GE 2169360, 2236420, 2275938 Technical Manual

Technical
Publications
Direction 2173229-100
Revision 6
AMX-4+ Schematics
GE Medical Systems
(Model 2169360, 2236420 &
2275938 Series)
Copyrighte 1996, 1997, 1999, 2000, 2004 By General Electric Co.
Operating Documentation


AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
D THIS SERVICE MANUAL IS AVAILABLE IN ENGLISH ONLY.
WARNING
AVERTISSEMENT
D IF A CUSTOMER'S SERVICE PROVIDER REQUIRES A LANGUAGE OTHER
THAN ENGLISH, IT IS THE CUSTOMER'S RESPONSIBILITY TO PROVIDE
TRANSLATION SERVICES.
D DO NOT ATTEMPT TO SERVICE THE EQUIPMENT UNLESS THIS SERVICE
MANUAL HAS BEEN CONSULTED AND IS UNDERSTOOD.
D FAILURE TOHEED THIS WARNING MAY RESULT IN INJURYTO THE SERVICE
PROVIDER, OPERATOROR PATIENT FROM ELECTRIC SHOCK, MECHANICAL
OR OTHER HAZARDS.
D CE MANUEL DE MAINTENANCE N'EST DISPONIBLE QU'EN ANGLAIS.
D SI LE TECHNICIEN DU CLIENT A BESOIN DE CE MANUEL DANS UNE AUTRE
LANGUE QUE L'ANGLAIS, C'EST AU CLIENT QU'IL INCOMBE DE LE FAIRE
TRADUIRE.
D NE PAS TENTER D'INTERVENTION SUR LES ÉQUIPEMENTS TANT QUE LE
MANUEL SERVICE N'A PAS ÉTÉ CONSULTÉ ET COMPRIS.
D LE NONĆRESPECT DE CET AVERTISSEMENT PEUT ENTRAÎNER CHEZ LE
TECHNICIEN, L'OPÉRATEUR OU LE PATIENT DES BLESSURES DUES À DES
DANGERS ÉLECTRIQUES, MÉCANIQUES OU AUTRES.
WARNUNG
AVISO
D DIESES KUNDENDIENST-HANDBUCH EXISTIERT NUR IN ENGLISCHER
SPRACHE.
D FALLS EIN FREMDER KUNDENDIENST EINE ANDERE SPRACHE BENÖTIGT,
IST ES AUFGABE DES KUNDEN FÜR EINE ENTSPRECHENDE ÜBERSETZUNG
ZU SORGEN.
D VERSUCHEN SIE NICHT, DAS GERÄT ZU REPARIEREN, BEVOR DIESES
KUNDENDIENST-HANDBUCH NICHT ZU RATE GEZOGEN UND VERSTANDEN
WURDE.
D WIRD DIESE WARNUNG NICHT BEACHTET, SO KANN ES ZU VERLETZUNGEN
DES KUNDENDIENSTTECHNIKERS, DES BEDIENERS ODER DES PATIENTEN
DURCH ELEKTRISCHE SCHLÄGE, MECHANISCHE ODER SONSTIGE
GEFAHREN KOMMEN.
D ESTE MANUAL DE SERVICIO SÓLO EXISTE EN INGLÉS.
D SI ALGÚN PROVEEDOR DE SERVICIOS AJENO A GEMS SOLICITA UN IDIOMA
QUE NO SEA EL INGLÉS, ES RESPONSABILIDAD DEL CLIENTE OFRECER UN
SERVICIO DE TRADUCCIÓN.
D NO SE DEBERÁ DAR SERVICIO TÉCNICO AL EQUIPO, SIN HABER
CONSULTADO Y COMPRENDIDO ESTE MANUAL DE SERVICIO.
D LA NO OBSERVANCIA DEL PRESENTE AVISO PUEDE DAR LUGAR A QUE EL
PROVEEDOR DE SERVICIOS, EL OPERADOR O EL PACIENTE SUFRAN
LESIONESPROVOCADASPORCAUSASELÉCTRICAS,MECÁNICASODEOTRA
NATURALEZA.
i
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
D ESTE MANUAL DE ASSISTÊNCIA TÉCNICA SÓ SE ENCONTRA DISPONÍVEL
ATENÇÃO
AVVERTENZA
EM INGLÊS.
D SE QUALQUER OUTRO SERVIÇO DE ASSISTÊNCIA TÉCNICA, QUE NÃO A
GEMS, SOLICITAR ESTES MANUAIS NOUTRO IDIOMA, É DA
RESPONSABILIDADE DO CLIENTE FORNECER OS SERVIÇOS DE TRADUÇÃO.
D NÃO TENTE REPARAR O EQUIPAMENTO SEM TER CONSULTADO E
COMPREENDIDO ESTE MANUAL DE ASSISTÊNCIA TÉCNICA.
D O NÃO CUMPRIMENTO DESTE AVISO PODE POR EM PERIGO A SEGURANÇA
DO TÉCNICO, OPERADOR OU PACIENTE DEVIDO A` CHOQUES ELÉTRICOS,
MECÂNICOS OU OUTROS.
D IL PRESENTE MANUALE DI MANUTENZIONE È DISPONIBILE SOLTANTO IN
INGLESE.
D SE UN ADDETTO ALLA MANUTENZIONE ESTERNO ALLA GEMS RICHIEDE IL
MANUALE IN UNA LINGUA DIVERSA, IL CLIENTE ÈTENUTO A PROVVEDERE
DIRETTAMENTE ALLA TRADUZIONE.
D SI PROCEDA ALLA MANUTENZIONE DELL'APPARECCHIATURA SOLO DOPO
AVER CONSULTATO IL PRESENTE MANUALE ED AVERNE COMPRESO IL
CONTENUTO.
D NON TENERE CONTO DELLA PRESENTE AVVERTENZA POTREBBE FAR
COMPIERE OPERAZIONI DA CUI DERIVINO LESIONI ALL'ADDETTO ALLA
MANUTENZIONE, ALL'UTILIZZATORE ED AL PAZIENTE PER
FOLGORAZIONE ELETTRICA, PER URTI MECCANICI OD ALTRI RISCHI.
ii

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
Direction 2173229-100
Revision 6
AMX-4+ Schematics
(Model 2169360, 2236420 & 2275938 Series)
IMPORTANT! . . . XĆRAY PROTECTION
XĆray equipment if not properly
used may cause injury. AccordĆ
ingly, the instructions herein
contained should be thoroughly
read and understood by everyĆ
one who will use the equipment
before you attempt to place this
equipment in operation. The
General Electric Company, MediĆ
cal Systems Group, will be glad
to assist and cooperate in placing
this equipment in use.
Although this apparatus incorpoĆ
rates a high degree of protection
against xĆradiation other than the
useful beam, no practical design of
equipment can provide complete
protection. Nor can any practical
design compel the operator to take
adequate precautions to prevent
the possibility of any persons
carelessly exposing themselves or
others to radiation.
It is important that everyone having
anything to do with xĆradiation be
properly trained and fully acĆ
quainted with the recommendaĆ
tions of the National Council on
Radiation Protection and MeasureĆ
ments as published in NCRP
Reports available from NCRP PubliĆ
cations, 7910 Woodmont Avenue,
Room 1016, Bethesda, Maryland
20814, and of the International
Commission on Radiation ProtecĆ
iii
tion, and take adequate steps to
protect against injury.
The equipment is sold with the
understanding that the General
Electric Company, Medical SysĆ
tems Group, its agents, and
representatives have no responsiĆ
bility for injury or damage which
may result from improper use of the
equipment.
Various protective material and
devices are available. It is urged
that such materials or devices be
used.
CAUTION: United States Federal
law restricts this device to use by or
on the order of a physician.

