Page 1
GE Healthcare
URGENT MEDICAL DEVICE CORRECTION
3000 N. Grandview Blvd.
-
W440
Safety
Hot screws from the
“Heater Head” of the IWS
could
fall onto
the bed
if the
“Heater Head” assembly has
Safety
The potential for this issue to occur is only present if the “Heater Head” assembly has been improperly
Affected
Refer to
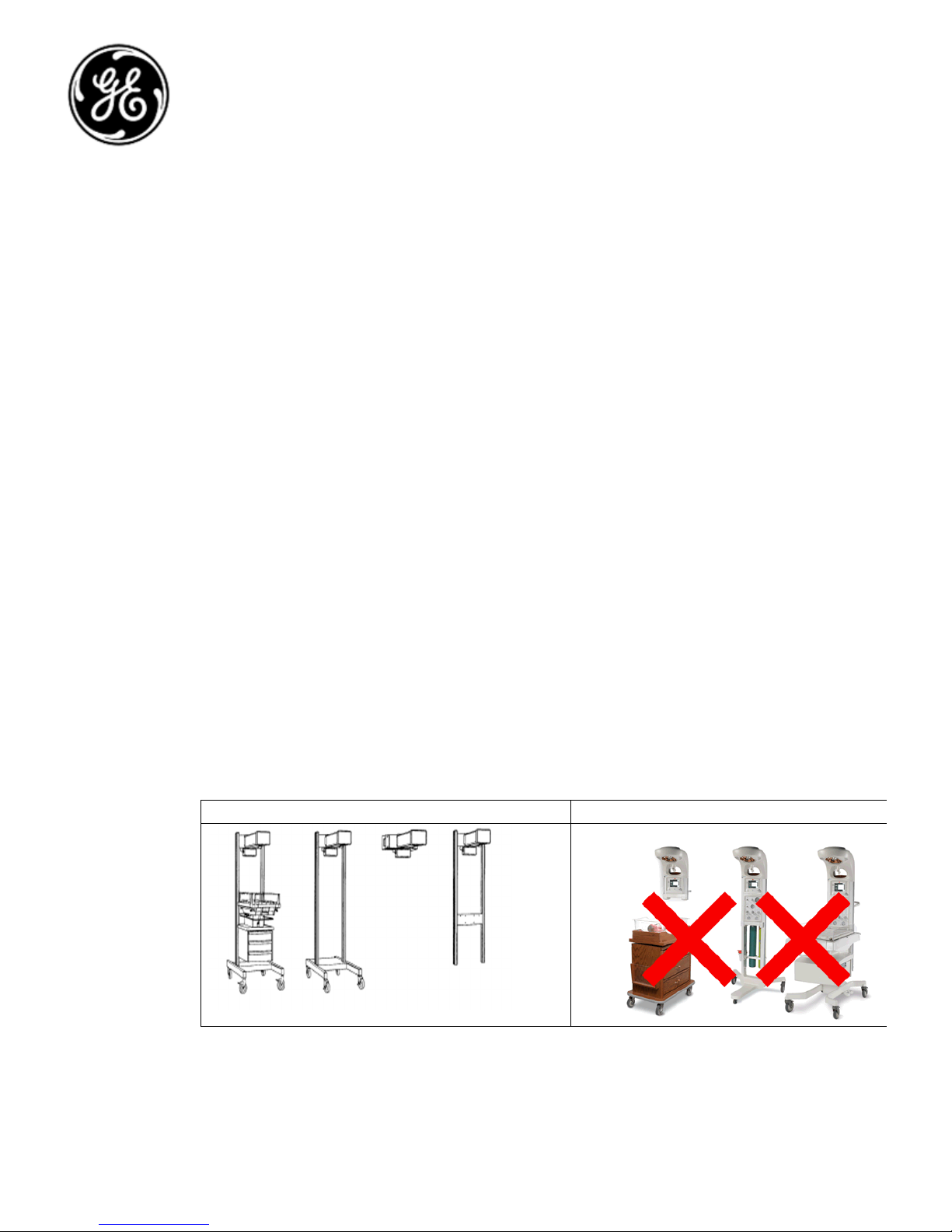
Product Images
below
for
images of
A
ffected and Not affected
products
.
