

Thank you for purchasing our product.
Before using this product, read this operation manual thoroughly for correct
handing and operation.
Safety Precautions
Read the “Safety Precautions” thoroughly before use to ensure correct and
safe use of the product. Make sure to follow the precautions indicated below,
as these are important messages related to safety.
Failure to follow this message may cause
DANGER
WARNING
immediate threat of death or serious injury, or
complete failure of the equipment.
Failure to follow this message may result in death
or serious injury, or complete failure of the
equipment.
CAUTION
NOTE
Failure to follow this message may cause injury or
failure of the equipment.
A note is not related to product safety, but provides
information about the correct use and operating
procedures to prevent incorrect operation and
malfunction of the equipment.
Precaution from Fukuda Denshi
Fukuda Denshi is responsible for the safety, reliability, and performance of its
equipment only if;
Maintenance, modifications, and repairs are carried out by authorized
personnel. Components are used in accordance with Fukuda Denshi oper ating
instructions.
If the equipment is used incorrectly and become unusable, Fukuda Denshi is
not liable for the malfunction. Use the equipment only for the purpose specified
in this manual.
i

Intended Use of this Equ ipment
This equipment is designed for the following <Intended Use >.
<Intended Use>
This equipment is intended for measuring parameters of ECG, respiration in surgery
room, ICU, ward, emergency room in the medical facility and transmitting the
measured data by wireless network to the central monitor continuously.
This equipment is intended for monitoring one patient. It is not intended for
monitoring multiple patients.
For specification of this equipment, refer to "Chapter 14 Specification" of this
manual.
This equipment is intended to be used by healthcare professionals. Users should
have a thorough knowledge of the function and operation before usi ng this
equipment. The maintenance of this equipment should be performed by skilled
personnel who received a training of possible hazards and measures to avoid those
hazards. Also, your local regulation must be followed. If this equipment is used for
the purpose other than intended, or if the user does not follow the safety
instructions, the following hazard may result.
ȷ Hazard to the Life and Health of the Patient or the User
ȷ A Problem Related to Medical Practice
ȷ Damage to the Equipment
ii

Graphic Symbol
The following symbols are used for this equipment.
LX-8100 Symbols indicated on the main unit
Symbol Description
Warning (indicated in yellow)
Follow operating instructions (Warning); (indicated in blue)
Indicates that the failure to follow operating instructions
could place the patient or operator at risk.
Type CF Applied Part with Defibrillation-Proof
Indicates that the degree of protection against electric
shock is Type CF Applied Part with defibrillation-proof.
Indicates the power ON/OFF status.
Indicates the point to close the battery compartment lid.
Indicates the battery type and direction
Name and Address of Manufacturer
Date of Manufacture
Indicates the name and address of manufacturer, and
date of manufacture.
Non-ionizing electromagnetic radiation
Indicates the radio transmitting device.
iii

LX-8100 Symbols displayed on the display screen
Symbol Description
Synchronized Mark
This mark flashes synchronizing to the heartbeat.
Indicates the remaining battery level.
iv

Precautions for Safe Operation of Medical Electrical Equipment
Cautions described here are regarding the general instructions for safety use
to the patient and the users. As for cautions about the LX-8100, please refer
to the following pages.
CAUTION
1. Users should have a thorough knowledge of the operation before
using this equipment.
2. Pay attention to the following when installing or storing the equipment.
y Do not install or store in a place where the equipment will be subjected to
splashing water.
y Do not install or store in an area where the environmental conditions,
such as atmospheric pressure, temperature, humidity, ventilation,
sunlight, dust, sodium, sulfur, will adversely affect the system.
y Place the equipment on a stable surface where there is no inclination,
vibration, or shock (including during transportation).
y Do not install or store in an area where chemicals are stored or gas may
generate.
3. Before operating the equipment, verify the following items.
y Check the cable connection and polarity to ensure proper operation of the
equipment.
y Ensure that all cables are firmly and safely connected. Especially, recheck
the attachment and connection condition of electrodes and transducers.
y Pay special attention when the equipment is used in conjunction with other
equipment as it may cause erroneous diagnosis and danger.
y Check the remaining battery level.
y When replacing the battery, make sure that the battery polarity is correct.
Do not charge the battery.
4. During operation of the equipment, verify the following items.
y Do not operate the equipment beyond the time period required for
diagnosis and medical care.
y Do not pick up and/or swing the equipment pulling/grabbing the probe
(sensor) or cable part. It may damage the equipment and lead to
measurement error.
y Always observe the equipment and patient to ensure safe operation of the
equipment.
y If any abnormality is found on the equipment or patient, take appropriate
measures such as ceasing operation of the equipment and/or detaching the
probe (sensor) and/or electrode, in the safest way for the patient.
y Do not allow the patient to come in contact with other equipment.
v
CAUTION
5. After using the equipment, verify the following items.
y Return all operating switches, knobs etc to the position before using the
equipment, and then switch off the power.
y When unplugging the cables, do not apply excessive force on the cable
and pull from its connector.
y Clean the accessories and cables, and keep them together in one place.
y Keep the equipment clean to ensure proper operation for the next use.
y Make sure to remove the battery if the equipment is not used for a long
time. The leakage from the battery may damage the equipment or an
explosion from the battery may occur.
6. If the equipment is damaged and in need of repair, ensure patient
safety by immediately turning the equipment off and remove the
electrodes and/or probe from the patient. Users should not attempt
service. Label the unit “OUT OF ORDER” and contact Fukuda Denshi
representative.
7. Do not disassemble or remodel the eqipment.
8. Maintenance
y Make sure to periodically check the equipment and accessories.
y Before reusing the equipment that has been left unused for a while, make
sure that the equipment works normally and safely.
9. When using electrosurgical knives or defibrillator with this equipment,
take care of the following.
y To prevent burn injury to the patient, verify proper attachment of the patient
ground plate, the ECG electrode type for the electrosurgical knives, and the
quantity of gel, output energy for the defibrillator. Also, verify that a proper
ground is selected.
y Some types of equipment other than above may cause accidental hazards
to the patient and operator due to the conditions of the equipment. Read
the operation manual attached to each equipment and understand the
precautionary instructions prior to use.
Non-Explosion Proof
z Never operate the equipment in the presence of flammable
anesthetics, high concentration of oxygen. It may cause an explosion or
fire.
z Never operate the equipment inside a hyperbaric chamber. It may
cause an explosion or fire.
z Never operate the equipment where flammable gas or fluid such as
anesthetic, oxygen, and hydrogen are used. It may cause an explos ion
or fire.
DANGER
vi

Precautions about Magnetic Resonance Imaging (MRI)
WARNING
z Do not operate this equipment in magnetic resonance imaging (MRI)
environments.
z When conducting MRI test, remove the electrodes and sensors
connected to the patient (test subject).
The local heating caused by the induced electromotive force may
cause burn injury to the patient (subject). For details, refer to the
operation manual for the MRI testing device.