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
THIS PAGE INTENTIONALLY LEFT BLANK.
iv

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
If you have any comments, suggestions or corrections to the information in this document,
please write them down, include the document title and document number, and send them to:
GENERAL ELECTRIC COMP ANY
MEDICAL SYSTEMS
MANAGER – INFORMA TION INTEGRATION, AMERICAS W–622
P.O. BOX 414
MIL WAUKEE, WI 53201–0414
CERTIFIED ELECTRICAL CONTRACTOR STATEMENT
All electrical installations that are
preliminary to positioning of the
equipment at the site prepared for
the equipment shall be performed by
licensed electrical contractors. In
addition, electrical feeds into the
Power Distribution Unit shall be
performed by licensed electrical
contractors. Other connections beĆ
tween pieces of electrical equipment,
calibrations, and testing shall be
DAMAGE IN TRANSPORTATION
All packages should be closely
examined at time of delivery. If
damage is apparent, have notation
damage in shipment" written on
all copies of the freight or express
bill before
signed for" by a General Electric
representative or a hospital receivĆ
ing agent. Whether noted or
concealed, damage MUST be
reported to the carrier immediately
delivery is accepted or
performed by qualified GE Medical
personnel. The products involved
(and the accompanying electrical
installations) are highly sophistiĆ
cated, and special engineering
competence is required. In performĆ
ing all electrical work on these
products, GE will use its own
specially trained field engineers. All
of GE's electrical work on these
products will comply with the
upon discovery, or in any event,
within 14 days after receipt, and the
contents and containers held for
inspection by the carrier. A transĆ
portation company will not pay a
claim for damage if an inspection is
not requested within this 14 day
period.
Call Traffic and Transportation,
Milwaukee, WI (414) 827-3449Ă/
requirements of the applicable elecĆ
trical codes.
The purchaser of GE equipment
shall only utilize qualified personnel
(i.e., GE's field engineers, personnel
of thirdĆparty service companies with
equivalent training, or licensed elecĆ
tricians) to perform electrical servicĆ
ing on the equipment.
8*285-3449 immediately after
damage is found. At this time be
ready to supply name of carrier,
delivery date, consignee name,
freight or express bill number, item
damaged and extent of damage.
Complete instructions regarding
claim procedure are found in
Section S" of the Policy &
Procedure Bulletins.
6/17/94
v

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
THIS PAGE INTENTIONALLY LEFT BLANK.
vi

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
TABLE OF CONTENTS
SECTION TITLE PAGE
1 INTRODUCTION
Identification 1-1................................................
General 1-2.....................................................
Assembly Designators 1-2.........................................
2 AMX-4 OVERALL WIRING 2-1....................................
AMX-4 OVERALL WIRING 2115090SCH 2-3.........................
3 AMX1 A1 A1 DISPLAY CONTROLLER 46-264982G1 3-1..............
AMX1 A1 A1 DISPLAY CONTROLLER 46-264982-S 3-3..............
4 AMX1 A1 A2 DISPLAY 46-232832G1 4-1.............................
AMX1 A1 A2 DISPLAY 46-232832-S 4-3.............................
5 AMX1 A2 A1 CONTROLLER 46-264974G2 5-1.......................
AMX1 A2 A1 CONTROLLER 46-264974-S 5-3.......................
6 AMX1 A3 A1 BATTERY CHARGER 46-288786G2 6-1.................
AMX1 A3 A1 BATTERY CHARGER 46-288786-S 6-3.................
7 AMX1 A3 A2 ROTOR CONTROLLER 46-232786G2 7-1...............
AMX1 A3 A2 ROTOR CONTROLLER 46-232786-S 7-3...............
8 AMX1 A4 A1 1 KHZ INVERTER 46-288764G1 8-1.....................
AMX1 A4 A1 1 KHZ INVERTER 46-288764-S 8-3....................
9 AMX1 A4 A2 KVP/FIL CONTROL 46-264986G1 9-1...................
AMX1 A4 A2 KVP/FIL CONTROL 46-264986-S 9-3...................
10 AMX1 A4 A3 CAPACITOR BOARD 46-288504G1 10-1..................
AMX1 A4 A3 CAPACITOR BOARD 46-288504-S 10-3..................
11 AMX1 A5 A1 DRIVE CONTROLLER 46-232834G1 11-1................
AMX1 A5 A1 DRIVE CONTROLLER 46-232834-S 11-3................
12 AMX1 A5 A2 DRIVE POWER AMPLIFIER 46-232836G2 12-1...........
AMX1 A5 A2 DRIVE POWER AMPLIFIER 46-232836-S 12-3...........
13 AMX1 A6 A1 HIGH VOLTAGE TRANSFORMER 46-270954G1 13-1.....
AMX1 A6 A1 HIGH VOLTAGE TRANSFORMER 46-270954-S 13-3.....
14 AMX1 A2 S1 HALL EFFECT TUBE PARKED
SENSOR 46-288962G1 14-1..........................................
AMX1 A2 S1 HALL EFFECT TUBE PARKED
SENSOR 46-288962-S 14-3..........................................
AMX1 A2 S1 HALL EFFECT TUBE PARKED
SENSOR 2173060SCH 14-4...........................................
15 AMX2 A3 A1 BATTERY SENSE CIRCUIT 46-321370G1 15-1............
AMX2 A3 A1 BATTERY SENSE CIRCUIT 46-321370-S 15-3............
AMX2 A3 A1 BATTERY SENSE CIRCUIT 2334738 15-5.................
AMX2 A3 A1 BATTERY SENSE CIRCUIT 2334738SCH 15-7.............
16 RELAY CHASSIS ASSEMBLY 2178054 16-1............................
17 HANDSWITCH WIRING 46-270800G5 17-1...........................
vii

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
THIS PAGE INTENTIONALLY LEFT BLANK.
viii

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
REVISION HISTORY
REV DATE REASON FOR CHANGE
0 Dec 13. 1996 Initial release.
1 Mar. 7 1997 Cnanges to Section 2.
2 June 24 1997 Added Section 16 (Relay Chassis Assembly); added 2173060SCH to Section 14;
corrected 2115090sch in Section 2.
3 Aug. 15, 1997 Updated schematic 2115090sch. High Impact Inspection.
4 Apr. 12, 1999 Updated schematics 2115090sch, 46-232786-s.
5 Nov. 8, 2000 Added 2275938 series. Updated schematics.
6 Jan, 30, 2004 Added Battery Sense board 2334738
LIST OF EFFECTIVE PAGES
PAGE REVISION PAGE REVISION PAGE REVISION
NUMBER NUMBER NUMBER NUMBER NUMBER NUMBER
Title Page 6
i thru x 6
1-1 and 1-4 6
2-1 thru 2-12 6
3-1 thru 3-4 6
4-1 thru 4-4 6
5-1 thru 5-10 6
6-1 thru 6-6 6
7-1 and 7-4 6
8-1 and 8-4 6
9-1 thru 9-6 6
10-1 thru 10-4 6
15-1 thru 15-8 6
16-1 thru 16-2 6
17-1 thru 17-2 6
Back Page -
11-1 thru 11-6 6
12-1 thru 12-6 6
13-1 thru 13-4 6
14-1 thru 14-4 6
ix

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
THIS PAGE INTENTIONALLY LEFT BLANK.
x