Rendered PDF File Page 2 of 4 DOC1881503, Rev:1
Waukesha, WI 53188, USA
<Date of Letter Deployment> GEHC Ref# 32047
To: Nurse Managers, Labor & Delivery/NICU
Bio-Medical Engineering Department Managers
Risk Management Directors
RE: Infant Warmer System (IWS) Hot “Heater Head” Screw could Fall onto the Bed
GE Healthcare has recently become aware of a potential safety issue related to loose screws in the “Heater Head” of certain
Infant Warmer System (IWS) devices. Please ensure that all potential users, as well as those servicing these units, in your
facility are made aware of this safety notification and the recommended actions.
Issue
Instructions
Product
Details
been improperly serviced. This situation can be clinically hazardous because thermal injury to a patient
could result. Two injuries have been reported as a result of this issue.
serviced.
As a result, if “Heater Head” service has been performed in the past, check to ensure that the screws are
tightened as soon as the unit becomes available.
If the “Heater Head” assembly has not been serviced, check to ensure that the screws are tightened as part
of your next regularly scheduled annual Preventive Maintenance check.
The attached Service Manual Addendum provides instructions for checking and tightening the screws.
During each annual Preventive Maintenance check, continue to ensure that the screws are tight.
Affected model numbers: Model 2001 IWS (International), 3000 IWS, 3050 IWS, 3100 IWS, 3150 IWS, 3300
IWS, 3500 IWS, 4000 IWS, 4300 IWS, 4400 IWS, 5000 IWS.
Affected Products Not Affected Products
Released
Page 1 of 3
Page 2
Product
Attached to this letter
, we provide
instructi
ons
as part of a
Service Manual Addendum
on how to
correct
Contact
Rendered PDF File Page 3 of 4 DOC1881503, Rev:1
Correction
Information
Please be assured that maintaining a high level of safety and quality is our highest priority. If you have any questions, please
contact us immediately per the contact information above.
Sincerely,
James W. Dennison Jeff Hersh, M.D.
Vice President - Quality & Regulatory Chief Medical Officer
GE Healthcare GE Healthcare
the issue. Please add this new Addendum to the Service Manual of your device(s) and train the affected
users accordingly.
Please acknowledge that you have received this letter and that you understand that an action needs to be
taken on your part to correct this issue by filling out and returning the attached “Customer Response”
form.
If you have any questions or concerns regarding this notification, please contact GE Healthcare Service at
1-800-437-1171 or your local Service Representative.
Released
Page 2 of 3
Page 3
Please return this form using one of the following methods:
Rendered PDF File Page 4 of 4 DOC1881503, Rev:1
GE Healthcare
MEDICAL DEVICE CORRECTION CONFIRMATION GE REF: 32047
CUSTOMER RESPONSE REQUIRED
We request that you PLEASE COMPLETE and return this form to GE Healthcare within two (2) weeks.
Customer/Consignee Name: _____________________________________________________________
Street Address: _____________________________________________________________
City/State/ZIP/Country: _____________________________________________________________
Email Address: _____________________________________________________________
Phone Number: _____________________________________________________________
It is important that we confirm our customers have received this correction notice. Please check one of the following and complete the
requested information and send back via one of the methods below.
We acknowledge receipt and understanding of the Medical Device Correction Notice and have alerted the appropriate personnel
at our facility regarding the safety issue and instructions. We will perform the actions as requested in the attached Medical
Device Correction Notice on all potentially affected systems.
List all Device/System Serial Number(s) known (attachment can be used):__________________________________
_____________________________________________________________________________________________
_____________________________________________________________________________________________
We acknowledge receipt and understanding of the Medical Device Correction Notice and no longer have a system affected by this
Medical Device Correction Notice. (Please check appropriate disposition. If multiple systems or further information, attachment
can be used.)
Sold Returned Scrapped Other: _______________
Device/System Serial Number(s): ___________________________________________________
New Owner, if known: ____________________________________________________________
Contact Name: __________________________________________________________________
Street Address: __________________________________________________________________
City/State/Country: ______________________________________________________________
Contact (i.e. Email, Phone): _________________________________________________________
Please provide the name of the individual with responsibility for risk and compliance.