Electrosurgery Safety
WARNING
The monitoring system contains protection against interference generated
by electrosurgical instruments. However, depending on the operating
conditions, surgery site with respect to the location of ECG electrodes,
ground plate attachment condition, or the type of instrument used, it may
cause burn injury at the electrode site or noise on the ECG. The noise is
generated at the tip of the electrosurgical knife and is difficult to completely
eliminate because of the frequency components of the ECG. T o reduce
electrosurgical interference, take the following precautions:
Location:
Locate the electrosurgical unit as far as possible from this equipment and
the patient cable. This will help reduce interference on the ECG through
the monitor or cables.
Electrode Placement:
The amount of noise interference is considerably different depending on
the electrode position and surgery site. Place the ECG electrodes as far
away as possible from the surgery site and the ground plate. Do not
place electrodes in the path between the surgery site and the groun d
plate. If the electrodes are placed in this path, the amount of interference
will be quite large. Position (+) and (–) electrodes as close as possible to
each other.
Ground Plate:
When using electrosurgical instruments, make sure the contact between
the patient and the ground plate is secure. If the connection is
incomplete, the patient may suffer from burn at the electrode site.
vii

Defibrillation Safety
WARNING
z Use only the lead cable specified by Fukuda Denshi when defibrillating.
If used by unspecified lead cable, the equipment may be damaged,
resulting in a safety hazard.
z When using the defibrillator, keep away from the electrodes or
medicament applied to the patient chest. If this is not possible, remove
the electrodes or medicament before using it.
If the defibrillator paddles are directly in contact with the electrodes or
medicament, an electrical shock may result from the discharged
energy.
z When using the defibrillator, do not touch the patient and the metal p art
of the equipment or cables. Electric shock may result from the
discharged energy.
Precautions about the Pacemaker
WARNING
z Minute ventilation rate-adaptive implantable pacemakers can
occasionally interact with certain cardiac monitoring a nd diagnostic
equipment, causing the pacemakers to pace at their maximum
programmed rate. The cardiac monitoring and diagnostic equipment
may possibly send wrong information.
If such event occurs, disconnect the cardiac monitoring and diagnostic
equipment, or follow the procedures described in the operation manual
of the pacemaker.
(For more details, contact FUKUDA DENSHI personnel, your
institution’s professionals, or your pacemaker distributors.)
z Rate meters may continue to count the pacemaker rate during
occurrences of cardiac arrest or some arrhythmias. Do not rely entirely
upon rate meter alarms. Keep pacemaker patients under close
surveillance.
Reference
“Minute Ventilation Rate-Adaptive Pacemakers”
FDA alerts health professionals that minute ventilation rate-adaptive
implantable pacemakers can occasionally interact with certain cardiac
monitoring and diagnostic equipment, causing pacemakers to pace at their
maximum programmed rate.
[October 14, 1998 – FDA]
viii
Precautions about the LX-8100
z Do not connect cables not authorized by Fukuda Denshi to any I/O
connector. If done so by mistake, the LX-8100 cannot deliver its
maximum performance and may be damaged, resulting in a safety
hazard.
z Do not use this equipment with multiple patients simultaneously.
z This equipment itself has no alarm function. Do not use it if an alarm
function is necessary. The alarm function with the receiving monitor,
refer to the operation manual of the receiving monitor.
Do not pick up and/or swing the LX-8100 pulling/grabbing th e probe
(sensor) or cord part. The cable could break or get disconnected from the
LX-8100. And it may hit people or damage other equipm ent around.
WARNING
CAUTION
Precautions about Waterproof
z Replace the “Battery Compartment Lid” of the LX-8100 regularly to
keep the performance of waterproof. If not regularly replaced, the
quality of the lid will deteriorate and cannot keep the waterproof
performance. For details about the regular replacement, contact your
local Fukuda Denshi service representative.
z The lid may be damaged from high impact. If the LX-8100 is dropped or
is subjected to a high impact, make sure that the lid is not damaged.
z Do not use the LX-8100 wet. Always wipe the LX-8100 with a soft cloth
and dry it thoroughly before use.
CAUTION
ix
Precautions about ECG
z When removing electrodes from the patient, remove them carefully and
slowly. Do not apply excessive force to remove them. Otherwise, it may
damage the skin.
z If any electrodes get detached from the patient after being connected to
the lead cable and the patient monitor, pay attention that the metal part
of the electrode does not get in touch with any metal parts of the bed or
any conductive parts. Also, the operator should not touch any
conductive parts with bare hands. Otherwise, it may cause electric
shock to the patient and/or operator due to excessive leakage current.
z The indication for continuous use of the electrode is about one day.
z Replace the electrode if the skin contact gets loosen due to
perspiration, etc.
z When an electrode is attached to the same location for a long period,
some patients may develop skin irritation. Check the patient's skin
condition periodically and change the electrode site as required.
z For stable ECG monitoring, verify proper electrode placement, lead,
and waveform size. If not properly selected, it may cause erroneous
detection.
z There are some cases when the pacemaker pulse can not be detected
depending on the pacemaker type, pulse voltage, pulse width,
electrode lead type (unipolar, bipolar), or electrode placement which
causes the pacemaker pulse amplitude to decrease, and disabl es the
pacemaker pulse detection.
z If signals similar to a pacemaker pulse are present, such as electric
blanket noise or excessive AC frequency noise, these ma y be
erroneously detected and displayed as a pacemaker pulse. In this
case, check the condition of the electrodes and ECG lead cable to
resolve the cause or turn off the pacemaker detection setting on the
receiving monitor.
z If a pacemaker pulse is continuously detected due to AC frequency
interference, QRS detection will be suspended and the heart rate will
be reduced.
CAUTION
Precautions about Output Signal
WARNING
Do not use the output signal of the monitor that receives radio wave signal
from the LX-8100 as the trigger signal for IABP, MRI echocardiographic, or
defibrillator for the following reasons.
It may lead to a delay of operating timing due to the delay time of
waveform transmission.
A trigger signal unrelated to the heart rate may be generated due to
the interfusion of spike noise at weak electric field.
x
Precautions about Accessories and Optional Accessories
WARNING
Use only the accessories such as disposable electrodes and electrode
codes specified by Fukuda Denshi. Otherwise, this equipment cannot
deliver its maximum performance and may be damaged, resulting in a
safety hazard.
CAUTION
z Do not reuse disposable products.
z Store the disposable products properly as mentioned in their user
manuals.
Precautions about Battery
WARNING
z Use new "AA" size (“LR6” size) alkaline cell.
z Install the battery with the correct polarity.
z Do not charge the battery. Any attempt to charge the battery may
cause it to leak or break.
z Do not short the (+) and (-) terminals. It may result in exothermic heat
and fire.
z Do not throw the battery into fire. It may explode.
Precautions about Disposing of Equipment, Accessories, or
Components
CAUTION
z When disposing of equipment, accessories, or components, use an
industrial waste distributor. Do not dispose of as ordinary waste.
z Used disposal items (ECG electrodes, etc.) shall be discarded as
medical waste.
