Frontiers From Glycerol to Value-Added User Manual

FROM GLYCEROL TO
VALUE-ADDED PRODUCTS
EDITED BY : Patrick Cognet and Mohamed Kheireddine Aroua
PUBLISHED IN : Frontiers in Chemistry

Frontiers eBook Copyright Statement
The copyright in the text of
individual articles in this eBook is the
property of their respective authors
or their respective institutions or
funders. The copyright in graphics
and images within each article may
be subject to copyright of other
parties. In both cases this is subject
to a license granted to Frontiers.
The compilation of articles
constituting this eBook is the
property of Frontiers.
Each article within this eBook, and
the eBook itself, are published under
the most recent version of the
Creative Commons CC-BY licence.
The version current at the date of
publication of this eBook is
CC-BY 4.0. If the CC-BY licence is
updated, the licence granted by
Frontiers is automatically updated to
the new version.
When exercising any right under the
CC-BY licence, Frontiers must be
attributed as the original publisher
of the article or eBook, as
applicable.
Authors have the responsibility of
ensuring that any graphics or other
materials which are the property of
others may be included in the
CC-BY licence, but this should be
checked before relying on the
CC-BY licence to reproduce those
materials. Any copyright notices
relating to those materials must be
complied with.
Copyright and source
acknowledgement notices may not
be removed and must be displayed
in any copy, derivative work or
partial copy which includes the
elements in question.
All copyright, and all rights therein,
are protected by national and
international copyright laws. The
above represents a summary only.
For further information please read
Frontiers’ Conditions for Website
Use and Copyright Statement, and
the applicable CC-BY licence.
ISSN 1664-8714
ISBN 978-2-88963-577-1
DOI 10.3389/978-2-88963-577-1
About Frontiers
Frontiers is more than just an open-access publisher of scholarly articles: it is a
pioneering approach to the world of academia, radically improving the way scholarly
research is managed. The grand vision of Frontiers is a world where all people have
an equal opportunity to seek, share and generate knowledge. Frontiers provides
immediate and permanent online open access to all its publications, but this alone
is not enough to realize our grand goals.
Frontiers Journal Series
The Frontiers Journal Series is a multi-tier and interdisciplinary set of open-access,
online journals, promising a paradigm shift from the current review, selection and
dissemination processes in academic publishing. All Frontiers journals are driven
by researchers for researchers; therefore, they constitute a service to the scholarly
community. At the same time, the Frontiers Journal Series operates on a revolutionary
invention, the tiered publishing system, initially addressing specific communities of
scholars, and gradually climbing up to broader public understanding, thus serving
the interests of the lay society, too.
Dedication to Quality
Each Frontiers article is a landmark of the highest quality, thanks to genuinely
collaborative interactions between authors and review editors, who include some
of the world’s best academicians. Research must be certified by peers before entering
a stream of knowledge that may eventually reach the public - and shape society;
therefore, Frontiers only applies the most rigorous and unbiased reviews.
Frontiers revolutionizes research publishing by freely delivering the most outstanding
research, evaluated with no bias from both the academic and social point of view.
By applying the most advanced information technologies, Frontiers is catapulting
scholarly publishing into a new generation.
What are Frontiers Research Topics?
Frontiers Research Topics are very popular trademarks of the Frontiers Journals
Series: they are collections of at least ten articles, all centered on a particular subject.
