “Try these conditions first”. If the
results obtained are unsatisfactory, consider the following:
Action Eff ect
Change pH/buf fer salt (see Changes selectivity, gives
Figure 1 and Figure 2 for buf fers) weaker/stronger binding.
Change salt, counter ions Changes selectivity.
and/or co-ions
Decrease the sample load Improves resolution.
Decrease the flow rate Improves resolution.
Change gradient slope Shallower gradients improve selectivity but
broaden peaks (decrease efficiency).
A steeper gradient will sharpen peaks, but
move them closer together.
For more information, please refer to the handbook “Ion exchange
chromatography, Principles & Methods”, which can be ordered from GE Healthcare
or downloaded from our web site.
Cleaning
It is recommended to reverse the direction of flow during column cleaning so that
contaminants do not need to pass through the entire length of the column.
Regular cleaning
Flow: 0.5 ml/min at room temperature
1. Wash with 2 column volumes (CV) of 2 M NaCl.
2. Wash with 4 CV of 1 M NaOH
3. Wash with at least 2 CV of 2 M NaCl
4. Rinse with at least 2CV of distilled water until the UV-baseline and the eluent
pH are stable.
4. Wash with at least 4 CV of start buffer or storage buf fer until pH and
conductivity values have reached the required values.
More rigorous cleaning
Remove strongly hydrophobically bound proteins, lipoproteins and lipids by
washing with 4 column volumes (CV) of 30% isopropanol or 70% ethanol at
0.25 ml/min. Remove precipitated proteins with 1 CV of 1 mg/ml pepsin in
0.5 M NaCl, 0.1 M acetic acid (leave overnight) or wash with 2 CV of 6 M Guanidine
hydrochloride at 0.25 ml /min.
Depending on the nature of contaminant cleaning solution in the section ”Buffers
and solvent resistance” may be appropriate. After cleaning the column wash
with at least 2 CV of distilled water and 4 CV of star t buffer or storage buffer. For
more information on how to clean your column, please refer to the handbook ”Ion
exchange chromatography & Chromatofocusing, Principles & Methods”.
As an alternative to more rigorous cleaning or if column performance still not
restored change the filter at the top of the column. (Since contaminants are
introduced with the liquid flow, many of them are caught by the filter.) Instructions
for changing the f ilter are supplied with the Filter Kit. Clean the column after filter
change according to regular cleaning.
Troubleshooting
Symptom Remedy
Increased back-pressure Reverse the flow direction and pump 5 ml elution
over the column buffer at a flow rate of 0.5 ml/min through the
column. Return to normal flow direction and run for
5 minutes at 1 ml/min through the column. If high
backpressure persists, clean the column.
Loss of resolution and/or Clean the column according to the procedure
decreased sample recovery described in the section “More rigorous cleaning”.
Air in the column Reverse the flow direction and pump 10 ml well de-
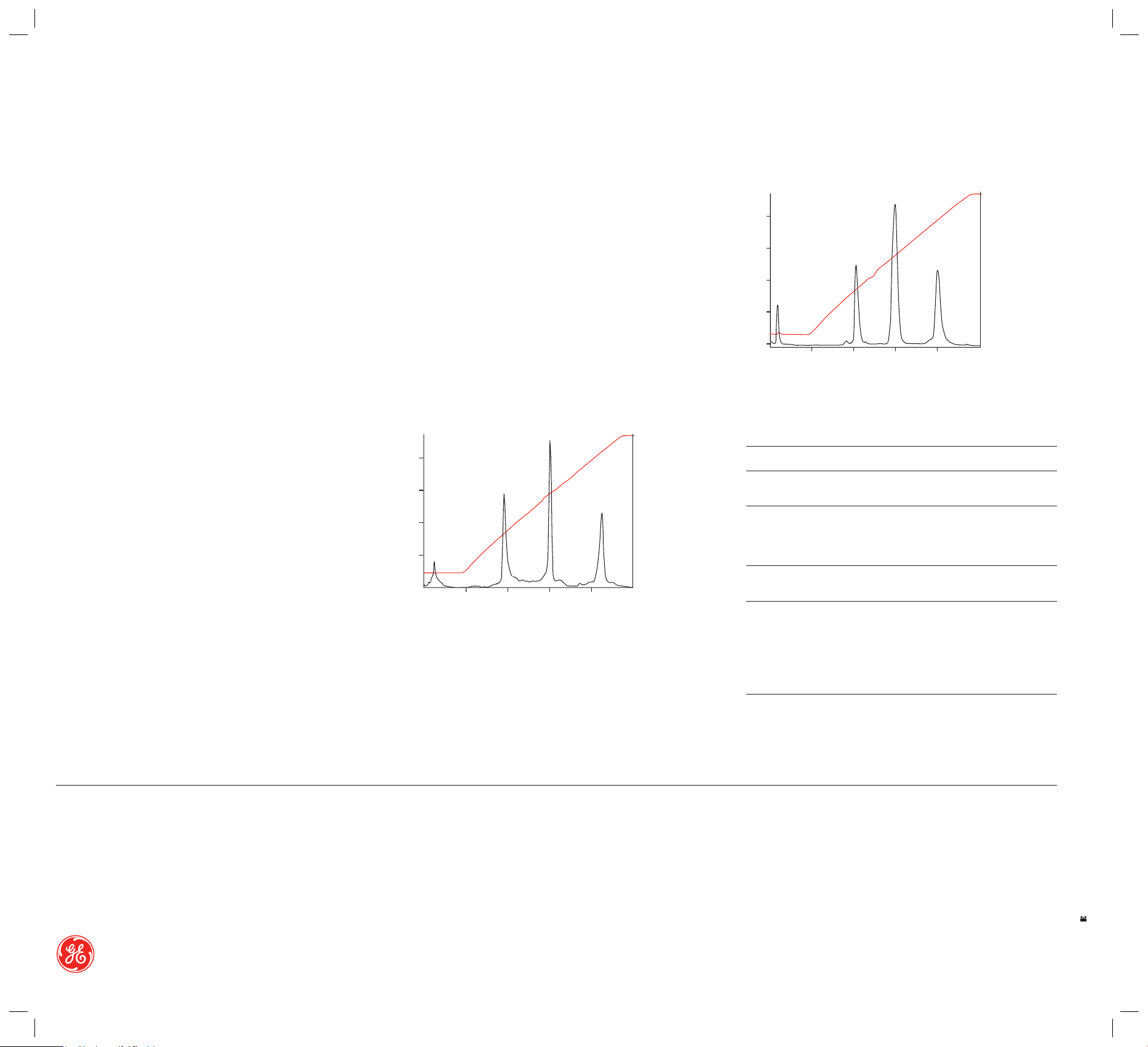
Function test of Mono S 5/50 GL
Sample: 1 . Ribonuclease A (1.5 mg/m l)
2. Cytochrome C (0.4 mg /ml)
3. Lysozyme ( 0.4 mg/ml)
Sample volume : 100 μl
Gradie nt: 0–100% elution b uffer in 20 CV
Start buffer: 20 mM so dium phospha te, pH 6.8
Elution buf fer: 20 mM sodiu m phosphate + 0.4 M N aCl, pH 6.8