Precautions about Disposing of Battery
CAUTION
Follow the local municipal rule to dispose the used dry cell batteries.
xi
Precautions for Use of Medical Telemeter
WARNING
z The LX-8100 transmitter must not be co-located or operated in
conjunction with any other antenna or transmitter.
z This equipment complies with FCC/IC radiation exposure limits set forth
for an uncontrolled environment and meets the FCC radio frequency
(RF) Exposure Guidelines and RSS-102 of the IC radio frequency (RF)
Exposure rules. This equipment has very low levels of RF energy that
are deemed to comply without testing of specific absorption rate (SAR).
z Operation of LX-8100 requires the prior coordination with a frequency
coordinator designated by the FCC for the Wireless Medical Telemetry
Service.
z This radio frequency device is susceptible to interference from outside
sources. Interference may prevent the monitoring of patients connected
to this equipment. If a problem exists, contact your local service
representative.
z The LX-8100 transmits vital signs to the receiving monitor using radio
wave signal. Under unstable radio wave signals, the receiving monitor
will not generate any alarms. This situation may miss sudden change in
the patient's condition and may cause a serious accident. Under
unstable radio wave signals, check the patient status consistently under
this situation. To get stable radio wave signals, make sure to have a
proper telemetry installation.
CAUTION
For installation, make sure of the following.
y The medical institution (hereinafter referred to as the “Institution”) must
decide the telemetry installation plan for the medical department in order to
prevent interference and interference between transmitters (telemetry
based on destination country’s radio law). When telemetry has already
been installed and been used, radio format, frequency, and antenna power
are required to be examined to prevent interference.
y When laying receiver antenna for each transmitter, the Institution has to
examine the installation so that electronic interference does not occur.
y Based on the above examination result, the Institution should install each
receiver antenna as required.
xii
CAUTION
For management, make sure to follow the precautions below.
y The Institution should appoint a person (hereinafter referred to as the
“Coordinator”) to manage the wireless channels for the whole Institution.
y The Coordinator must be selected from people who understand the
characteristics and functionality of telemetry systems, and are skilled in
operating telemetry.
y When installing telemetry, the Coordinator has to understand the
precautions for use of telemetry in advance.
y The Coordinator is responsible for maintenance of wireless channels and
storage and maintenance of telemeter in the overall medical facilities to
give proper instructions to the telemetry users.
y The Coordinator should create a management log (hereinafter referred to
as the “log”), which contains a list of the management status of the wireless
channels for the whole Institution. When changing a wireless channel,
register it in the log and give proper instructions to the user.
y The telemetry user verifies operation of the transmitter/receiver before use.
y When interference or breakdown occurs in telemetry communication, the
user is required to inform the Coordinator of the problems. The Coordinator
is to deal with the problem properly and/or contact their nearest Fukuda
Denshi representative for service.
xiii

Electromagnetic Compatibility
This equipment complies with IEC 60601-1-2 (2014), safety standard
regarding the electromagnetic distrubances of medical electrical equipment. To
ensure maximum performance against the electromagnetic distrubances,
make sure to follow the precautions for installation and usage described in this
manual.
ŪThis equipment is intended for use in the medical facility (except inside the
shield room of MRI device), and satisfies the immunity level for professional
healthcare facility environment stipulated in IEC 606 01-1-2.
ŪAn excessive magnetic disturbance may degrade the HR measur ement
accuracy (refer to 14. Specification, ECG, HR measurement detection),
which is the essential performance of this equipment, and may cause delay
in treatment or inaccurate diagnosis.
ŪWhen using this equipment, interference with other medical electrical
equipments or non-medical electrical equipments may oocur. Make sure that
no interference is present before usage.
ŪTo ensure basic safety and essential performance related to
electromagnetic distrubances during the expected service life of this
equipment, “Daily Check” and “Periodic Check” must be performed.
(refer to 12.Maintenance and Inspection)
Precautions for Safe Operation under Electromagnetic Influence
CAUTION
If any sorts of electromagnetic wave, magnetic field, or static electricity
exist around the equipment, noise interference or malfunction of the
equipment may occur. If any unintended malfunction or noise occurs during
monitoring, check the magnetic influence and take appropriate
countermeasures.
The followings are examples of the common cause and countermeasures.
ەMobile Phone
The radio wave may cause malfunction to the equipment.
Mobile phones and radio sets should be turned off in the room
(building) where medical device is locate d.
ەStatic Electricity
In a dry environment (room), static electricity is likely to occur. Take
the following countermeasures.
y Both operator and patient should remove any static electricity before
entering the room.
y Humidify the room.
xiv

CAUTION
z If this equipment is installed close to, or stacked with other equipment,
malfunction may occur. Make sure to verify that the equipments
operate properly in a used location.
z Use of accessories, probes, or cables other than specified may cause
increase in electromagnetic emission or decrease in electromagnetic
immunity resulting in malfunction of the equipment.
z The portable RF communications equipment (including antenna cable
and peripheral equipment such as external antenna) with the specified
cable should be used in a location at least 30 cm apart from any part of
this equipment. Otherwise, it may result in performance degradation of
this equipment.
xv
EMC Guidance
This equipment complies with IEC 60601-1-2 (2014). However, if portable
transmitter or wireless LAN equipment is used extremely nearby, the
electromagnetic influence may largel y exceed the compliance level and may
cause unexpected phenomenon such as nois e interference on the waveform,
etc.
Therefore, this equipment should be used in a location specified by each
medical institution. If any unexpected noise interference on the waveform or
failure to the peripheral device occurs, stop using the equipment and follow the
instruction of the technician.
The following is the information relating to EMC (Electromagnetic
Compatibility).
(When using this equipment, verify that it is used within the environment
specified below.)
䖃Compliance to the Electromagnetic Emissions
This equipment complies with the following emission standard.
Emission test Compliance
RF Emission
CISPR 11
Group 1 Class A
CAUTION
The emission performance of this equipment is suitable for use in industrial
environment and hospital environment (CISPR 11 Class A). To use in
home environment (generally, CISPR 11 Class B is required), this
equipment may not be properly protected from wireless frequency
communication service. It may be necessary to take measures such as
changing the installation location or equipment orientation.
xvi
䖃Compliance to the Electromagnetic Immunity
The LX-8100 is intended for use in the electromagnetic environment specified
below. The customer or the user of the LX-8100 should assure that it is used in
such an environment.
Basic EMC standard or
test method
Electrostatic discharge
IEC 61000-4-2
Radiated RF EM fields
IEC 61000-4-3
Proximity fields from RF
wireless communications
equipment
IEC 61000-4-3
Conducted disturbances
induced by RF fields
IEC 61000-4-6
Rated power frequency
magnetic fields
IEC 61000-4-8
r8kV contact
r2kV, r4kV, r8kV, r15kV air
10V/m
80MHz to 2.7GHz
1kHz 80%AM
Refer to the following table.
3V
0.15MHz to 80MHz
1kHz 80%AM
6V
0.15MHz to 80MHz
(in ISM bands between 0.15 MHz and 80MHz)
1kHz 80%AM
30A/m
50Hz
Immunity test specifications for RF wireless communications equipment.
Test frequency
(MHz)
Modulation
Maximum
Immunity test levels
power
(W)
Distance
(m)
Immunity test level
(V/m)
710, 745, 780 PM, 217Hz 0.2 0.3 9
810, 870, 930 PM, 18Hz 2 0.3 28
1720, 1845, 1970 PM, 217Hz 2 0.3 28
2450 PM, 217Hz 2 0.3 28
5240, 5500, 5785 PM, 217Hz 0.2 0.3 9
xvii

Contact
If you need more information, please contact the following.