With their unique mix of varied contributions from Original Research to Review
Articles, Frontiers Research Topics unify the most influential researchers, the latest
key findings and historical advances in a hot research area! Find out more on how
to host your own Frontiers Research Topic or contribute to one as an author by
contacting the Frontiers Editorial Oce: researchtopics@frontiersin.org
Frontiers in Chemistry 1 March 2020 | From Glycerol to Value-Added Products

FROM GLYCEROL TO VALUE-ADDED PRODUCTS
Topic Editors:
Patrick Cognet, National Polytechnic Institute of Toulouse, France
Mohamed Kheireddine Aroua, Sunway University, Malaysia and
Lancaster University, UK
Citation: Cognet, P., Aroua, M. K., eds. (2020). From Glycerol to Value-Added
Products. Lausanne: Frontiers Media SA. doi: 10.3389/978-2-88963-577-1
Frontiers in Chemistry 2 March 2020 | From Glycerol to Value-Added Products

Table of Contents
05 Editorial: From Glycerol to Value-Added Products
Mohamed Kheireddine Aroua and Patrick Cognet
07 A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel
Additives
Amin Talebian-Kiakalaieh, Nor Aishah Saidina Amin, Neda Najaafi and
Sara Tarighi
32 Experimental Determination of Optimal Conditions for Reactive Coupling
of Biodiesel Production With in situ Glycerol Carbonate Formation in a
Triglyceride Transesterification Process
Luma Sh. Al-Saadi, Valentine C. Eze and Adam P. Harvey
43 Selective Electrooxidation of Glycerol Into Value-Added Chemicals: A
Short Overview
Christophe Coutanceau, Stève Baranton and Roméo S. Bitty Kouamé
58 Extending Catalyst Life in Glycerol-to-Acrolein Conversion Using
Non-thermal Plasma
Lu Liu, Xiaofei Philip Ye, Benjamin Katryniok, Mickaël Capron, Sébastien Paul
and Franck Dumeignil
71 Selective Electrochemical Conversion of Glycerol to Glycolic Acid and
Lactic Acid on a Mixed Carbon-Black Activated Carbon Electrode in a
Single Compartment Electrochemical Cell
Ching Shya Lee, Mohamed Kheireddine Aroua, Wan Ashri Wan Daud,
Patrick Cognet, Yolande Pérès and Mohammed A. Ajeel
82 Catalytic Dehydration of Glycerol to Acrolein in a Two-Zone Fluidized Bed
Reactor
Benjamin Katryniok, Roger Meléndez, Virginie Bellière-Baca, Patrick Rey,
Franck Dumeignil, Nouria Fatah and Sébastien Paul
94 Glycerol to Glyceraldehyde Oxidation Reaction Over Pt-Based Catalysts
Under Base-Free Conditions
Ayman El Roz, Pascal Fongarland, Franck Dumeignil and Mickael Capron
103 Esterification of Glycerol With Oleic Acid Over Hydrophobic
Zirconia-Silica Acid Catalyst and Commercial Acid Catalyst: Optimization
and Influence of Catalyst Acidity
Pei San Kong, Yolande Pérès, Wan Mohd Ashri Wan Daud, Patrick Cognet
and Mohamed Kheireddine Aroua
114 Peculiarities of Glycerol Conversion to Chemicals Over Zeolite-Based
Catalysts
Oki Muraza
125 A Novel Strategy for Selective O-Methylation of Glycerol in Subcritical
Methanol
Sophie Bruniaux, Rajender S. Varma and Christophe Len
Frontiers in Chemistry 3 March 2020 | From Glycerol to Value-Added Products

132 Recent Progress in Synthesis of Glycerol Carbonate and Evaluation of its
Plasticizing Properties
Pascale de Caro, Matthieu Bandres, Martine Urrutigoïty, Christine Cecutti
and Sophie Thiebaud-Roux
145 Two-Step Purification of Glycerol as a Value Added by Product From the
Biodiesel Production Process
Abdul Aziz Abdul Raman, Hooi W. Tan and Archina Buthiyappan
154 Techno-Economic Analysis of Glycerol Valorization via Catalytic
Applications of Sulphonic Acid-Functionalized Copolymer Beads
Luma Sh. Al-Saadi, Valentine C. Eze and Adam P. Harvey
Frontiers in Chemistry 4 March 2020 | From Glycerol to Value-Added Products

Editorial: From Glycerol to
5
Value-Added Products
published: 11 February 2020
doi: 10.3389/fchem.2020.00069
EDITORIAL
Edited and reviewed by:
Florent Allais,
AgroParisTech Institut des Sciences et
Industries du Vivant et de
L’environnement, France
*Correspondence:
Patrick Cognet
patrick.cognet@ensiacet.fr
Specialty section:
This article was submitted to
Green and Sustainable Chemistry,
a section of the journal
Frontiers in Chemistry
Received: 15 January 2020
Accepted: 21 January 2020
Published: 11 February 2020
Citation:
Aroua MK and Cognet P (2020)
Editorial: From Glycerol to
Value-Added Products.