Signature: _____________________________________________________________
Printed Name: _____________________________________________________________
Title: _____________________________________________________________
Date (DD/MM/YYYY): _____________________________________________________________
1. Scan or take photo of completed form and email to MIC.Recall@ge.com
Note: QR code can be used to email the form: click QR code, attach photo to email, click Send
2. Take photo of completed form and send via SMS text to +1-410-972-8096
Note: QR code can be used to text the form: click QR code, attach photo to text, click Send
3. Fax completed form to Fax Number: +1-410-630-5938
QR (text)
QR (email)
Released
32047 – XXXX
Page 3 of 3
Page 4

Addendum
2098189-001 A
Infant Warmer System Heater Reflector
Screws Maintenance Check
For future reference, add this addendum to your
Infant Warmer System Service Manual.
© 2016 General Electric Company
Page 5

Language Disclaimer
(EN)
(BG)
(ZH-CN)
(ZH-HK)
(ZH-TW)
WARNING:
This service document is available in English only.
• If a customer’s service provider requires a language other than English, it is the customer’s
responsibility to provide translation services.
• Do not attempt to service the equipment unless this service manual has been consulted
and is understood.
• Failure to heed this warning may result in injury to the service provider, operator, or patient
from electric shock, mechanical hazards, or other hazards.
ПРЕДУПРЕЖДЕНИЕ
Това упътване за работа е налично само на английски език.
• Ако доставчикът на услугата на клиента изиска друг език, задължение на клиента е да
осигури превод.
• Не използвайте оборудването, преди да сте се консултирали и разбрали упътването за
работа.
• Неспазването на това предупреждение може да доведе до нараняване на доставчика
на услугата, оператора или пациентa в резултат на токов удар, механична или друга
опасност.
警告
本维修手册仅提供英文版本。
• 倘若客戶的服務供應商需要英文以外之服務手冊,客戶有責任提供翻譯服務。
•
除非已參閱本服務手冊及明白其內容,否則切勿嘗試維修設備。
•
不遵從本警告或會令服務供應商、網絡供應商或病人受到觸電、機械性或其他的危險。
警告
本服務手冊僅提供英文版本。
• 倘若客戶的服務供應商需要英文以外之服務手冊,客戶有責任提供翻譯服務。
•
除非已參閱本服務手冊及明白其內容,否則切勿嘗試維修設備。
•
不遵從本警告或會令服務供應商、網絡供應商或病人受到觸電、機械性或其他的危險。
警告
本維修手冊僅有英文版。
• 若客戶的維修廠商需要英文版以外的語言,應由客戶自行提供翻譯服務。
•
請勿試圖維修本設備,除非 您已查閱並瞭解本維修手冊。
•
若未留意本警告,可能導致維修廠商、操作員或病患因觸電、機械或其他危險而受傷。
2 2098189-001 Rev A IWS Service Manual Addendum
Page 6

VÝSTRAHA
WAARSCHUWING
(HR)
(CS)
(DA)
UPOZORENJE
Ovaj servisni priručnik dostupan je na engleskom jeziku.
• Ako davatelj usluge klijenta treba neki drugi jezik, klijent je dužan osigurati prijevod.
• Ne pokušavajte servisirati opremu ako niste u potpunosti pročitali i razumjeli ovaj servisni
priručnik.
• Zanemarite li ovo upozorenje, može doći do ozljede davatelja usluge, operatera ili pacijenta
uslijed strujnog udara, mehaničkih ili drugih rizika.
Tento provozní návod existuje pouze v anglickém jazyce.
• V případě, že externí služba zákazníkům potřebuje návod v jiném jazyce, je zajištění
překladu do odpovídajícího jazyka úkolem zákazníka.
• Nesnažte se o údržbu tohoto zařízení, aniž byste si přečetli tento provozní návod a
pochopili jeho obsah.
• V případě nedodržování této výstrahy může dojít k poranění pracovníka prodejního servisu,
obslužného personálu nebo pacientů vlivem elektrického proudu, respektive vlivem
mechanických či jiných rizik.
ADVARSEL
Denne servicemanual findes kun på engelsk.
• Hvis en kundes tekniker har brug for et andet sprog end engelsk, er det kundens ansvar at
sørge for oversættelse.
• Forsøg ikke at servicere udstyret uden at læse og forstå denne servicemanual.
• Manglende overholdelse af denne advarsel kan medføre skade på grund af elektrisk stød,
mekanisk eller anden fare for teknikeren, operatøren eller patienten.