(1) Fukuda Denshi Co., Ltd., Head Office
3-39-4 Hongo, Bunkyo-ku, Tokyo, Japan
Tel: +81-3-5684-1455 Fax: +81-3-3814-1222
E-mail: info@fukuda.co.jp
Home Page: http://www.fukuda.com
(2) Sales Representative
Write the name, address, phone, fax number of your local sales
representative.
(Name of Sales Representative, Address, Phone/Fax)
xviii

Contents
Safety Precautions ............................................................................................. i
Precaution from Fukuda Denshi ................................................................ i
Intended Use of this Equipment ............................................................... ii
Graphic Symbol ...................................................................................... iii
Precautions for Safe Operation of Medical Electrical Equipment ............. v
Non-Explosion Proof ............................................................................... vi
Precautions about Magnetic Resonance Imaging (MRI) ........................ vii
Electrosurgery Safety ............................................................................. vii
Defibrillation Safety ............................................................................... viii
Precautions about the Pacemaker ........................................................ viii
Precautions about the LX-8100 ............................................................... ix
Precautions about Waterproof ................................................................ ix
Precautions about ECG ........................................................................... x
Precautions about Output Signal ............................................................. x
Precautions about Accessories and Optional Accessories ..................... xi
Precautions about Battery ....................................................................... xi
Precautions about Disposing of Equipment, Accessories, or Components
........................................................................................................... xi
Precautions about Disposing of Battery .................................................. xi
Precautions for Use of Medical Telemeter ............................................. xii
Electromagnetic Compatibility ................................................................... xiv
Precautions for Safe Operation under Electromagnetic Influence ......... xiv
EMC Guidance ...................................................................................... xvi
Contact ......................................................................................................... xviii
1. General Description ...................................................................................... 1
2. Names of Parts and Their Functions ............................................................. 3
3. Preparation ................................................................................................... 5
(1) Installing the Battery ........................................................................... 5
(2) Operating Power Switch ..................................................................... 9
4. ECG Monitoring .......................................................................................... 11
ŠConnecting the ECG Lead Cable and Electrodes .............................. 11
ŠAttaching the Electrodes .................................................................... 14
ŠConnecting the ECG Lead Cable to the LX-8100 ............................... 15
5. Respiration Monitoring ................................................................................ 17
6. Measurement .............................................................................................. 19
ŠStarting Screen ................................................................................... 19
ŠWaveform Display Screen .................................................................. 19
ŠBattery Level Check ........................................................................... 21
ŠWaveform Display .............................................................................. 22
7. Operation .................................................................................................... 31
ŠChanging Setup .................................................................................. 31
ŠRestarting the display ......................................................................... 37

ŠPressing the EVENT button ................................................................ 37
8. Other Setting Items ..................................................................................... 39
ŠChanging the Time Constant .............................................................. 40
ŠChanging the Detection Sensitivity of the Pacemaker Pulse .............. 40
ŠChanging QRS Detection ................................................................... 41
ŠChanging the Respiration Detection Signal ON/OFF ......................... 42
ŠChanging the display Brightness ........................................................ 42
ŠChanging Display Timeout Duration ................................................... 42
ŠChanging Sound ON/OFF .................................................................. 43
9. Changing the Transmitter Channel and Group ID ....................................... 45
ŠChanging the Transmitter Channel ..................................................... 45
ŠChanging the Group ID ...................................................................... 45
10. Troubleshooting ........................................................................................ 47
ŠList of Displayed messages ................................................................ 47
ŠIn Case of Dropping the LX-8100 into Water ...................................... 51
11. Cleaning and Disinfection ......................................................................... 53
ŠCleaning ............................................................................................. 53
ŠDisinfection ......................................................................................... 53
ŠCleaning the ECG lead cable ............................................................. 54
12. Maintenance and Inspection ..................................................................... 55
ŠRepairing the Equipment .................................................................... 63
ŠReplacing the Battery Compartment Lid Unit ..................................... 63
13 . Standard and Optional Accessories ......................................................... 67
ŠStandard Accessories ........................................................................ 67
ŠOptional Accessories .......................................................................... 68
14. Specification .............................................................................................. 69
ŠSpecification ....................................................................................... 69
ŠDisplays .............................................................................................. 73
ŠDetails of the “Electrode” Message .................................................... 75
ŠList of Setup Items .............................................................................. 76
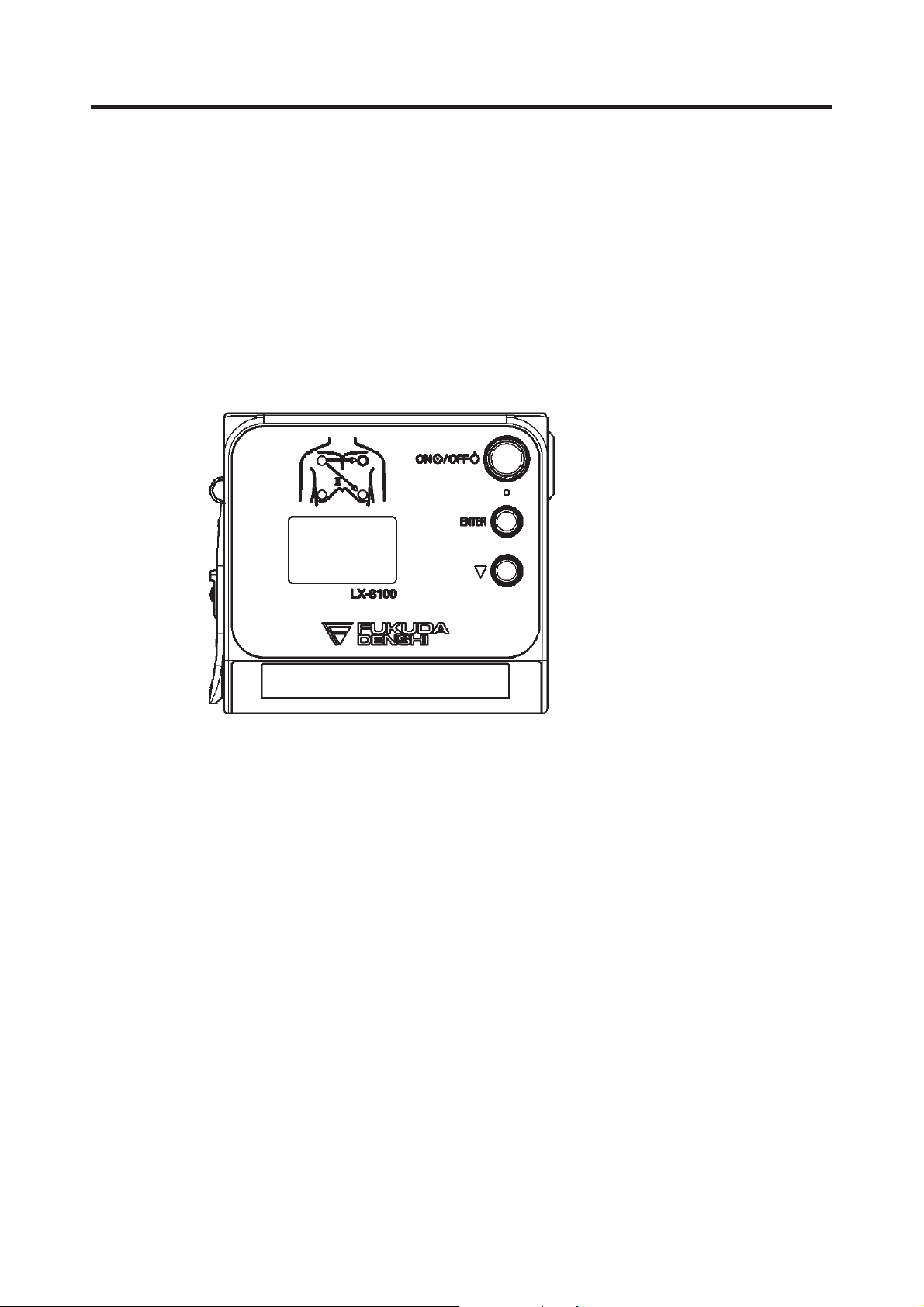
1. General Description
The LX-8100 is a telemetry transmitter designed to measure the ECG and
respiration waveform with a “AA” size (“LR6” size) alkaline battery.