Flow rat e: 1.0 ml/min (r oom temperatu re)
mAU (280 nm) % Elution Buffer
100
gassed start buffer through the column at a flow
rate of 0.5 ml/min.
200
Column performance control
Check the performance of the column when new by running the separation
described in Figures 4 and 5. Figures 4 and 5 shows a typical chromatogram run
on an optimized system. Since the system can profoundly affect the resolution,
it is meaningful to compare runs done on the same system. Check the column at
regular intervals and whenever you suspect a problem.
Function test of Mono Q 5/50 GL
Sample: 1. Conalbumin (3 mg/ml)
2. α-lactalbumin, bovine milk (4 mg/ml)
3. Soyb ean trypsin inhibit or (6 mg/ml)
Sample volume: 200 μl
Gradient: 0–100% elution buffer in 20 CV
Start buffer: 20 mM Tris-HCl, pH 7.0
Elution buf fer: 20 mM Tris-HCl + 0.25 M NaCl, pH 7.0
Flow rat e: 1.0 ml/min (r oom temperatu re)
mAU (280 nm) % Elution Buffer
200
150
▼▼
100
50
0
0.0 5.0 10.0 15.0 20.00ml
100
Fig 4. Typical chromatograms from a function test of Mono Q 5/50 GL.
150
100
50
0
0.0 5.0 10.0 15.0 20.00ml
Fig 5. Typical chromatograms from a function test of Mono S 5/50 GL.
Ordering information
Product No. per pack Code No.
Mono Q 5/50 GL 1 17-5166-01
Mono S 5/50 GL 1 17-5168-01
Related products
Product No. per pack Code No.
Mono Q 10/100 GL 1 17-5167-01
Mono Q 4.6 /100 PE 1 17-5179-01
Mono S 10/100 GL 1 17-5169-01
Mono S 4.6 /100 PE 1 17-5180-01
TM
HiTrap
Desal ting 5 × 5 ml 17-1408-01
Accessories
Product No. per pack Code No.
Tubing connectors:
Fingertig ht connector 1 /16” male 10 18-1112-55
Tricorn 5 f ilter kit* 1 18-1153-02
Filter Too l 1 18-1153-20
Union M6 fe male/1/16” male 8 18-1112-58
On-li ne filter (1/16”) 1 18-1118-01
Handbook:
Ion Exchange Chromatography & Chromatofocusing,
Princi ples and Methods 1 11-0004 -21
* includes t op and bottom f ilters and O- rings, 5 of each .
▼
www.gehealthcare.com/protein-purifi cation
www.gehealthcare.com
GE Healthcare Bio-Sciences AB
Björkgatan 30
751 84 Uppsala
Sweden
GE Healthcare Europe GmbH
Munzinger Strasse 5, D-79111 Freiburg, Germany
GE Healthcare UK Ltd
Amersham Place, Little Chalfont, Buckinghamshire, HP7 9NA, UK
GE Healthcare Bio-Sciences Corp
800 Centennial Avenue, P.O. Box 1327, Piscataway, NJ 08855-1327, USA
GE Healthcare Bio-Sciences KK
Sanken Bldg, 3-25-1, Hyakunincho, Shinjuku-ku, Tokyo 169-0073, Japan
71-5017-88 AC 03/2006
Tricorn, Mono Q, Mono S, HiTrap, ÄKTA and Drop Design are trademarks of GE Healthcare
companies. GE and GE Monogram are trademarks of General Electric Company.
The Tricorn column and components are protected by US design patents USD500856, USD506261,
USD500555, USD495060 and their equivalents in other countries.
All goods and services are sold subject to the terms and conditions of sale of the company within
GE Healthcare which supplies them. General Electric Company reserves the right, subject to any
regulatory and contractual approval, if required, to make changes in specifi cations and features
shown herein, or discontinue the product described at any time without notice or obligation.
© 2006 General Electric Company – All rights reserved.
GE Healthcare Bio-Sciences AB, a General Electric Company.
Elanders Östervåla 2006
 Loading...
Loading...