Front. Chem. 8:69.
doi: 10.3389/fchem.2020.00069
Mohamed Kheireddine Aroua
1
Centre for Carbon Dioxide Capture and Utilisation, School of Science and Technology, Sunway University, Subang Jaya,
Malaysia,2Department of Engineering, Faculty of Science and Technology, Lancaster University, Bailrigg, United Kingdom,
3
Laboratoire de Génie Chimique, Université de Toulouse, CNRS, INPT, UPS, Toulouse, France
Keywords: glycerol, green chemistry, catalysis, process, activation, added value bio-based products,
electrochemical conversions
1,2
and Patrick Cognet
3
*
Editorial on the Research Topic
From Glycerol to Value-Added Products
Increases in biodiesel production and the demand for oleochemical-based products have led to the
generation of huge amounts of crude glycerol, whichhasgiven birth to new challenges regarding its
sustainable use. Although there is a wide range of potential uses for crude glycerol, there are limited
by its degree of purity, which affects its physical, chemical, and biological properties. The chemical
transformation of glycerol has thus become a major point of interest for crude glycerol valorization.
High added value products can be obtained from glycerol through different pathways, such
as oxidation, carbonylation, reforming, acetalyzation, etherification, esterification, dehydration,
hydrogenolysis, etc. Starting from a poly-hydroxylated molecule, all these chemical routes generally
lead to complex mixtures and are not selective. In order to develop further industrial processes,
progresses must be achieved to increase yield and selectivity, reduce reaction times, and ensure
that work in media is as clean as possible. The catalyst choice is also of great importance since it
impacts the selectivity. Heterogeneous ones must be preferred for an industrial process because
they can be easily separated. One other important aspect is the quality of the starting glycerol; it
has a great influence on the synthesis performance. This special issue gathers some contributions
focused on recent advances in some key aspects of glycerol transformation processes: the crude
glycerol purification prior to use for chemical transformation, the use of new synthesis media,
the use of non-thermal activation techniques such as electrochemistry and plasma, as well as the
synthesis, use, and characterization of new heterogeneous catalysts. These principles are applied to
the optimization of the synthesis of key added-value products, such as glycerol carbonate, glycerol
oleates, glyceraldehyde, acrolein, glycolic acid, and lactic acid. Process aspects are also considered,
such as the purification process or fluidized bed technology.
This special issue is a collection of 3 critical reviews and 10 original research articles.
The use of an electron as a clean reagent is of great interest to the goal of transforming glycerol
in added-value products in a sustainable manner. As direct electron transfer for a polyhydroxylated
molecule like glycerol would lead to the creationof a variety of products, an indirect electrocatalytic
process is envisaged in this special issue.
lack-activated carbon electrodes for glycolic and lactic acid production. Glycolic acid was then
b
Lee et al. have investigated the use of mixed carbon
obtained with good yield and selectivity. On the other hand, Coutanceau et al. have proposed an
overview of different catalytic systems and conditions to control the products selectivity obtained
from glycerol electrooxidation.
Catalyst deactivation is also a crucial issue to overcome on the road to a sustainable
industrial process. Liu et al. have demonstrated the benefits of non-thermal plasma technology
to avoid silice-supported silicotungstic acid catalyst deactivation during glycerol dehydration for
acrolein production.
Glycerol purity is crucial for its further selective transformation into various products.
Therefore, in order to valorize crude glycerol for synthetic purposes, a purification step is necessary.
Frontiers in Chemistry | www.frontiersin.org 1 February 2020 |
Volume 8 | Article 69

Aroua and Cognet Editorial: From Glycerol to Value-Added Products
6
Abdul Raman et al. propose, in this special issue, a dual-step
purification method t hat includes acidification and ion exchange
operations, which allows it to reach a 98.2% purity.