Information such as ECG measurements, respiration waveform, battery level,
and the conditions of the ECG electrodes are displayed on the front panel.
ECG lead selection is available using the two buttons (ENTER and ť) on the
front panel (In case of using a 3-electrode lead cable or a 5-electrode chest
lead cable).
Before using the LX-8100, read also the operation manual of the patient
monitor at the receiving side thoroughly.
External Appearance
1

1. General Description
2
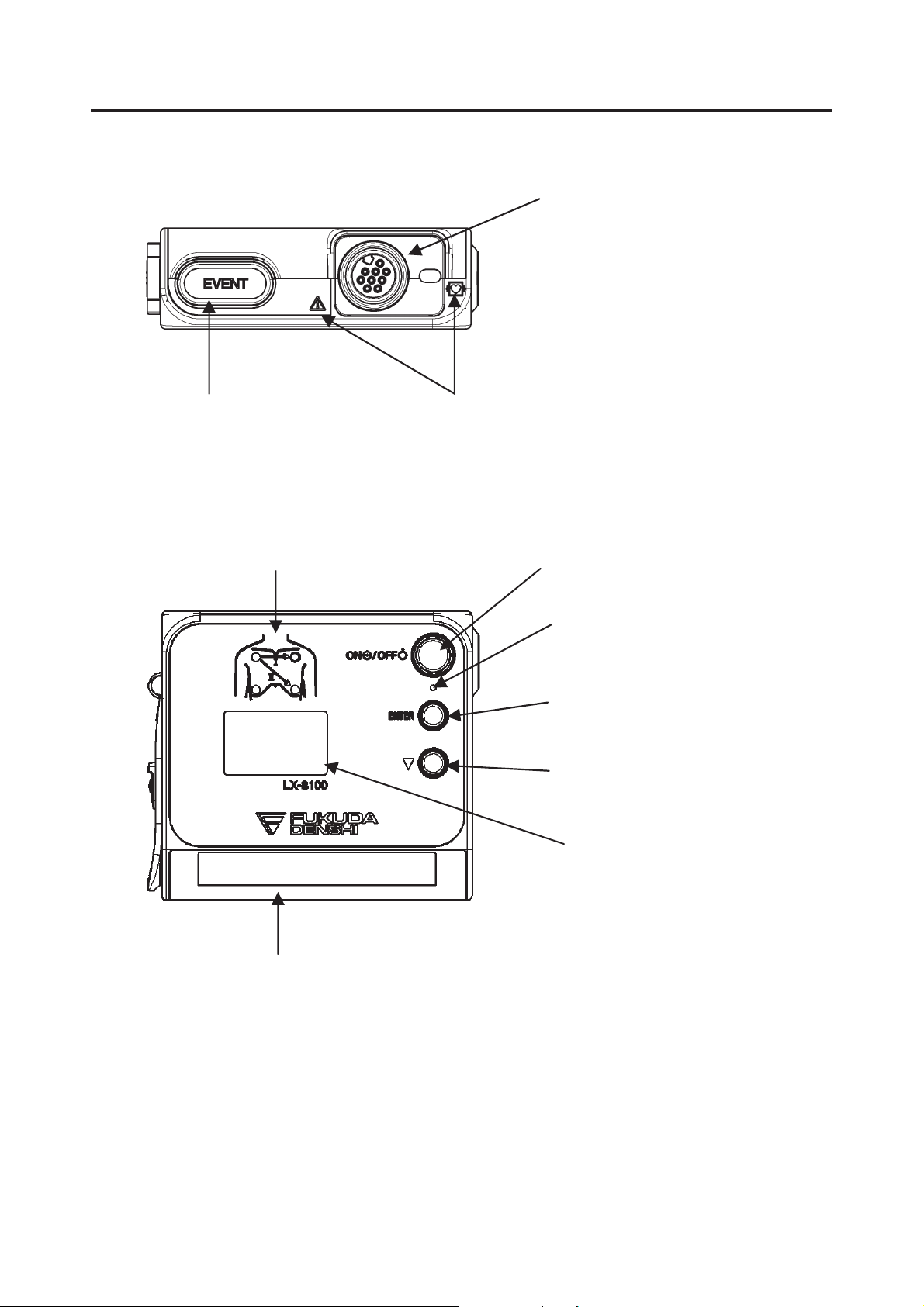
2. Names of Parts and Their Functions
EVENT Button
Transmits EVENT Information
Refer to “Safety
Precautions in this
manual’s preface”
Electrode Position Label
Indicates standard ECG
electrode position.
ECG/RESP Input Connector
Connects the ECG lead cable to
measure ECG and respiration
waveform.
Power Switch
Turns ON/OFF the power
Power Supply LED
Indicates the power supply status
x Light ON: In nomal operation
x Light OFF: Power Off
ENTER Button
For Setup
ۃButton
For Setup
Channel Number
Indicates transmitter channel
number.
Display
Displays measurement
waveform and
transmitter information.