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
AMX-4+
SCHEMATICS
SECTION 1
INTRODUCTION
1-1 Indentification
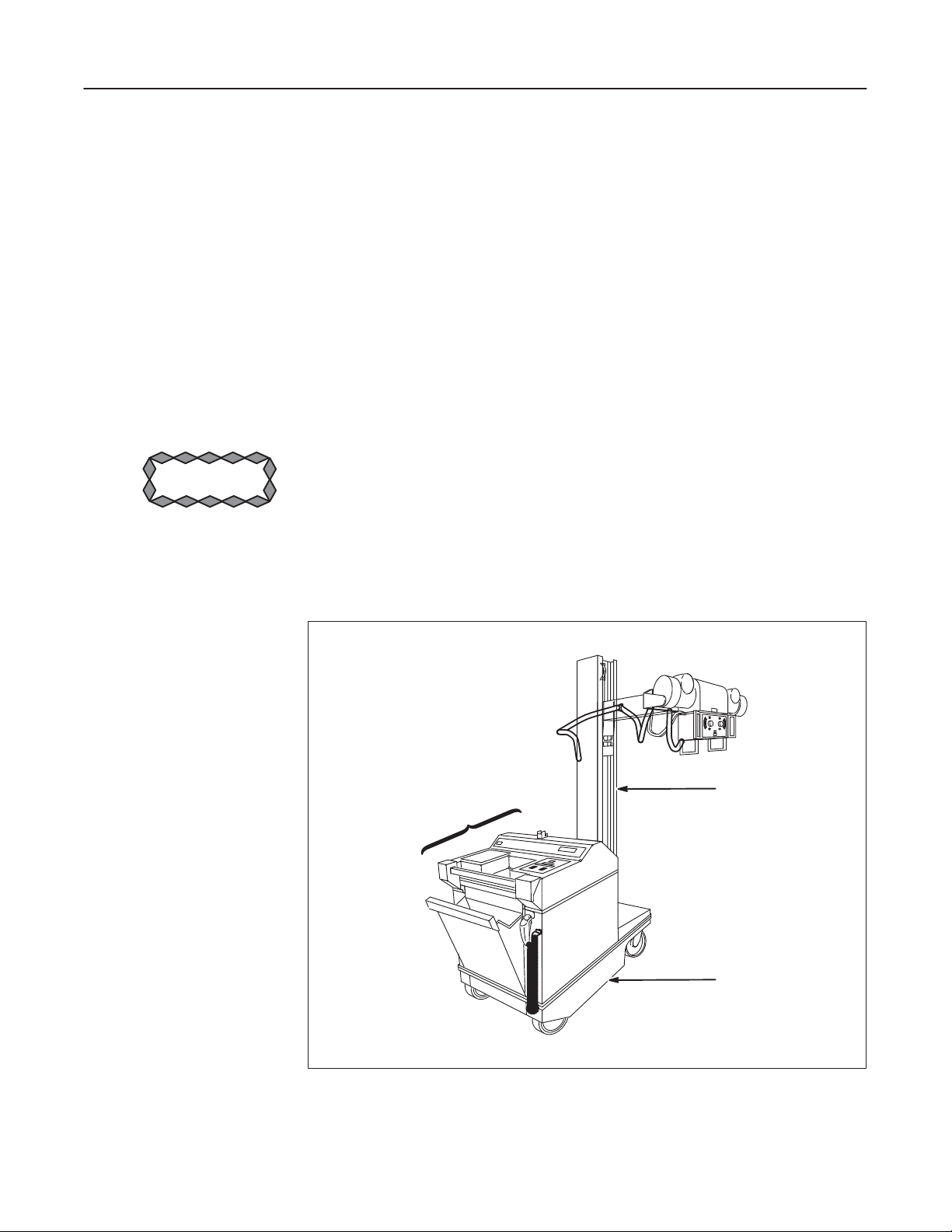
See Illustration 1. The AMX-4+ is identified by Model Number on the rating plate
located on the top cover. Model part and catalog numbers are identified in Table 1.
This direction contains schematic diagrams for these units.
TABLE 1
AMX-4+ MODELS
DESCRIPTION PART NUMBER
DOMESTIC 2169360-7 A0659F 2236420-7 & 2275938-7 A0659JF
DOMESTIC, AEC 2169360-8 A0659FA 2236420-8 & 2275938-8 A0659JG
DOMESTIC, TECH SWITCH 2169360-9 A0659FC 2236420-9 & 2275938-9 A0659JH
DOMESTIC, AEC, TECH SWITCH 2169360-10 A0659FB 2236420-10 & 2275938-10 A0659JJ
IEC, EMC 2169360 A0659A 2236420 & 2275938 A0659J
IEC, EMC, AEC 2169360-2 A0659AA 2236420-2 & 2275938-2 A0659JA
IEC, EMC, TECH SWITCH 2169360-3 A0659AB 2236420-3 & 2275938-3 A0659JB
IEC, EMC, AEC, TECH SWITCH 2169360-4 A0659AC 2236420-4 & 2275938-4 A0659JC
JAPAN 2169360-5 A0659C 2236420-5 & 2275938-5 A0659JD
JAPAN SHORT COLUMN 2169360-6 A0659D 2236420-6 & 2275938-6 A0659JE
ILLUSTRATIONă1
AMX-4+ IDENTIFICATION
CATALOG
NUMBER
PART NUMBER
CATALOG
NUMBER
1-1
RATING
PLATE
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
1-2 General
The AMX-4+ contains operating safeguards providing maximum safety. Before
servicing, be certain proper operating procedures are being used. Refer to Direction
2166913-100 AMX-4+ Operation or to Direction 2166911-100 AMX-4+
International Operation for proper operating procedures.
Satisfactory equipment performance requires the use of service personnel specially
trained on x-ray apparatus. The GE Medical Systems, is responsible for the effects on
safety, reliability, and performance only if the following conditions are met:
The electrical wiring of the relevant rooms complies with all national and local
codes.
All assembly operations, extensions, re-adjustments, modifications, or repairs
are carried out by GE Medical Systems, authorized service representatives.
The equipment is used in accordance with the instructions for use. Refer to
Direction 2166913-100 AMX-4+ Operation or to Direction 2166911-100
AMX-4+ International Operation for proper operating procedures.
CAUTION
1-3 Assembly Designators
ILLUSTRATIONă2
ASSEMBLY DESIGNATORS
Only trained and qualified personnel should be permitted access to the internal parts
of this equipment.
AMX1
GENERATOR
AMX3
TUBE COLUMN
1-2
AMX2
BASE

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
Schematics and Terminal Strips are arranged in Assembly Designator order.
AsĆsembly Designators are codes which simplify component identification. The code
is a convenient shorthand which defines each assembly or component. Assembly
Designators are derived from the location of components and assemblies within a
major assembly. Refer to Illustration 2 and the following list for AMX-4+ codes:
AMX1 Generator
AMX1 A1 Top Cover
AMX1 A2 Top Deck (under cover)
AMX1 A3 Left Side
AMX1 A4 Right Side
AMX1 A5 Rear
AMX1 A6 High Voltage Transformer
AMX2 Base
AMX2 A1 Rear
AMX2 A2 Front
AMX2 A3 Battery
AMX3 Column
AMX3 A1 Column
AMX3 A2 Tube Arm
AMX3 A3 Tube
AMX3 A4 Collimator
1-3

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
THIS PAGE INTENTIONALLY LEFT BLANK.
1-4

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
SECTION 2
AMX-4 OVERALL WIRING
MODEL 2169360 SERIES:
2169360 2169360-6
2169360-2 2169360-7
2169360-3 2169360-8
2169360-4 2169360-9
2169360-5 2169360-10
MODEL 2236420 SERIES:
2236420 2236420-6
2236420-2 2236420-7
2236420-3 2236420-8
2236420-4 2236420-9
2236420-5 2236420-10
MODEL 2275938 SERIES:
2275938 2275938-6
2275938-2 2275938-7
2275938-3 2275938-8
2275938-4 2275938-9
2275938-5 2275938-10
NO BOARD DRAWING
2-1

THIS PAGE INTENTIONALLY LEFT BLANK.
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-2
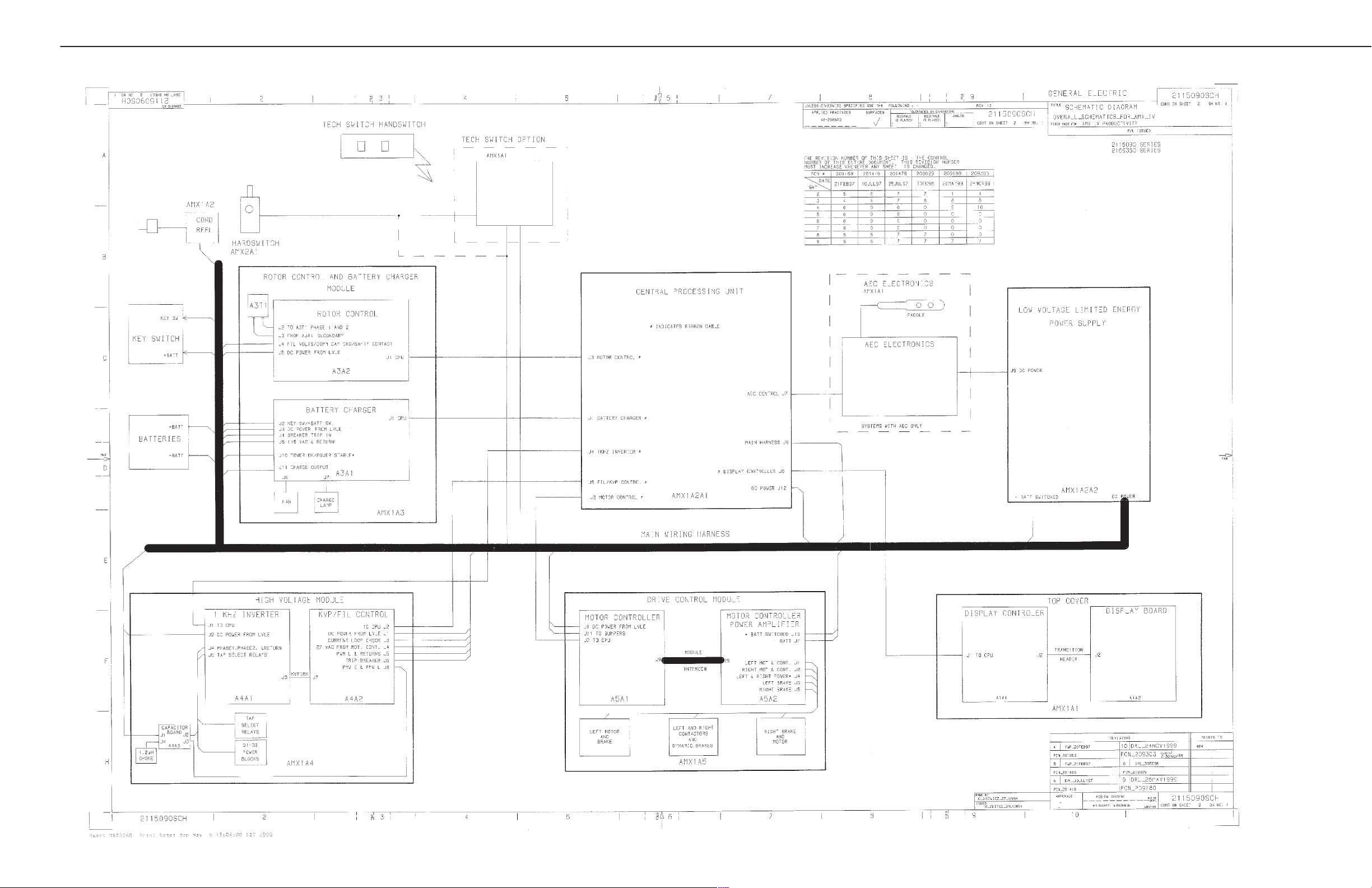
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-3