(NL)
(ET)
Deze onderhoudshandleiding is enkel in het Engels verkrijgbaar.
• Als het onderhoudspersoneel een andere taal vereist , dan is de klant verantwoordelijk voor
de vertaling ervan.
• Probeer de apparatuur niet te onderhouden alvorens deze onderhoudshandleiding werd
geraadpleegd en begrepen is.
• Indien deze waarschuwing niet wordt opgevolgd, zou het onderhoudspersoneel, de
operator of een patiënt gewond kunnen raken als gevolg van een elektrische schok,
mechanische of andere gevaren.
IATUS
See teenindusjuhend on saadaval ainult inglise keeles
• Kui klienditeeninduse osutaja nõuab juhendit inglise keelest erinevas keeles, vastutab klient
tõlketeenuse osutamise eest .
• Ärge üritage seadmeid teenindada enne eelnevalt käesoleva teenindusjuhendiga tutvumist
ja sellest aru saamist .
• Käesoleva hoiatuse eiramine võib põhjustada teenuseosutaja, operaatori või patsiendi
vigastamist elektrilöögi, mehaanilise või muu ohu tagajärjel.
IWS Service Manual Addendum 2098189-001 Rev A 3
Page 7

(FI)
(FR)
(DE)
(EL)
VAROITUS
Tämä huolto-ohje on saatavilla vain englanniksi.
• Jos asiakkaan huoltohenkilöstö vaatii muuta kuin englanninkielistä materiaalia, tarvittavan
käännöksen hankkiminen on asiakkaan vastuulla.
• Älä yritä korjata laitteistoa ennen kuin olet varmasti lukenut ja ymmärtänyt tämän huolto-
ohjeen.
• Mikäli tätä varoitusta ei noudateta, seurauksena voi olla huoltohenkilöstön, laitteiston
käyttäjän tai potilaan vahingoittuminen sähköiskun, mekaanisen vian tai muun
vaaratilanteen vuoksi.
ATTENTION
Ce manuel d’installation et de maintenance est disponible uniquement en anglais.
• Si le technicien d’un client a besoin de ce manuel dans une langue autre que l’anglais, il
incombe au client de le faire traduire.
• Ne pas tenter d’intervenir sur les équipements tant que ce manuel d’installation et de
maintenance n’a pas été consulté et compris.
• Le non-respect de cet avertissement peut entraîner chez le technicien, l’opérateur ou le
patient des blessures dues à des dangers électriques, mécaniques ou autres.
WARNUNG
Diese Serviceanleitung existiert nur in englischer Sprache.
• Falls ein fremder Kundendienst eine andere Sprache benötigt , ist es Aufgabe des Kunden
für eine entsprechende Übersetzung zu sorgen.
• Versuchen Sie nicht diese Anlage zu warten, ohne diese Serviceanleitung gelesen und
verstanden zu haben.
• Wird diese Warnung nicht beachtet , so kann es zu Verletzungen des
Kundendiensttechnikers, des Bedieners oder des Patienten durch Stromschläge,
mechanische oder sonstige Gefahren kommen.
ΠΡΟΕΙΔΟΠΟΙΗΣΗ
Το παρόν εγχειρίδιο σέρβις διατίθεται μόνο στα αγγλικά.
• Εάν ο τεχνικός σέρβις ενός πελάτη απαιτεί το παρόν εγχειρίδιο σε γλώσσα εκτός των
αγγλικών, αποτελεί ευθύνη του πελάτη να παρέχει τις υπηρεσίες μετάφρασης.
• Μην επιχειρήσετε την εκτέλεση εργασιών σέρβις στον εξοπλισμό αν δεν έχετε
συμβουλευτεί και κατανοήσει το παρόν εγχειρίδιο σέρβις.
• Αν δεν προσέξετε την προειδοποίηση αυτή, ενδέχεται να προκληθεί τραυματισμός στον
τεχνικό σέρβις, στο χειριστή ή στον ασθενή από ηλεκτροπληξία, μηχανικούς ή άλλους
κινδύνους.
4 2098189-001 Rev A IWS Service Manual Addendum
Page 8

AÐVÖRUN
(HU)
(IS)
(IT)
FIGYELMEZTETÉS
Ezen karbantartási kézikönyv kizárólag angol nyelven érhető el.