3
2. Names of Parts and Their Functions
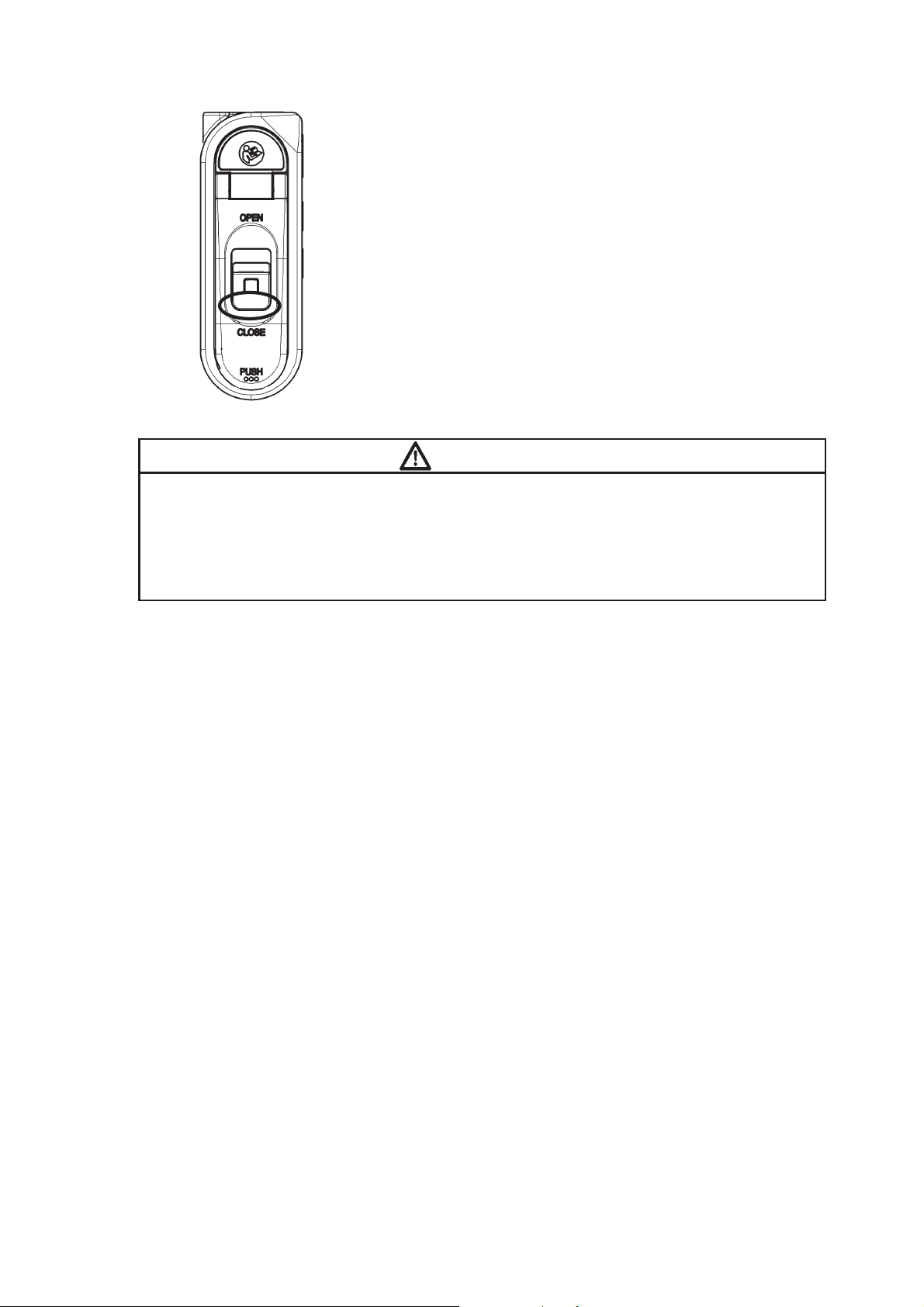
Battery Compartment Lid Open/Close Lever
Battery Lid Lock Button
Refer to “Safety Precautions” in
this manual’s preface.
To open/close the battery compartment lid,
slide this lever up/down.
Unlocks the battery compartment lid.
Strap Hole
Attaches the strap.
4
3. Preparation
(1) Installing the Battery
The LX-8100 functions with an “AA” size (“LR6” size) alkaline battery. Use the
battery specified by Fukuda Denshi.
Use a newßAA” size (“LR6” size) alkaline battery.
Specified types of batteries
Type Product Manufacturer Operation time
“AA” size (“LR6” size)
alkaline battery
*1: When using a new battery.
(ECG measurement, RESP measurement, Default setting, Operating
environments in 23qC)
*The operation times are estimated for operations with specified types of
batteries by Fukuda Denshi.
MX1500 DURACELL
Approximately
6 days
*1
WARNING
z Unplug the ECG lead cable when the battery compartment lid is
opened. Otherwise, patient leakage current beyond the allowable value
may occur.
z Use a new ”AA” size (“LR6” size) alkaline battery.
z Do not short out the (+) and (-) terminals. It may result in exothermic
heat and fire, the leakage from the batteries may damage the
equipment, or an explosion from the batteries may occur.
z Install the batteries with the correct polarity.
z Do not charge alkaline batteries. Any attempt to charge the batteries
may cause them to leak or break.
z Do not use disassembled or damaged batteries due to drop or shock.
The leakage from the batteries may damage the equipment, or an
explosion from the batteries may occur.
5
3. Preparation
z Remove the exhausted batteries immediately. The leakage from the
batteries may damage the equipment, or an explosion from the
batteries may occur.
z If the equipment is not in use for a long period of time, remove the
batteries and store the equipment in an appropriate plac e. If the
batteries are left in the equipment for a long period of time, the leakage
from the batteries may damage the equipment or an explosion from the
batteries may occur.
z Use alkaline battery (AA) specified by Fukuda Denshi.
z Do not replace the batteries with wet hands.
z In case of storing the used or unused batteries, make sure that the
terminals are not touching other batteries or metal parts.
z Use the same type of battery as its setting otherwise the accurate
battery level cannot be displayed.