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-4
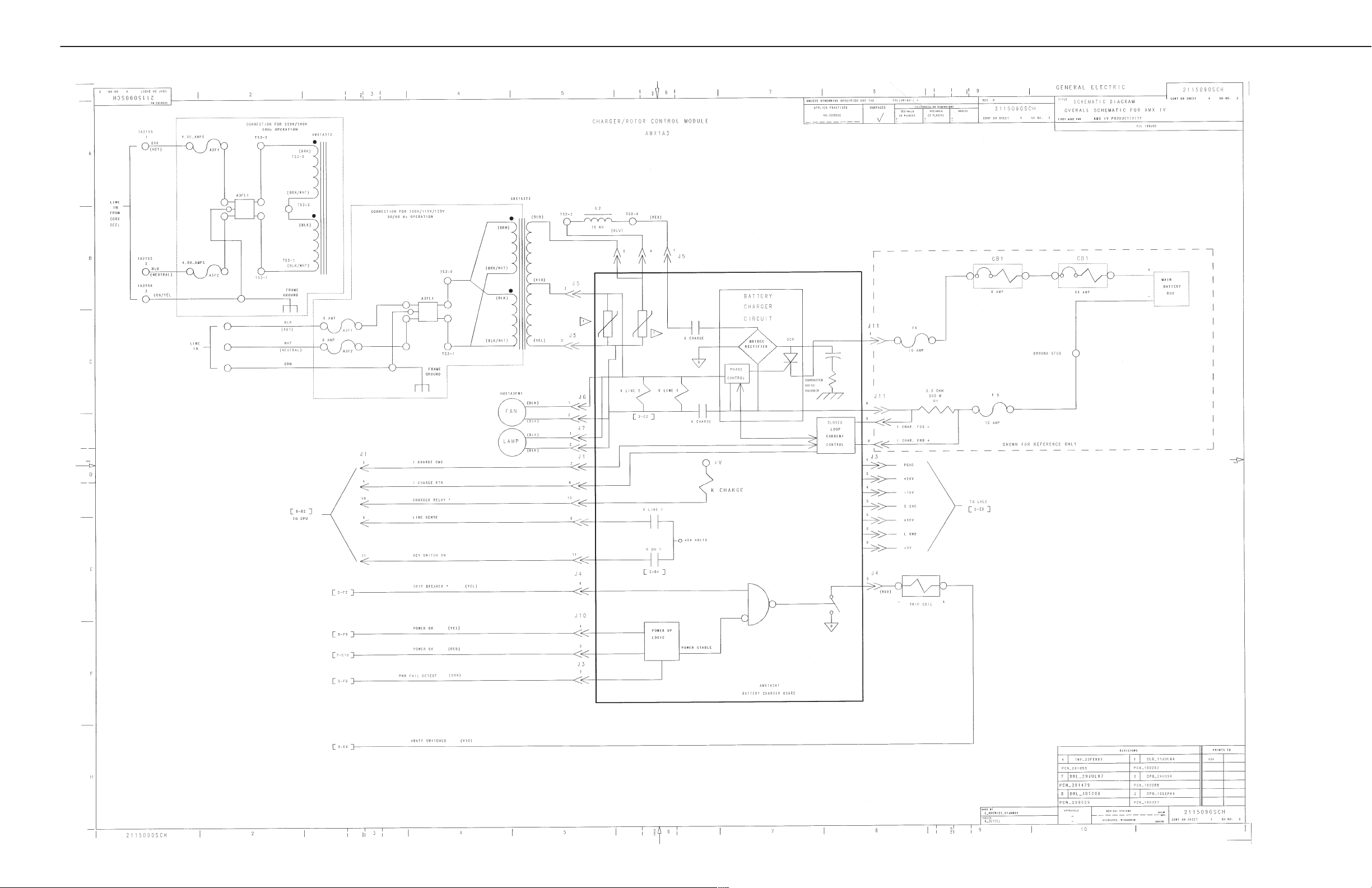
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-5

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-6
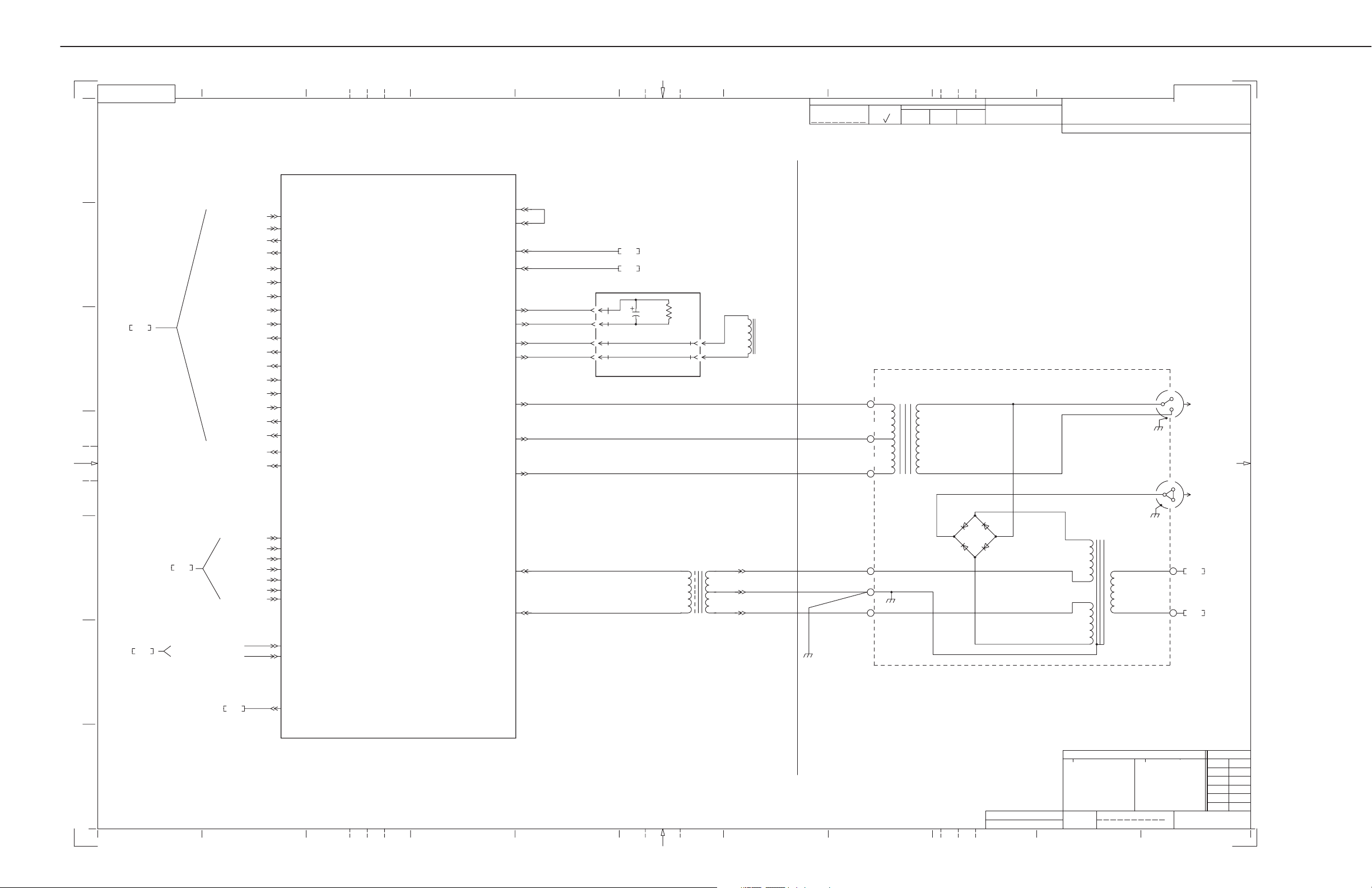
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
SH NO.
65
CONT ON SHEET
2115090SCH
DRAWING NO.
23
FOLD
6745
FOLD
APPLIED PRACTICES
46–208600
8
SURFACES
FOLLOWING : – UNLESS OTHERWISE SPECIFIED USE THE
TOLERANCES ON DIMENSIONS
DECIMALS DECIMALS
(2 PLACES) (3 PLACES)
++
–
–
9
FOLD
REV
0
ANGLES
+
–
2115090SCH
CONT ON SHEET
65
GENERAL ELECTRIC
TITLE
SCHEMATIC DIAGRAM
OVERALL_SCHEMATICS_FOR_AMX_IV
SH NO.
FIRST MADE FOR
AMX_IV_PRODUCTIVITY
P/L ISSUED
2115090SCH
CONT ON SHEET
SH NO.
65
A
FIL/KVP BOARD
J2
AMX1A4A2
MAS
3
4
5
6
21
7
8
11
12
9
10
22
17
19
15
25
26
13
14
LEAKAGE COMP +
LEAKAGE COMP –
LEAKAGE COMP FDBK +
B
TO CPU
8–F1
C
FOLD
D
LEAKAGE COMP FDBK –
FIL DMD +
FIL DMD –
KVP DMD +
KVP DMD –
FILAMENT FEEDBACK +
FILAMENT FEEDBACK –
X–RAY ON
2 KHZ PHASE 1
2 KHZ PHASE 2
16 KHZ CLK
FILAMENT
SHORTED
PRENEAT
+KVP DMN FDBK
–KVP DMN FDBK
J3
3
LOOP FOR CURRENT CHECK
4
J7
1
KVP FEEDBACK +
2
KVP FEEDBACK –
J8
1
FILT CAP+
2
FILT
CAP–
3
PWM E
4
PWM L
J5
1
(BRN)
2
(GRN)
3
(ORN)
(RED)
(WHT)
(BLK)
(BLK)
(VIO/W)
(VIO)
J3
3
5
2
6–B1
6–B1
AMX1A4
A3
X–RAY CONTROL MODULE
AMX1A4
R8
C6
1K
16000uF
2W
60V
J4
11
CAPACITOR
2
BOARD
PWM E
L3
1.2 MH
1
PWM RETURN
PWM1
PWM RETURN 2
XS1
XS2
HIGH VOLTAGE TRANSFORMER
AMX1A6
CATHODE
ANODE
L
C
C
L
S
S
TO RAY TUBE
FOLD
TO RAY TUBE
FILAMENT
XC
FILAMENT
J1
(YEL)
(BLU)
(YEL)
J4
J6
1
2
4
5
6
8
9
1
2
2
J3
1
2
MA+
MA–
AMX1A4T2
(RED) (BLU)
(RED/WHT)
J2
1
2
(WHT)
3
(BLK)
BLK
CHASSIS
BRASS STUD
GROUND
MA+
MA–
46–270954
PRIMARY VOLTS +
PRIMARY VOLTS –
P1
6–A7
P2
6–A11
PRINTS TOREVISIONS
404
POWER GROUND
+ 24 VDC
TO LVLE
E
4–C10
2–E5
27VAC SQUARE WAVE
27VAC SQUARE WAVE RTN
– 15 VDC
SIGNAL GROUND
+ 15 VDC
LOGIC GROUND
+ 5 VDC
F
TRIP BREAKER*
3–E3
H
2115090SCH
MADE BY
C_DREWICZ_27JUN94
ISSUED
N_ZETTEL
2
FOLD
4
653
FOLD
8
97
FOLD
APPROVALS
–
1010
MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN
DIV OR
DEPT.
LOCATION
CONT ON SHEET
2115090SCH
SH NO.
65
2-7