• Ha a vevő szolgáltatója angoltól eltérő nyelvre tart igényt , akkor a vevő felelőssége a
fordítás elkészíttetése.
• Ne próbálja elkezdeni használni a berendezést , amíg a karbantartási kézikönyvben
leírtakat nem értelmezték.
• Ezen figyelmeztetés figyelmen kívül hagyása a szolgáltató, működtető vagy a beteg
áramütés, mechanikai vagy egyéb veszélyhelyzet miatti sérülését eredményezheti.
Þessi þjónustuhandbók er aðeins fáanleg á ensku.
• Ef að þjónustuveitandi viðskiptamanns þarfnast annas tungumáls en ensku, er það skylda
viðskiptamanns að skaffa tungumálaþjónustu.
• Reynið ekki að afgreiða tækið nema að þessi þjónustuhandbók hefur verið skoðuð og skilin.
• Brot á sinna þessari aðvörun getur leitt til meiðsla á þjónustuveitanda, stjórnanda eða
sjúklings frá raflosti, vélrænu eða öðrum áhættum.
AVVERTENZA
Il presente manuale di manutenzione è disponibile soltanto in lingua inglese.
• Se un addetto alla manutenzione richiede il manuale in una lingua diversa, il cliente è
tenuto a provvedere direttamente alla traduzione.
• Procedere alla manutenzione dell’apparecchiatura solo dopo aver consultato il presente
manuale ed averne compreso il contenuto.
• Il mancato rispetto della presente avvertenza potrebbe causare lesioni all’addetto alla
manutenzione, all’operatore o ai pazienti provocate da scosse elettriche, urti meccanici o
altri rischi.
(JA)
このサービスマニュアルには英語版しかありません。
• サービスを担当される業者が英語以外の言語を要求される場合、翻訳作業はその業者
の 責任で行うものとさせていただきます。
• このサービスマニュアルを熟読し理解せずに、装置のサービスを行わないでください
。
この警告に従わない場合、サービスを担当される方、操作員あるいは患者 さんが、感
•
電や機械的又はその他の危険により負傷する可能性があります。
경고
본 서비스 매뉴얼은 영어로만 이용하실 수 있습니다.
(KO)
• 고객의 서비스 제공자가 영어 이외의 언어를 요구할 경우, 번역 서비스를 제공하는
것은 고객의 책임입니다.
• 본 서비스 매뉴얼을 참조하여 숙지하지 않은 이상 해당 장비를 수리하려고 시도하지
마십시오.
• 본 경고 사항에 유의하지 않으면 전기 쇼크, 기계적 위험, 또는 기타 위험으로 인해
서비스 제공자, 사용자 또는 환자에게 부상을 입힐 수 있습니다.
IWS Service Manual Addendum 2098189-001 Rev A 5
Page 9

ĮSPĖJIMAS
OSTRZEŻENIE
(LV)
(LT)
(NO)
BRĪDINĀJUMS
Šī apkopes rokasgrāmata ir pieejama tikai angļu valodā.
• Ja klienta apkopes sniedzējam nepieciešama informācija citā valodā, klienta pienākums ir
nodrošināt tulkojumu.
• Neveiciet aprīkojuma apkopi bez apkopes rokasgrāmatas izlasīšanas un saprašanas.
• Šī brīdinājuma neievērošanas rezultātā var rasties elektriskās strāvas trieciena, mehānisku
vai citu faktoru izraisītu traumu risks apkopes sniedzējam, operatoram vai pacientam.
Šis eksploatavimo vadovas yra tik anglų kalba.
• Jei kliento paslaugų tiekėjas reikalauja vadovo kita kalba – ne anglų, suteikti vertimo
paslaugas privalo klientas.
• Nemėginkite atlikti įrangos techninės priežiūros, jei neperskaitėte ar nesupratote šio
eksploatavimo vadovo.
• Jei nepaisysite šio įspėjimo, galimi paslaugų tiekėjo, operatoriaus ar paciento sužalojimai
dėl elektros šoko, mechaninių ar kitų pavojų.
ADVARSEL
Denne servicehåndboken finnes bare på engelsk.
• Hvis kundens serviceleverandør har bruk for et annet språk, er det kundens ansvar å sørge
for oversettelse.