This special issue also focuses on targeted molecules derived
from glycerol. de Caro et al. have presented the recent advances
concerning glycerol carbonate (GC) synthesis. Amongst the
different routes, DMC and glycerol are good precursors,
leading to GC through transcarbonation under mild conditions.
Bruniaux et al. have reported the selective conversion of
glycerol into 3-methoxypropan-1,2diol in mild yields. Al-Saadi
et al. have investigated a reactive coupling that associates the
transesterification of rapeseed oil into a fatty acid methyl ester
and glycerol carbonate in a one-step process by introducing
triazabicyclodecene guanidine as a catalyst.
Viable industrial chemical processes imply the use of
heterogeneous catalysts for easy product recovery and catalyst
recycling. Moreover, catalyst activity strongly relies on its
physical properties, such as hydrophilicity/hydrophobicity,
acidity, stability to water, etc. Muraza et al. have investigated
the performances of natural zeolites and natural clays as lowcost catalysts. For a specific application, such as esterification
with oleic acid, new catalysts must be developed, and these
should exhibit good proprieties in terms of acidity and
hydrophobicity. Kong et al. have developed, characterized,
and studied hydrophobic zirconia-silica acid catalysts. They
obtained 80% glycerol conversion together with 60% monooleate selectivity. Pt-based solid catalysts deposited on various
supports have been studied by El Roz et al. when applied to the
synthesis of glyceraldehyde from glycerol. The best activity was
obtained for Pt/g-Al2O3, whereas best selectivity was obtained
using Pt/SiO2. Sulphonic acid-functionalized copolymer beads
were also synthetized, characterized, and used for solketal
synthesis from glycerol. Al-Saadi et al. succeeded in optimizing
the process using a two-step acetone feeding process. A technicoeconomic analysis revealed that this process could compete
with the current industrial one. This reaction—the acetalization
of glycerol through acid catalysis—was also investigated by
Talebian-Kiakalaieh et al. They give, in this special issue, a
comprehensive study of the impact of the different operating
parameters—a prerequisite for biorefinery development.
To succeed in these industrial implementations, a chemical
engineering approach has to be coupled with a chemical one.
For this purpose, Katryniok et al. de veloped a two-zone fluidized
bed reactor to carry out the gas-phase dehydration of glycerol
to acrolein, using phosphotungstic acid supported on silica
as a catalyst. The fluidization quality, the catalyst mechanical
stability, and the influence of the operating conditions were
successively studied, thus showing, for example, the crucial
part the O2/glycerol ratio plays for the purposes of conversion
and selectivity.
The contributions made to this special issue of "From Glycerol
to Value-Added Products” underline the variety of the research
work carried out in the field of the valorization of glycerol and
processing of raw material at low cost, and they tackle both
the re actional aspects as catalysis, processes, and even economic
aspects. Many chemical applications have been covered in this
special issue, thus showcasing all the potential of glycerol. We
hope that the issue will inspire the readers to further contribute
to this exciting field of glycerol valorization.
Finally, we would like to thank all authors for their valuable
contributions to this special issue, and we wish them success in
their research.
AUTHOR CONTRIBUTIONS
MA and PC thank all contributors for their original articles
submissions to this special issue.
ACKNOWLEDGMENTS
This special issue is the result of a long term collaboration
between University of Malaya and Sunway University in Malaysia
and Toulouse University in France. We strongly acknowledge the
French Embassy in Malaysia for its support and all contributors
to this collection.
Conflict of Interest: The authors declare that the research was conducted in the
absence of any commercial or financial relationships that could be construed as a
potential conflict of interest.
Copyright © 2020 Aroua and Cognet. This is an open-access article distributed
under the terms of the Creative Commons Attribution License (CC BY). The use,
distribution or reproduction in other forums is permitted, provided the original
author(s) and the copyright owner(s) are credited and that the original publication
in this journal is cited, in accordance with accepted academic practice. No use,
distribution or reproduction is permitted which does not comply with these terms.