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
6
7
SH NO.
A
CONT. ON SHEET
2115090SCH
DRAWING NO.
32
FOLD
54 76
FOLD
–
5–E11
UNLESS OTHERWISE SPECIFIED USE THE FOLLOWING : –
APPLIED PRACTICES
46–208600
PRIMARY
VOLTS+
8
SURFACES
TOLERANCES ON DIMENSIONS
–
DECIMALSDECIMALS
(3 PLACES)(2 PLACES)
++
–
9
FOLD
0
REV
ANGLES
+
–
2115090SCH
CONT. ON SHEET
7
X–RAY CONTROL MODULE
.25
90W
R7
K6
37
37
K5
K4
37
AMX1A4
K3
36K236K136
GENERAL ELECTRIC
TITLE
SCHEMATIC DIAGRAM
OVERALL_SCHEMATICS_FOR_AMX_IV
SH NO.
6
FIRST MADE FOR
K4
84K584K684
CONT. ON SHEET
AMX_IV_PRODUCTIVITY
P/L ISSUED
5–F11
PRIMARY
VOLTS–
2115090SCH
SH NO.
7
6
3
K3
1KHZ BOARD
B
KVP FEEDBACK+
5–B5
KVP FEEDBACK–
C
TO CPU
8–E1
FOLD
D
(VIO/WHT)
(VIO)
1KHZ PHASE 1
1KHZ PHASE 2
STAT
STOP
TAP 1
TAP 2
TAP 3
TAP 4
TAP 5
TAP 6
TAP 1 FEEDBACK
TAP 2 FEEDBACK
TAP 3 FEEDBACK
TAP 4 FEEDBACK
TAP 5 FEEDBACK
TAP 6 FEEDBACK
1 KHZ READY
INVERTER OK
J3
1
2
J1
24
26
25
23
20
18
16
14
10
8
19
17
15
13
9
7
12
22
E
J2
POWER GROUND
+24 VDC
TO LVLE
2–E5
F
–15 VDC
SIGNAL GROUND
+15 VDC
LOGIC GROUND
+5 VDC
1
2
4
5
6
8
9
AMX1A4A1
J6
J4
J5
J5
J4
(BLK)
1
(BLK)
3
(WHT)
1
3
5
11
13
7
9
9
8
7
2
6
5
4
1
11
(BLK)
(WHT)
(WHT)
(BLK)
(WHT)
(BLK)
(BRN)
(WHT)
(ORN)
(RED)
(YEL)
(VIO)
(BLU)
(BLK)
G2
G1
START
STOP
1
K1
2
1
K4
2
2–E2
4–D10
4–D10
2–D9
2–A5
SAFETY CONTACTOR
4–D10
SAFETY CONTACTOR
4–D10
1
1
K3
K2
2
2
2
1
K5
K6
1
2
2–A5
+BATT SWITCHED
COM CAP CHARGE
COM CAP CHARGE RTN
–BATT
STOP CAP(WHT)
(#8AWG)
+BATT
(RED)
RTN (BLK)
(RED)
(RED)
–BATT
(#8AWG)
(BLK)
(VIO)
(BLK)
(WHT)
(BLK)
(BLK)
6
7
5
1
3
4
5
3
1
A114BA114B
J2
E
J1
J2
R21
250
55W
5
1
SAFETY
CONTACTOR
HOLD
283
OHM
(NC)
CR18
1N5062
PULL IN
13.1
OHM
3
K2
5
K7
1N5352
CR19
C15
1200
10%
250V
3
5
K1
F
80VAC
3
3
K6
5
AK
3
K5
K4
5
5
17
L2
10uH
T1
C5
12
20uF
500V
Q1
G
(WHT)
(BLK)
A AK K
K
G1
G2
5S
T1
L1
300uH
5F
A
G1
K1
K
K
R14
27K
2W
R16
50
12W
C11
1.0
10%
200V
C
J2
9
START
(WHT)
(BLK) (BLK)
BD
C10
CR20
+
400V
6A
–
R17
500
12W
CR7
1N1186
CR13
1N1186
770
10%
250V
C12
770
10%
250V
4
8
K4
6
6
4
K5
K6
6
1513121098222120191653762
14
AK
Q2
G
(WHT)
(BLK)
K
4F
T1
4S
Q3
G2
(WHT)
STOP
FOLD
K2
R9
27K
2W
R5
27K
2W
CAPACITOR
BOARD
AMX1A4
A
A3
REVISIONS PRINTS TO
404
H
2115090SCH
MADE BY
C_DREWICZ_27JUN94
ISSUED
2
356
FOLD
4
FOLD
79
8
N_ZETTEL
FOLD
APPROVALS
–
–
1010
MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN
DIV OR
DEPT.
LOCATION
2115090SCH
CONT. ON SHEET
SH. NO.
67
2-8