• Ikke forsøk å reparere utstyret uten at denne servicehåndboken er lest og forstått .
• Manglende hensyn til denne advarselen kan føre til at serviceleverandøren, operatøren
eller pasienten skades på grunn av elektrisk støt , mekaniske eller andre farer.
(PL)
(PT-BR)
Niniejszy podręcznik serwisowy dostępny jest jedynie w języku angielskim.
• Jeśli serwisant klienta wymaga języka innego niż angielski, zapewnienie usługi tłumaczenia
jest obowiązkiem klienta.
• Nie próbować serwisować urządzenia bez zapoznania się z niniejszym podręcznikiem
serwisowym i zrozumienia go.
• Niezastosowanie się do tego ostrzeżenia może doprowadzić do obrażeń serwisanta,
operatora lub pacjenta w wyniku porażenia prądem elektrycznym, zagrożenia
mechanicznego bądź innego.
AVISO
Este manual de assistência técnica encontra-se disponível unicamente em inglês.
• Se outro serviço de assistência técnica solicitar a tradução deste manual, caberá ao cliente
fornecer os serviços de tradução.
• Não tente reparar o equipamento sem ter consultado e compreendido este manual de
assistência técnica.
• A não observância deste aviso pode ocasionar ferimentos no técnico, operador ou
paciente decorrentes de choques elétricos, mecânicos ou outros.
6 2098189-001 Rev A IWS Service Manual Addendum
Page 10

ATENŢIE
UPOZORNENIE
(PT-PT)
(RO)
(RU)
ATENÇÃO
Este manual de assistência técnica só se encontra disponível em inglês.
• Se qualquer outro serviço de assistência técnica solicitar este manual noutro idioma, é da
responsabilidade do cliente fornecer os serviços de tradução.
• Não tente reparar o equipamento sem ter consultado e compreendido este manual de
assistência técnica.
• O não cumprimento deste aviso pode colocar em perigo a segurança do técnico, do
operador ou do paciente devido a choques eléctricos, mecânicos ou outros.
Acest manual de service este disponibil doar în limba engleză.
• Dacă un furnizor de servicii pentru clienţi necesită o altă limbă decât cea engleză, este de
datoria clientului să furnizeze o traducere.
• Nu încercaţi să reparaţi echipamentul decât ulterior consultării şi înţelegerii acestui manual
de service.
• Ignorarea acestui avertisment ar putea duce la rănirea depanatorului, operatorului sau
pacientului în urma pericolelor de electrocutare, mecanice sau de altă natură.
ОСТОРОЖНО!
Данное руководство по техническому обслуживанию представлено только на английском
языке.
(SR)
(SK)
• Если сервисному персоналу клиента необходимо руководство не на английском, а на
каком-то другом языке, клиенту следует самостоятельно обеспечить перевод.
• Перед техническим обслуживанием оборудования обязательно обратитесь к данному
руководству и поймите изложенные в нем сведения.
• Несоблюдение требований данного предупреждения может привести к тому, что
специалист по техобслуживанию, оператор или пациент получит удар электрическим
током, механическую травму или другое повреждение.
UPOZORENJE
Ovo servisno uputstvo je dostupno samo na engleskom jeziku.
• Ako klijentov serviser zahteva neki drugi jezik, klijent je dužan da obezbedi prevodilačke
usluge.
• Ne pokušavajte da opravite uređaj ako niste pročitali i razumeli ovo servisno uputstvo.
• Zanemarivanje ovog upozorenja može dovesti do povređivanja servisera, rukovaoca ili
pacijenta usled strujnog udara ili mehaničkih i drugih opasnosti.
Tento návod na obsluhu je k dispozícii len v angličtine.
• Ak zákazníkov poskytovateľ služieb vyžaduje iný jazyk ako angličtinu, poskytnutie
prekladateľských služieb je zodpovednosťou zákazníka.
• Nepokúšajte sa o obsluhu zariadenia, kým si neprečítate návod na obluhu a neporozumiete
mu.
• Zanedbanie tohto upozornenia môže spôsobiť zranenie poskytovateľa služieb, obsluhujúcej
osoby alebo pacienta elektrickým prúdom, mechanické alebo iné ohrozenie.