WARNING
CAUTION
6
3. Preparation
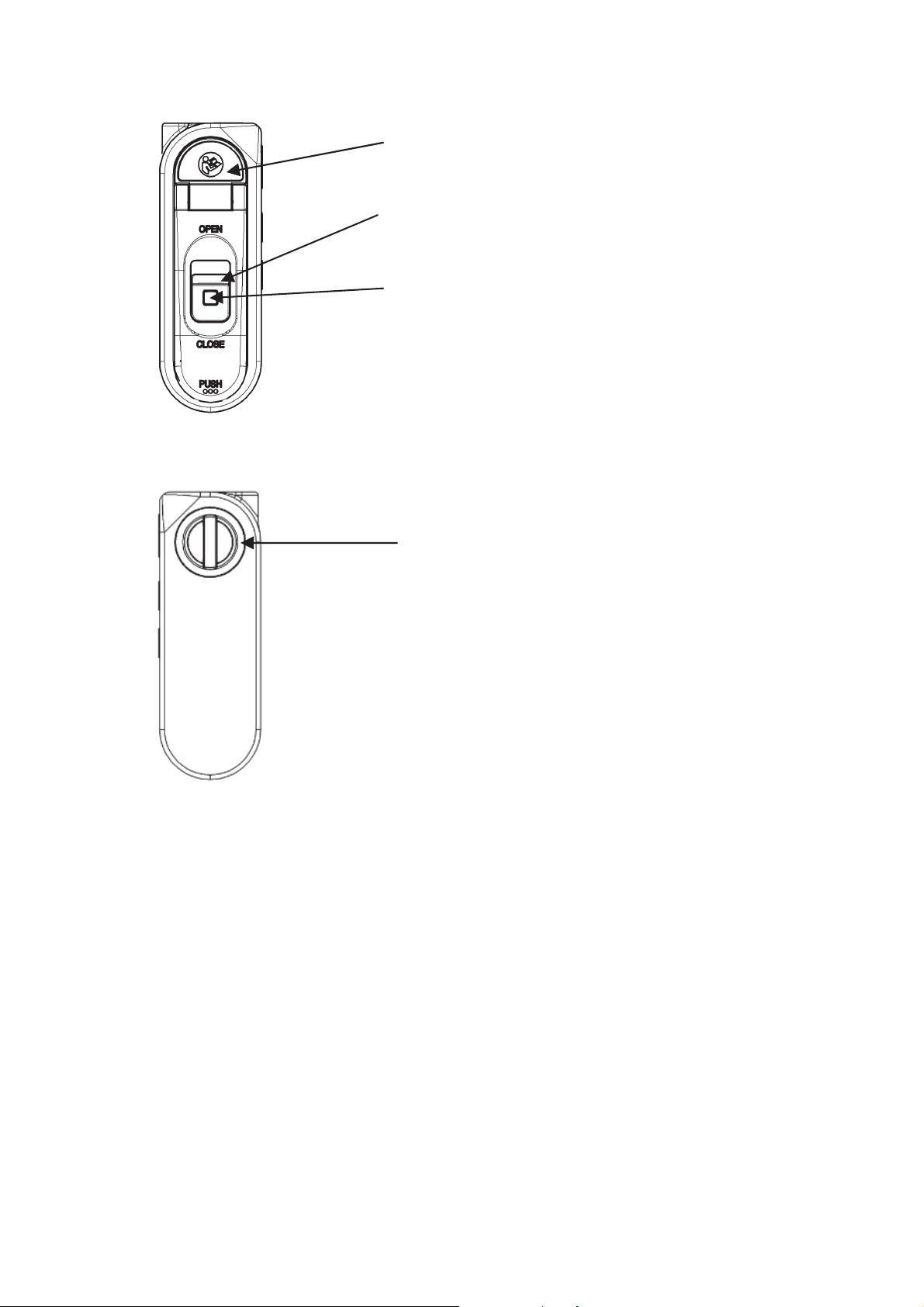
Ĭ
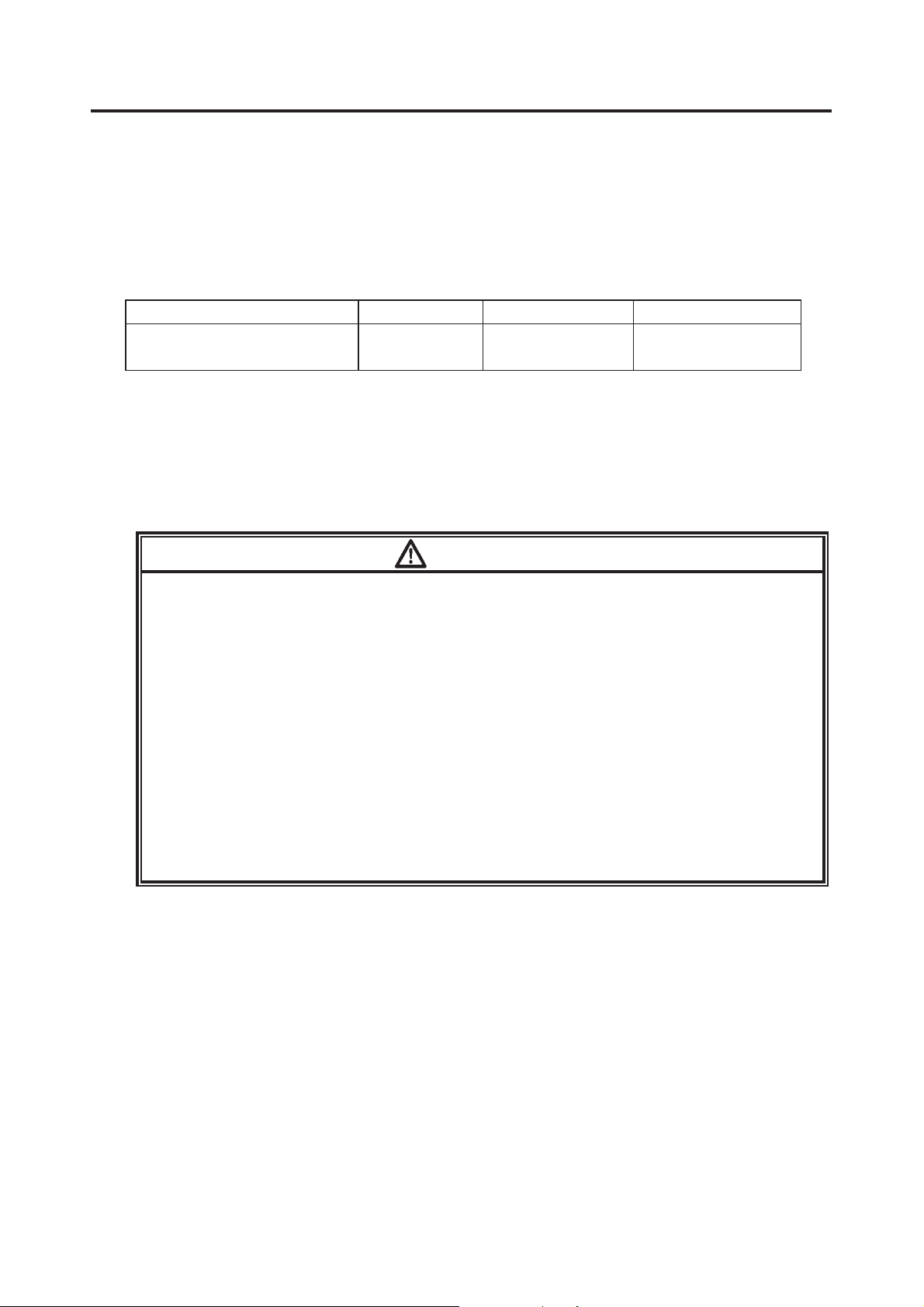
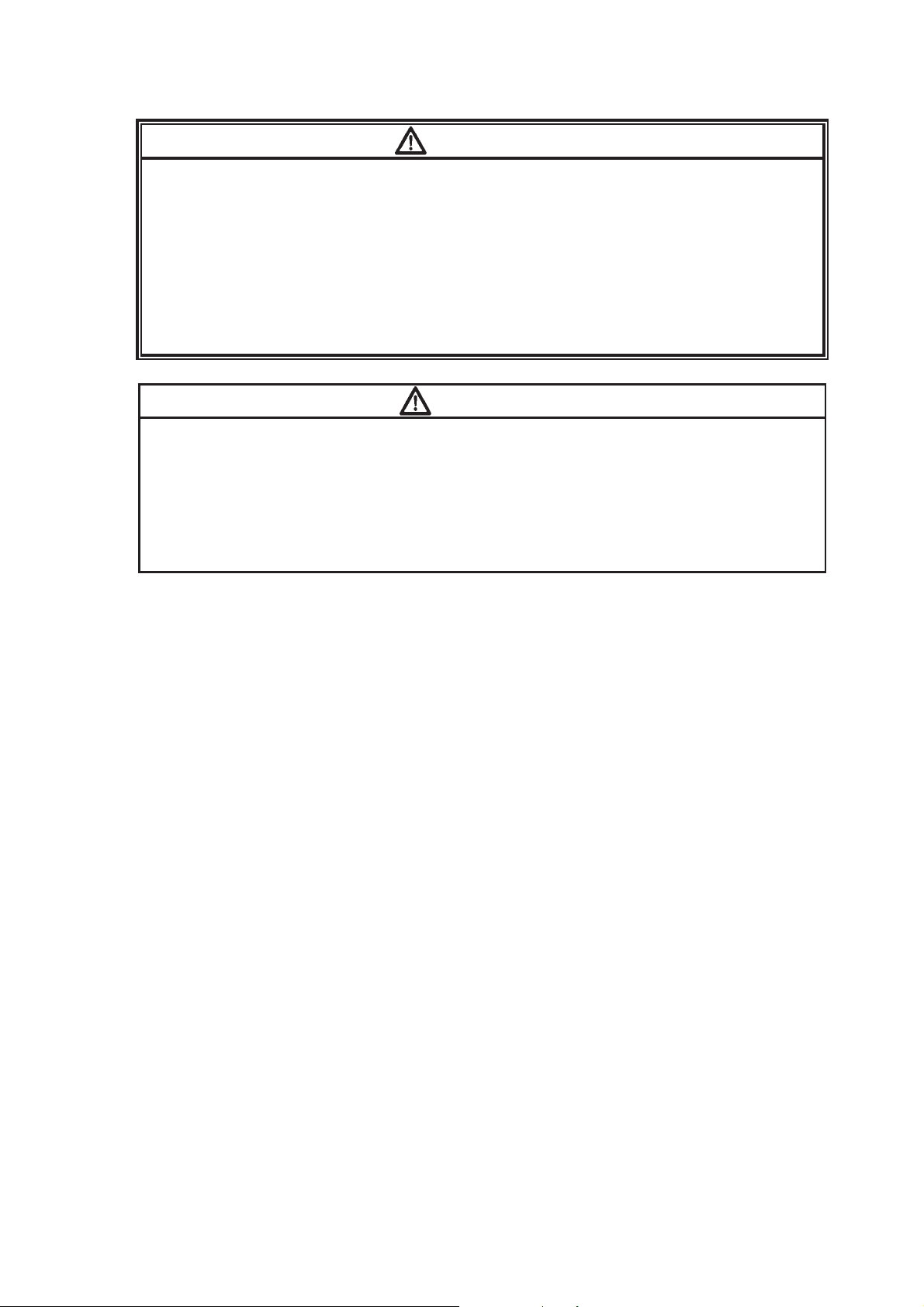
Unlock and open the battery compartment lid
by sliding the open/close lever towards
OPEN while pressing the lock button.
ĭ
Install a new battery according to the polarity
indication inside the battery compartment.
Ĭ
ĭ
After installing the battery, lock the battery
compartment lid by sliding the open/close
lever towards CLOSE while pressing over
“PUSH” on the lid.