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
SH NO.
87
CONT ON SHEET
2115090SCH
DRAWING NO.
23
FOLD
FOLD
AMX1A5
6745
APPLIED PRACTICES
46–208600
8
SURFACES
FOLLOWING : – UNLESS OTHERWISE SPECIFIED USE THE
TOLERANCES ON DIMENSIONS
DECIMALS DECIMALS
(2 PLACES) (3 PLACES)
++
–
–
9
FOLD
REV
0
ANGLES
+
–
2115090SCH
CONT ON SHEET
87
GENERAL ELECTRIC
TITLE
SCHEMATIC DIAGRAM
OVERALL SCHEMATICS FOR AMX IV
SH NO.
FIRST MADE FOR
AMX4_IV_PRODUCTIVITY
P/L ISSUED
2115090SCH
CONT ON SHEET
SH NO.
87
MOTOR DRIVE MODULE
A
LEFT DRIVE COMMAND +
LEFT DRIVE COMMAND –
RIGHT DRIVE COMMAND –
RIGHT DRIVE COMMAND +
LEFT DRIVE FEEDBACK +
B
8–C1
LEFT DRIVE FEEDBACK –
RIGHT DRIVE FEEDBACK +
RIGHT DRIVE FEEDBACK –
LEFT STALL
RIGHT STALL
FULL SPD ENA
BUMPER
MOTOR ENABBLE
DRV RESET
DRIVE ENA SW
J2
7
8
5
6
3
4
1
2
14
12
13
10
16
15
17
DRIVE CONTROL
AMX1A5A1
J9
3
J1
9
8
2
1
6
4
5
J11
1
5
2
6
C
INTERCONNECTRIBBON CABLE
BRAKE REL
J10
J5
1
2
1
2
DRIVE POWER AMP
(BLU) BRAKE RELEASE
2–A5
(VIO)
(RED)
(BLK)
BRAKE REL RTN
+BATT SWITCHED
2–F2
FOLD
D
AMX2A1BRK1
LEFT
BRAKE
J9
AMX1A5A2
1
2
1
2
J7
BRAKE REL
J6
BRAKE REL RTN
(BLK)
(BLK)
(RED)
(BLK)
+5
LOGIC GROUND
+ 24
POWER GROUND
+ 15
– 15
SIGNAL GROUND
–BATT
–BATT
2–B9
2–B9
TO LVLE
2–E5
LEFT BUMPER
(WHT)
LEFT BUMPER RETURN
(BLK)
RIGHT BUMPER
(WHT)
RIGHT BUMPER RETURN
(BLK)
POWER OK*
(RED)
3–F3
RIGHT
BRAKE
BUMPER
AMX2A2
1
C
AMX2A2S1
2
NO
1
C
AMX2A2S2
2
NO
AMX2A1BRK2
FOLD
DRIVE ENB.
A5K1 A5K2
3
1
1
A114B
2
A5K1A
(RED) (BLK)
E
F
+
AMX2A1MTR1
LEFT
MOTOR
–
(RED)
J 1
(BLK)
2
1
LEFT POWER*
3
7
(BLU)
10 OHM
25 WATT
A5R1
RIGHT POWER*
(BLK) (VIO)
A5K1A
3
(WHT)
A114B
(BLU)
(ORN)
5
VM–
1
J4
2
3
3
J1 J2
VM+ VM+
1
J1
J2
(GRN)
VM–
A5K1B
A5K2B
6
(VIO)
(BLK)
46
4
A5K2A
5
(YEL)(ORN)
(YEL)
(BLK)
3
2
2
1
3
A5F1
8 AMP
A5F2
8 AMP
3
7
A5K2A
10 OHM
25 WATT
A5R2
+BATT
(RED)
(RED)
1
J 2
3
(BLK)
(RED)(RED)
+BATT
2–C3
2–C3
–
RIGHT
MOTOR
+
AMX2A1MTR2
PRINTS TOREVISIONS
404
H
2115090SCH
MADE BY
C_DREWICZ_27JUN94
ISSUED
N_ZETTEL
2
FOLD
4
653
FOLD
8
97
FOLD
APPROVALS
1010
MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN
DIV OR
DEPT.
LOCATION
CONT ON SHEET
2115090SCH
SH NO.
87
2-9

AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
SH NO.
89
A
TO BATTERY CHARGER
AMX1A3A1
3–D2 3–D2
B
TO MOTOR CONTROLLER
AMX1A5A1
C
FOLD
D
E
TO 1KHZ INVERTER
AMX1A4A1
F
TO FIL/KVP CONTROL
H
2115090SCH
CONT ON SHEET
2115090SCH
DRAWING NO.
7–B1
TO ROTOR CONTROL
AMX1A3A2
4–A1 4–C24–B10
4–F104–F2
4–H2
6–C1
AMX1A4A2
5–C1
V–CHANGE CMND
V–CHANGE RTN
I–CHARGE CMND
I–CHARGE CMND RTN
BATTERY V & CHARGE CUR
BATTERY V & CHARGE CUR SEL
SAFETY CONTACTOR ENB * 10
LINE SENSE
TRIP BREAKER
CHARGE RELAY*
KEY SWITCH ON
CHARGE SCALE SELECT
RIGHT DRV FDBK +
RIGHT DRV FDBK –
RIGHT DRIVE CMND –
RIGHT DRV CMND +
X–RAY PRESS SW RTN
60HZ INV. RELAY*
FIELD LIGHT SW RTN
1K HZ INVERTER READY
+ LEAKAGE COMP FDBK
– LEAKAGE COMP FDBK
LOAD
LEFT DRV FDBK +
LEFT DRV FDBK –
LEFT DRV CMND +
LEFT DRV CMND –
BUMPER*
RIGHT STALL
FULL SPD ENA
LEFT STALL
DRV RESET
MOTOR ENA
DRIVE ENA SW
ROTOR INTERLOCK
60 HZ INV OK
X–RAY PRESS SW
ROTOR SELECT*
PREP SW
60 HZ PHASE 2
60 HZ PHASE 1
FIELD LIGHT SW
TAP 6 FDBK
TAP 6*
TAP 5 FDBK
TAP 5*
TAP 4 FDBK
TAP 4*
TAP 3 FDBK
TAP 3*
TAP 2 FDBK
TAP 2*
TAP 1 FDBK
TAP 1*
INVERTER OK
EXP STOP CMND
1K HZ PHASE 1
START
1K HZ PHASE 2
LEAKAGE COMP +
LEAKAGE COMP –
FIL +
FIL –
FIL FDBK +
FIL FDBK –
KVP +
KVP –
KVP DWN FDBK +
KVP DWN FDBK –
16 KHZ CLK
2 KHZ PHASE 1
2 KHZ PHASE 2
MAS
X–RAY ON
PREHEAT 26
2
32
FOLD
54 76
FOLD
AMX1A2
UNLESS OTHERWISE SPECIFIED USE THE FOLLOWING : –
APPLIED PRACTICES
46–208600
8
SURFACES
CONTROLLER MODULE
CONTROL PROCESSOR CONTROL PROCESSOR
J8–2
J8–1
1A2J2–1
TEST MODE CONNECTIONSOPERATIONAL CONNECTIONS
8
J1
J2
J3
5
J4
J5
3
4
5
6
7
8
9
10
11
12
13
14
15
17
19
21
22
25FILSHORTTEST
J6
1
2
3
4
5
6
7
8
10
11
12
14
1
2
3
4
5
6
7
8
10
12
13
14
15
16
17
3
6
7
9
11
12
13
15
16
17
7
8
9
10
12
13
14
15
16
17
18
19
20
22
23
24
25
26
AMX1A2A1 AMX1A2A1
356
FOLD
4
DISP DATA OUT
1
SW DATA INPUT
3
MAS UP
5
DISP DATA RESET*
6
MAS DOWN
7
X–RAY ON LAMP*
9
KVP UP
11
DISPLAY OK
12
KVP DOWN
13
+24 VDC
14
SONART*
15
16
PWR GND
17
+40 V V.F. DISP SUP
18
+5 V
LOGIC GND
20
XMIT OK*
21
LGND
23
+24V V.F.SUP
24
J7
AEC ON
1
AEC RTN
2
KVP 0*
3
KVP 1*
4
KVP 2*
5
KVP 3*
6
KVP 4*
7
GEN READY*
8
AEC EX EN
9
EXP START*
10
1112KVP5
KVP6
J12
PWR GND
1
+24 VDC
2
–15 VDC
4
SIGNAL GND
5
+15 VDC
6
LOGIC GND
8
+5 VDC
9
J8
(ORN) RGT MAN TDS OUT
1
(RED) HALL RGT REF
5
(BLK) HALL RGT REF RET
6
(WHT) LFT MAN TDS OUT
3
(RED) HALL LFT REF
7
(BLK) HALL LFT REF RET
8
(YEL) POWER OK
10
(BLU) TUBE PARKED SW
13
(BLK) TUBE PARKED SW RTN
14
(GRY) SERVICE SW
19
(GRY) SERVICE SW RTN
20
(GRN) FIELD LIGHT SW
21
(WHT) PREP SW
23
(BLK) HAND SW COM
24
(RED) EXP SW
25
(BLU) DRIVE ENA SW
28
(BLU) DRIVE ENA SW RTN
27
TO DISPLAY CONTROLLER AMX1A1A1
TO AEC OPTION
TO LVLE AMX1A2A2
J2
(WHT)
4
(RED)
3
(BLK)
5
J1
(WHT)
4
(RED)
3
(BLK)
5
3–F3
SERVICE
SWITCH
1A2J3
4 FIELD LIGHT SW
2
3
1
(BLU)
OUTPUT
REF
RTN
RIGHT HALL
OUTPUT
REF
RTN
LEFT HALL
1A2S8
ENABLE
SWITCH
1A2S2
1A2S3
1A2S5
LEFT
DRIVE
SWITCH
2–E5
PREP SW
HAND SW COM
EXP SW
(YEL)
FOLD
1A2TS4–6
9–H1
1A2TS21A2TS2
11
22
BLU
YEL
BLK
FOR TECH SWITCH OPTION
TECH SWITCH MODULE
AMX1A1
1A2S–6&7
HAND SWITCH
(YEL)
1A2J1–21A2J1–1
2–D7
(YEL)
(BRN)
(ORG)
(BLU)
1A2S11A2S1
TUBETUBE
PARKEDPARKED
SENSOR
1A2J2–2
1–F9
TUBE PARKED SENSOR
REPLACED TUBE PARKED
SWITCH END OF 1992
(BLU)
N.C.N.C.
TO LVLE
24V (BLU)
PWR GRD (BRN)
1A2S4
RIGHT
(YEL)
DRIVE
ENABLE
COMCOM
SWITCH
79
TOLERANCES ON DIMENSIONS
–
9
FOLD
REV
7
DECIMALSDECIMALS
ANGLES
(3 PLACES)(2 PLACES)
++
+
–
–
2115090SCH
CONT ON SHEET
GENERAL ELECTRIC
TITLE
SCHEMATIC DIAGRAM
SH NO.
OVERALL SCHEMATICS FOR AMX IV
89
FIRST MADE FOR
AMX4 IV PRODUCTIVITY
P/L ISSUED
2115090SCH
CONT ON SHEET
SH NO.
89
J11
1
PREP SW
2
EXP SW
3
SERVICE SW
4
LINE SENSE
5
BUMPER*
6
TUBE PARKED
7
DRIVE ENABLE SW
8
KEY SW ON
9
MAS UP
10
MAS DWN
11
KVP UP
12
KVP DWN
DISP. DATA OUT
13
14
DISP. DATA OUT RTN
15
FIELD LIGHT SW
17
+24 VDC
18
BUFFERED DISP. DATA
19
RGT HALL INPUT
21
LFT HALL INPUT
FOLD
REVISIONS PRINTS TO
FOLD
MADE BY
C_DREWICZ_27JUN94
ISSUED
N_ZETTEL
APPROVALS
1010
PCN_201169
PCN_201416
PCN_201479
MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN
TWP_21FEB975
DRL_11JULY976
DRL_29JUL977
DIV OR
DEPT.
CONT ON SHEET
LOCATION
404
2115090SCH
SH NO.
89
2-10
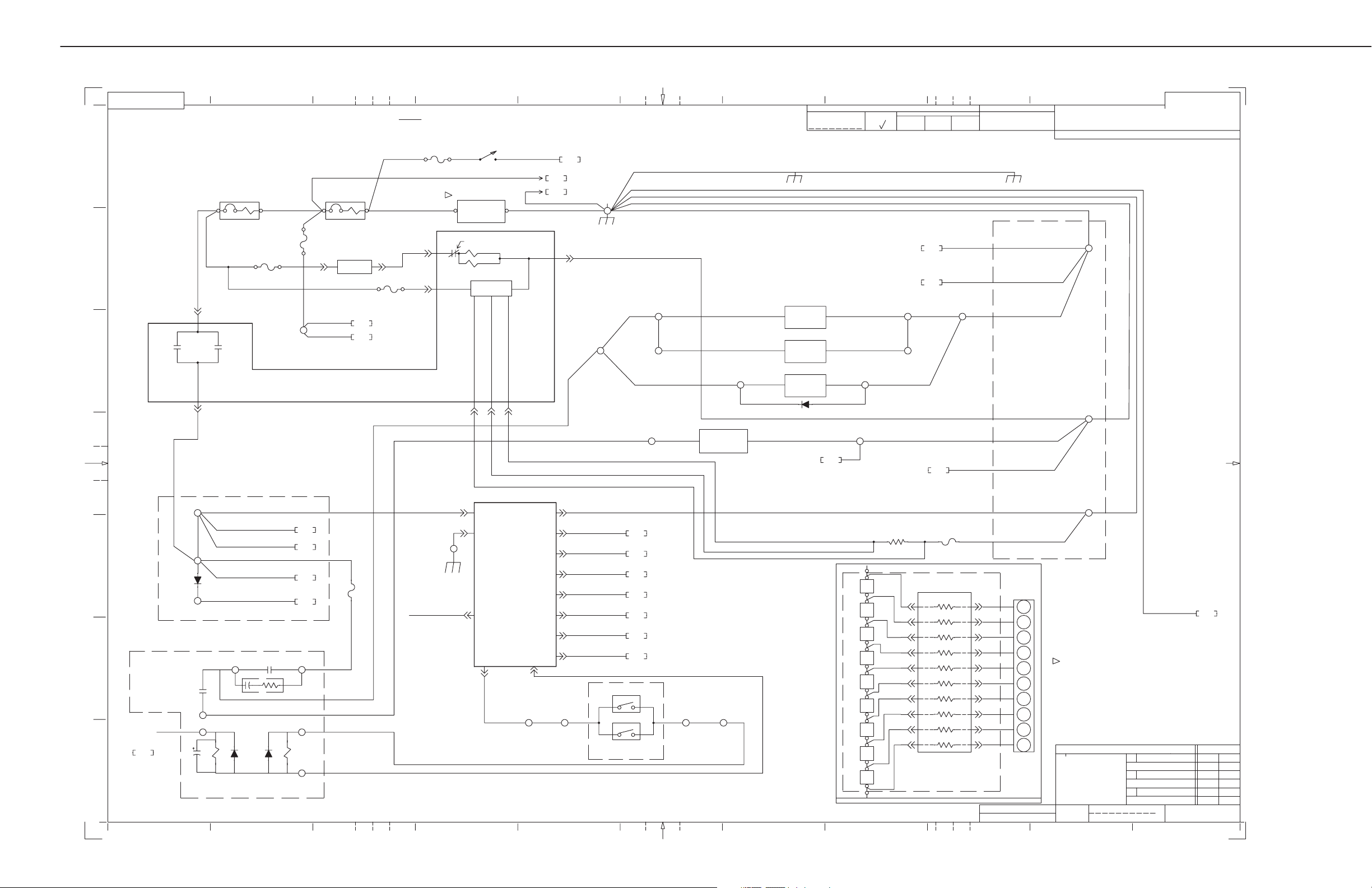
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
A
B
C
FOLD
D
9–
SH NO.
CONT ON SHEET
2115090SCH
DRAWING NO.
(RED)
8AMP
(ORN)
J2–5
K ON 2 K LINE 2
J2–9
(VIO)
A3F6
.5A
(RED)
15A
1A3F9
(BLU)
1A2TS1
3
BATTERY AMX1A3A1 BOARD
J1–2
1A5F1
1A5F2
1A3CB11A3CB1
64AMP
KEY SWITCH
(RED)
(RED)
7–E9
7–E9
32
FOLD
54 76
FOLD
NOTE: THIS SHEET FOR AMX4–PLUS MODELS WITH BASE NUMBER 2169360
(RED) (RED)
1A3F10
+BATT
J4–1
(BLU)
J1–1
(ORN)
J11–1
A3F4
10A
BRAKE
RELEASE
BRAKE RELEASE
(BLK)
(BLU)
(#8AWG)
2A
1A2S1
+BATT
1
MAIN
BATTERY
K CHARGE
BATT CHARGE
BOX
KON1
KON2
CIRCUIT
_+
(BLK)(BLK)
–BATT
7–C4
6–C6
TO X–RAY
CONTROL MODULE
6–E6
–BATT (#8AWG)
(BLK)
J2–7
(BLK)
1A2TS3
J11
489
RED
POWER DISTRIBUTION
GROUND STUD ON BASE
(WHT/BLK)
10
WHITE
8
(WHT)
(RED)
1A2TS2–4
3A1TS1
9
(RED)
2A2TS1–1
TUBE PARKED
LATCH
(RED)
(RED)
(BLK)
UNLESS OTHERWISE SPECIFIED USE THE FOLLOWING : –
APPLIED PRACTICES
46–208600
8
SURFACES
TOLERANCES ON DIMENSIONS
–
DECIMALSDECIMALS
(3 PLACES)(2 PLACES)
++
–
9
FOLD
7
REV
ANGLES
+
–
2115090SCH
CONT ON SHEET
GRN/YELGRN/YEL
COLUMN GROUND ARM GROUND
UPPER
BRAKE
ARM
BRAKE
LOWER
BRAKE
(BLK)(BLK)
(BLK)
(BLK)
COLUMN
COLUMN
2A2TS1–2
(BLK)
(BLK)
(BLK)
(BLK)
3A1TS1
11
(BLK)
10
(WHT)
7–D9
7–D9
(VIO)
1A2TS3
11
(BLK)
(BLK)
(BLK)
IN4936
1A2TS2–3
8–F6
6–F5
(BLK)
(BLK)
GENERAL ELECTRIC
TITLE
SCHEMATIC DIAGRAM
OVERALL_SCHEMATICS_FOR_AMX_IV
SH NO.
9–
1A2TS1
FIRST MADE FOR
AMX_IV_PRODUCTIVITY
4
5
P/L ISSUED
2115090SCH
CONT ON SHEET
SH NO.
9–
FOLD
E
F
H
K2 IS A SOLID
STATE RELAY.
1A2TS2–1
PARK INDICATOR
SIGNAL
8–F6
2115090SCH
1A2TS1
8
(VIO)
(VIO)
9
MR756
10
(VIO)
(VIO)
(VIO)
+BATT SWITCHED
2178054
TS4–3
K1
K2
TS4–5
TS4–6
BLUE
K2
470
FOR DETAILED DRAWING SEE SECTION 16
AMX1 A2 R1
1A5A2J10–2
1A2FL1
2
(VIO)
TS4–4
TS4–2
K1
TS4–1
6–F5
4–D1
3–H3
7–D4
RED
WHITE
YEL
BRN
1A3F3
J8–1
3A1TS1–131A2TS3–13
J1–2
J2
TO CPU
J3
TO BATT CHARGER
J4
TO ROTOR CONTROL
J5
TO MOTOR DRIVE
J6
TO FIL/KVP
J7
TO 1KHZ
J8–7
PWR FAIL DETECT
(ORN)
PGND
3A4
SW2
SW3
8–E4
3–D9
4–B2
7–A9
5–E2
6–F1
3–F5
3A1TS1–12
1A2TS3–12
(BRN)
(VIO)
J1–1
A2A2
LVLE
J1–3
GND STUD
2A
TO AEC MODULE
j9
J8–2
+24V
(BLU)
(YEL)
COLLIMATOR
356
FOLD
4
FOLD
79
A3R1
2.5
200W
+BATT
BATTERY
F1
BATTERY
F2
BATTERY
F3
BATTERY
F4
BATTERY
F5
BATTERY
F6
BATTERY COMPARTMENT
BATTERY
F7
BATTERY
F8
BATTERY
F9
J1–1
J2–1
J3–1
J4–1
J5–1
J6–1
J7–1
J8–1
J9–1
J10–1
–BATT
8
A3F5
10A
BATTERY HARNESS
BOARD AMX2A3A1
DETAIL DP
FOLD
(BLK)
(BLK)
J11–3
J11–1
J11–8
J11–9
J11–5
J11–4
J11–6
J11–7
J11–10
J11–11
MADE BY
C_DREWICZ_27JUN94
ISSUED
N_ZETTEL
AMX1A2TS6
1
2
3
4
5
6
7
8
9
10
6
–BATT
–BATT
1 DEE DETAIL DP FOR TEST HARNESS CONNECTIONS.
REVISIONS PRINTS TO
TWP_26FEB975
PCN_201169
DRL_11JULY976
PCN_201416
DRL_25JUL977
APPROVALS
1010
PCN_201479
MEDICAL SYSTEMS
MILWAUKEE, WISCONSIN
DIV OR
DEPT.
LOCATION
CONT ON SHEET
4–D1
404
2115090SCH
SH NO.
9–
2-11