IWS Service Manual Addendum 2098189-001 Rev A 7
Page 11

VARNING
DİKKAT
(ES)
(SV)
(SL)
ATENCION
Este manual de servicio sólo existe en inglés.
• Si el encargado de mantenimiento de un cliente necesita un idioma que no sea el inglés, el
cliente deberá encargarse de la traducción del manual.
• No se deberá dar servicio técnico al equipo, sin haber consultado y comprendido este
manual de servicio.
• La no observancia del presente aviso puede dar lugar a que el proveedor de servicios, el
operador o el paciente sufran lesiones provocadas por causas eléctricas, mecánicas o de
otra naturaleza.
Den här servicehandboken finns bara tillgänglig på engelska.
• Om en kunds servicetekniker har behov av ett annat språk än engelska, ansvarar kunden
för att tillhandahålla översättningstjänster.
• Försök inte utföra service på utrustningen om du inte har läst och förstår den här
servicehandboken.
• Om du inte tar hänsyn till den här varningen kan det resultera i skador på serviceteknikern,
operatören eller patienten till följd av elektriska stötar, mekaniska faror eller andra faror.
OPOZORILO
Ta servisni priročnik je na voljo samo v angleškem jeziku.·
• Če ponudnik storitve stranke potrebuje priročnik v drugem jeziku, mora stranka zagotoviti
prevod.·
• Ne poskušajte servisirati opreme, če tega priročnika niste v celoti prebrali in razumeli.·
• Če tega opozorila ne upoštevate, se lahko zaradi električnega udara, mehanskih ali drugih
nevarnosti poškoduje ponudnik storitev, operater ali bolnik.
(TR)
Bu servis kılavuzunun sadece ingilizcesi mevcuttur.
• Eğer müşteri teknisyeni bu kılavuzu ingilizce dışında bir başka lisandan talep ederse, bunu
tercüme ettirmek müşteriye düşer.
• Servis kılavuzunu okuyup anlamadan ekipmanlara müdahale etmeyiniz.
• Bu uyarıya uyulmaması, elektrik, mekanik veya diğer tehlikelerden dolayı teknisyen,
operatör veya hastanın yaralanmasına yol açabilir.
8 2098189-001 Rev A IWS Service Manual Addendum
Page 12

•
•
•
•
•
Maintenance Schedule
Perform this maintenance procedure annually to make sure the two heater reflector screws (front
and back) are tight. This schedule recommends the minimum frequencies. Always follow hospital and
local regulations.
WARNING: Failure to perform this maintenance per recommended frequency can result
in patient harm or injury.
Required Tools
One long Phillips screw driver
One 5/16” wrench or nut driver
One small step stool
One 5/64” hex wrench
One 3/8” nut driver
Heater Reflector Screws Maintenance Check Procedure
1. Turn off the Infant Warmer System and unplug its power cord from the outlet. Allow the heat
tube to cool down before proceeding with the next steps.
2. Locate the warmer unit serial number on the back of controller.
3. For units that have serial numbers starting with the prefix HAK, AAN, HAJ, or HCA (see Figure 1):
a. Use a 5/64” hex wrench to remove the four mounting screws for the front cover plate, and
b. Use a 3/8” nut driver to remove the four mounting screws for the front heater housing cap,
c. Proceed to step 5.
WARNING: Disconnect the power to the warmer and allow the heat tube to cool to
avoid the possibility of a burn.
remove the plate.
and remove the cap.
Figure 1: Heater Head Assembly – Units with HAK, AAN, HAJ, or HCA Serial Numbers
IWS Service Manual Addendum 2098189-001 Rev A 9
Page 13

4. For units that have serial numbers starting with the prefix HCC (see Figure 2):
a. Use a Phillips screw driver to remove the screw and lock washer from the top of the
cushioned heater head.
b. Use a Phillips screw driver to remove the screw and alarm light lens from the bottom of the
heater head. Now the cushioned heater head can be pulled off the front of the unit.
c. Use a wrench to remove the four screws from the support plate end cap, and remove the
plate.
d. Proceed to step 5.