Frontiers in Chemistry | www.frontiersin.org 2 February 2020 | Volume 8 | Article 69

published: 26 November 2018
7
doi: 10.3389/fchem.2018.00573
A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives
REVIEW
Mohamed Kheireddine Aroua,
Edited by:
Sunway University, Malaysia
Reviewed by:
Abdul Aziz Abdul Abdul Raman,
University of Malaya, Malaysia
Maan Hayyan,
University of Malaya, Malaysia
*Correspondence:
Nor Aishah Saidina Amin
noraishah@cheme.utm.my
Specialty section:
This article was submitted to
Green and Sustainable Chemistry,
a section of the journal
Frontiers in Chemistry
Received: 27 August 2018
Accepted: 05 November 2018
Published: 26 November 2018
Citation:
Talebian-Kiakalaieh A, Amin NAS,
Najaafi N and Tarighi S (2018) A
Review on the Catalytic Acetalization
of Bio-renewable Glycerol to Fuel
Additives. Front. Chem. 6:573.
doi: 10.3389/fchem.2018.00573
Amin Talebian-Kiakalaieh
1
Faculty of Petrochemicals, Iran Polymer and Petrochemical Institute (IPPI), Tehran, Iran,2Chemical Reaction Engineering
Group, Faculty of Chemical and Energy Engineering, Universiti Teknologi Malaysia (UTM), Skudai, Malaysia,3Iran Industrial
Design Company, Tehran, Iran
1,2
, Nor Aishah Saidina Amin
2
*
, Neda Najaafi3and Sara Tarighi
1
The last 20 years have seen an unprecedented breakthrough in the biodiesel industry
worldwide leads to abundance of glycerol. Therefore, the economic utilization of
glycerol to various value-added chemicals is vital for the sustainability of the biodiesel
industry. One of the promising processes is acetalization of glycerol to acetals
and ketals for applications as fuel additives. These products could be obtained by
acid-catalyzed reaction of glycerol with aldehydes and ketones. Application of different
supported heterogeneous catalysts such as zeolites, heteropoly acids, metal-based
and acid-exchange resins have been evaluated comprehensively in this field. In this
review, the glycerol acetalization has been reported, focusing on innovative and potential
technologies for sustainable production of solketal. In addition, the impacts of various
parameters such as application of different reactants, reaction temperature, water
removal, utilization of crude-glycerol on catalytic activity in both batch and continuous
processes are discussed. The outcomes of this research will therefore significantly
improve the technology required in tomorrow’s bio-refineries. This review provides
spectacular opportunities for us to use such renewables and will consequently benefit
the industry, environment and economy.
Keywords: glycerol, acetalization, fuel additives, heterogeneous catalysts, acetone, ketone
INTRODUCTION
In the early Twentieth century, petroleum exploitation and its cracking to simple hydrocarbons
was one of the most influential factors on human life. Fossil fuel has been the main source of energy
for almost a century. Current oil production rate reach approximately 12 Mt/day and its demand
is predicted to rise dramatically to around 16 Mt/day by 2030 due to the significant increase in
the world population and industrial development (Lin and Huber, 2009; Talebian-Kiakalaieh et al.,
2014). Various types of environmental concerns such as massive amount of carbon dioxide and the
depletion of fossil fuel resources have become the main concerns in maintaining sustainability. Low
cost supply of fossil fuel (<100 USD/b arrel) will no longer be available by 2040 (Posada et al., 2009).
Many efforts are geared toward finding new sources of alternative energy to supplant the current
non-renewable fossil fuels. Biomass is selected as a promising alternative source of energy to meet
the significant energy demand as well as to reduce environmental concerns. As a result, a new term,
“bio-refinery,” has emerged recently to describe a facility for converting biomass to food, fuel, and
value-added chemicals.