7
3. Preparation
z Make sure that any foreign particles, such as hairs, are not held on the
battery compartment lid and dust is not adhered to the edge of the lid to
prevent water entering into the battery compartment area.
z Do not keep the compartment lid unlocked as the battery may
unexpectedly get out from the compartment.
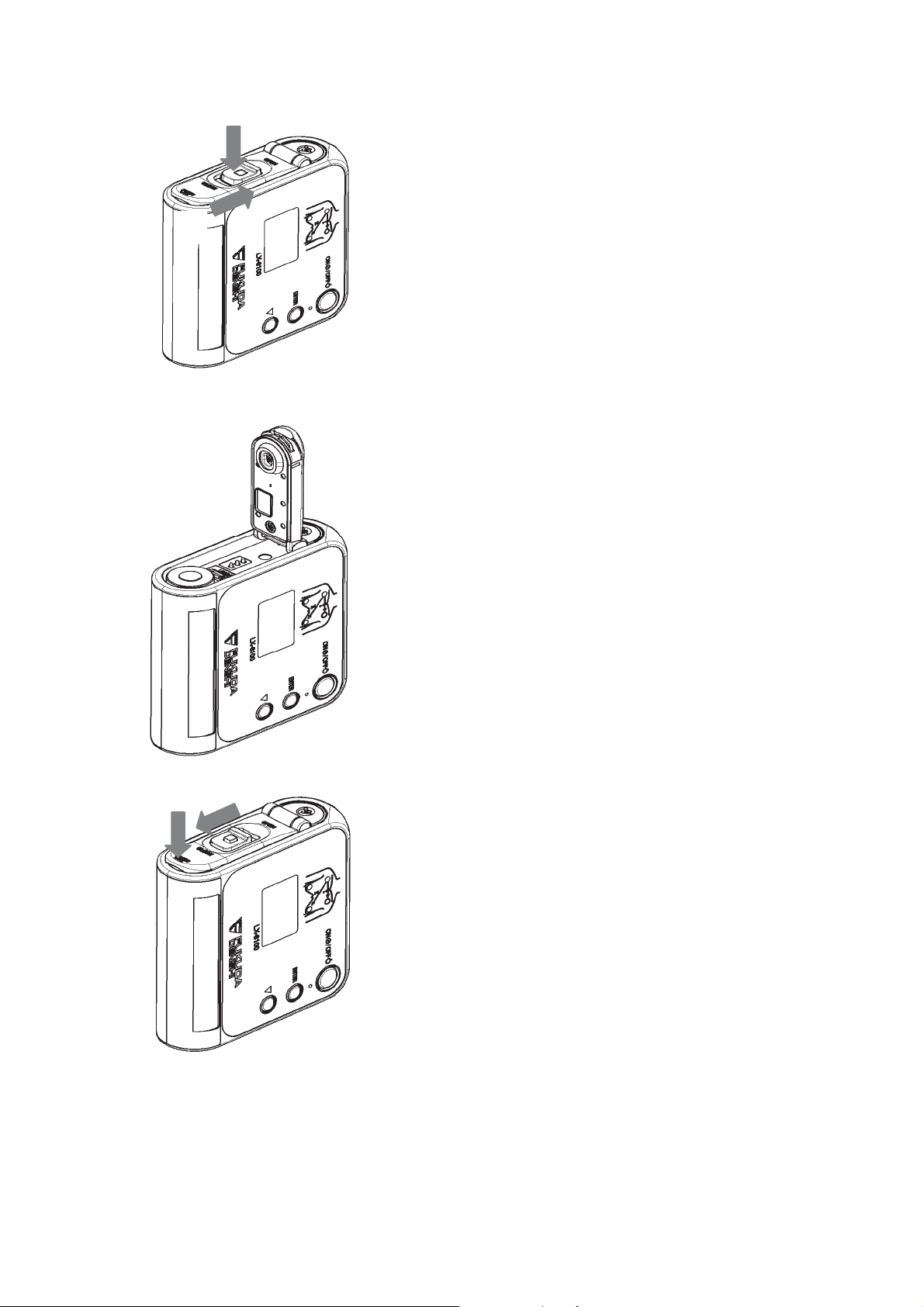
Make sure that the battery compartment lid
is locked. (If you can still see red, then it is
not locked properly.)
CAUTION
8
 Loading...
Loading...