Figure 2: Heater Head Assembly – Units with HCC Serial Numbers
5. Pull out the ventilation plate on the top of the heater housing assembly (Figure 3).
10 2098189-001 Rev A IWS Service Manual Addendum
Figure 3: Removing the Ventilation Plate
Page 14

Figure 4: Locating the Front Reflector Screw Nut
Figure 5: Locating the Back Reflector Screw Nut
6. Locate the two reflector screw nuts (front and back) on the top of the heater housing assembly.
(See Figure 4 and Figure 5).
7. Pass the tip of a long Phillips screw driver through the heater grille at an angle to engage the
front reflector screw, and then use a wrench or nut driver to loosen the nut about one turn
(Figure 6). Use care not to remove from screw. (You might need a small step stool to reach to the
top of the heater assembly.)
Figure 6: Engaging the Front Reflector Screw
8. While keeping the screw engaged with the screw driver, use your fingers to tighten the nut until it
is snug.
9. Use a 5/16” wrench or nut driver to tighten the nut about an additional one-third of a turn
(Figure 7).
Figure 7: Tightening the Nut in the Front Reflector Screw
IWS Service Manual Addendum 2098189-001 Rev A 11
Page 15

10. Pass the tip of a long Phillips screw driver through the heater grille at an angle to engage the
Side Tabs
Side Tabs
back reflector screw, and then use a wrench or nut driver to loosen the nut about one turn
(Figure 8). Use care not to remove from screw. (You might need a small step stool to reach to the
top of the heater assembly.)
Figure 8: Engaging the Back Reflector Screw
11. While keeping the screw engaged with the screw driver, use your fingers to tighten the nut until it
is snug.
12. Use a 5/16” wrench or nut driver to tighten the nut about an additional one-third of a turn.
13. Re-install the ventilation plate into the system. Push the side tabs down on the ventilation plate, if
necessary, for proper installation (Figure 9).
Figure 9: Re-installing the Ventilation Plate
14. Reverse step 3 or step 2 to re-install the heater head plate and cover.
NOTE: If the heater reflector screw or nut is lost, you can order the reflector screw by GE part number
0140-6124-114 and the nut by GE part number 6600-0382-400.
12 2098189-001 Rev A IWS Service Manual Addendum
Page 16

IWS Service Manual Addendum 2098189-001 Rev A 13
Page 17

World Headquarters
EC Representative
Tel 1 800 345 2700
Europe, Middle East , Africa
Germany
Fax +358 9 146 3310
Service 0800 4343258
Latin America Representatives
Asia Representative
Fax + (8621) 38777402
Brazil Only
Fax +55 11 3053 2573
South Eastern Asia (65) 6277 3444
Türkiye’ye İthalatçi
Addendum
Infant Warmer System
Maintenance Check
Printed
© 2016 General Electric Company
2098189-001
USA
GE Healthcare
9900 West Innovation Drive
Wauwatosa, WI 53226-4856
USA
GE Healthcare
P.O. Box 900
FIN-00031 GE
Finland
Tel +358 10 39411
GE Healthcare
3350 SW 148 Avenue
Suite 301
Miramar, Florida, 33027
USA
Tel + 1 954 744 5600
GE Healthcare Clinical Systems Equipamentos Médicos Ltda
Av. Paulista, 37 - 13º andar
CEP: 01311-902 - Cerqueira César
São Paulo, SP - Brasil
Tel +55 11 3053 2500
GE Medical Systems SCS
283 Rue de la miniere
78530 BUC - FRANCE
GE Medical Systems Information Technologies GmbH
Munzinger Str. 3-5
79111 Freiburg
Tel. 49 761 4543 570
Fax 49 761 4543 571
GE Healthcare
Shanghai GE (China) Hi-tech Park
No1 Huatuo Road, Zhangjiang Hi-tech Park Pudong, Shanghai,
P.R.China 201203
上海GE中国科技园
地址:中国上海市浦东张江高科技园华佗路1号, 201203
Tel + (8621) 38777888
Australia 1300 722 229
China 800 810 8188
India 1 800 425 7255
Korea (02) 1544 4564
GE Medical Systems Türkiye Ltd. Şti
Esentepe Mah. Harman Sok. No: 8
34394 Sisli-Istanbul
Türkiye
Ohmeda Medical,
a Division of Datex-Ohmeda, Inc.,
a General Electric Company
8880 Gorman Road
Laurel MD 20723
Heater Reflector Screws
in USA
Revision A
 Loading...
Loading...