THIS PAGE INTENTIONALLY LEFT BLANK.
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
2-12
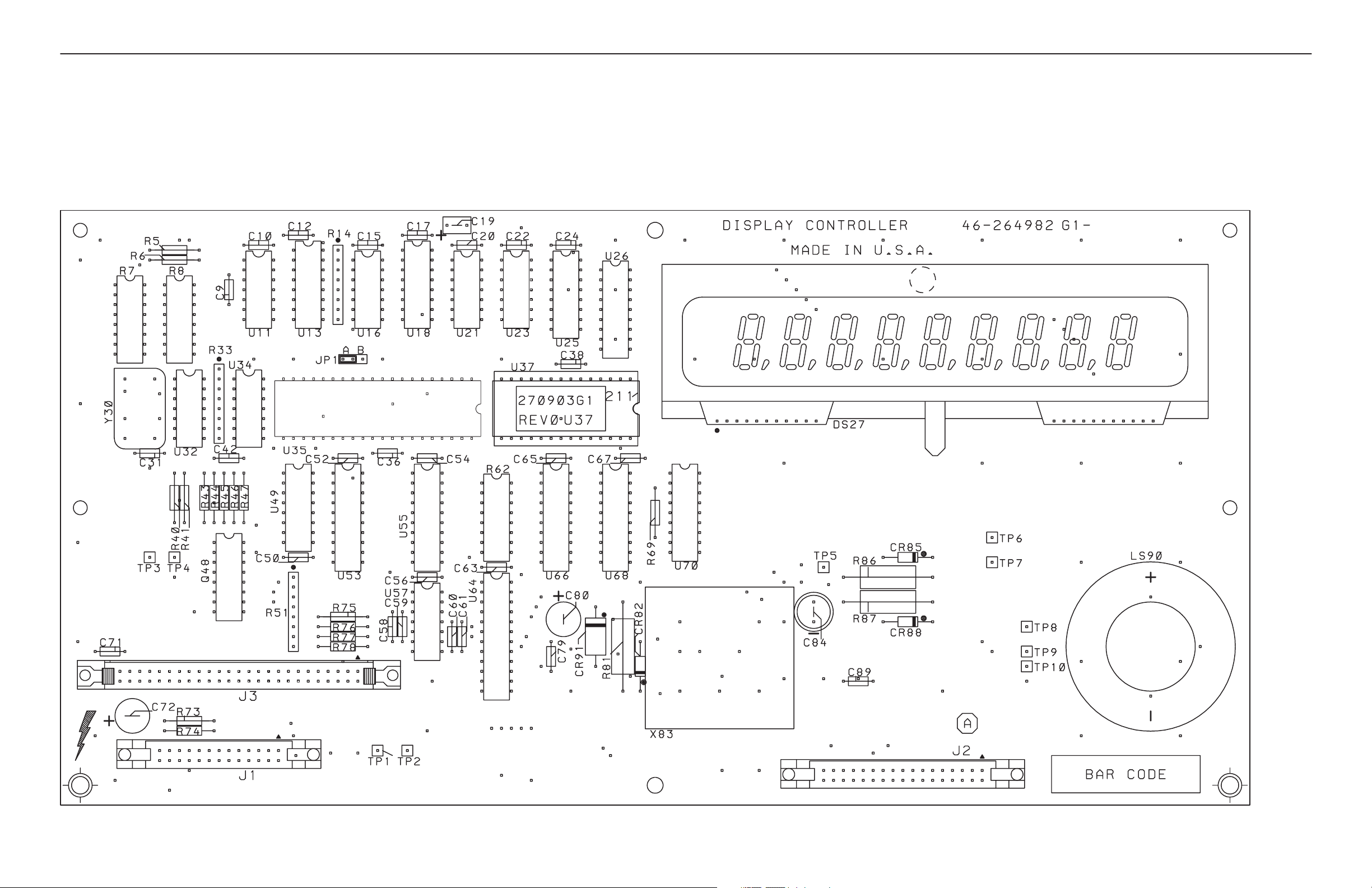
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
SECTION 3
DISPLAY CONTROLLER
AMX1 A1 A1
46-264982G1
B
3-1

THIS PAGE INTENTIONALLY LEFT BLANK.
AMX-4+ SCHEMATICS
GE MEDICAL SYSTEMS (MODEL 2169360, 2236420 & 2275938 SERIES)
REV 6 DIRECTION 2173229-100
3-2
 Loading...
Loading...