Frontiers in Chemistry | www.frontiersin.org 1 November 2018
| Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
8
Biodiesel is one of the most important and valuable alternative
liquid fuel in the transportation sector. As a substitute to fossil
fuels, biodiesel could reduce chemical emissions such as sulfur
dioxide (100%), unburned hydrocarbon (68%), and polycyclic
aromatic hydrocarbon (80–90%). In addition, biodiesel is
environmentally friendly, technically feasible and biodegradable
(
Fazal et al., 2011). The worldwide production of biodiesel is
edicted to increase to 141 billion liters by 2022 from 110
pr
billion liters in 2013, mainly due to the contribution of European
Renewable Energy Directive (EU-RED) and Renewable Fuel
Standard (RFS) in the United States. This would improve the
global production to almost 70% by the year of 2022, compared
to its average from 2010 to 2012 (SSI Review, 2014). Hence, it is
vital to enhance the economic feasibility of biodiesel production
through the modification of three major aspects of the process,
namely the raw materials used in the process, the synthesis
method, and the byproducts (De Torres et al., 2011).
Briefly, biodiesel is obtained via the transesterification of
animal fat or vegetable oils in the presence of methanol under
basic catalysis condition (Menchavez et al., 2017). Glycerol as a
byproduct is produced at a high 1:10 glycerol to biodiesel weight
ratio. The increasing demand for biodiesel caused a glycerol oversupply, thus reducing the commercial price of glycerol to almost
8 cents/lb recently compared to 25 cents/lb in 2004 (Clomburg
and Gonzalez, 2013). It is expected the global surge in biodiesel
production lead to production of >41.9 billion liter of crude
glycerol by 2020 (Nanda et al., 2014a). Fabrication of low cost
glycerol is important since it can be transformed to many valueadded chemicals (more than 2,000 products) in various reaction
pathways (Nanda et al., 2017; Nguyen et al., 2017; Tangestanifard
and Ghaziaskar, 2017). Traditionally, glycerol was produced from
the production of fatty acids (47%), followed by soaps (24%), fatty
alcohols (12%), and the biodiesel industry (9%). However, since
2009 the biodiesel industry is the main producer that supplies
over 64% of the glycerol (Abad and Turon, 2012). Thus, glycerol
consumption is expected to increase significantly by up to 50% in
2020. Its demand was 2,247.2 kilo tons in 2013 and is expected to
reach 3,469.2 kilo tons by 2020 (Ayoub and Abdullah, 2012; Villa
et al., 2015).
Traditional uses of glycerol include in the textiles (24%), food
and beverages (21%), cosmetics and toiletries (18%), drugs (18%),
tobacco (6%), and paper and printing (5%), and others, cannot
satisfy the dramatic surge in production of this compound. Thus,
it is necessary to find new routes of conversion for this chemical
in order to avoid market saturation. Table 1 lists the possible
catalytic processes and products that can be produced from
glycerol.
Undoubtedly, one of the most promising glycerol applications
is production of fuel additives such as cyclic acetals and ketals
with aldehydes and ketones, respectively (Deutsch et al., 2007).
Generally, fuel (Wang et al., 2004) and diesel additive (Ribeiro
et al., 2007) is a material that improves the cleanliness of different
parts of the engine (e.g., carburetor, fuel injector and intake
valve), promotes complete combustion, reduces fuel gelling and
choking of nozzle, as well as reducing corrosion impact on
different parts of the engine. The result is improved engine
performance, reduced emission and reduced fuel consumption.
It could significantly reduce the particulate emissions of diesel
fuel (Rakopoulos et al., 2008) (e.g., reduction of CO2and NO
emission) and increase oxygen and air concentration (Lin and
Chen, 2006). In addition, it could improve the thermal stability
of jet fuels as well as significantly reduce (1–70%) deposits in
jet engines (Forester et al., 2003). Methyl tertiary butyl ether
(MTBE) was widely used as octane accelerator in gasoline in
the early 1980’s (Franklin et al., 2000). For more than two
decades, it was the most economical oxygenate additive used
by the refineries to reduce production cost of Reformulated
Gasoline (RFG) (Romanow, 1999). However, the International
Agency of Research on Cancer (IARC) classified RFG as a major
health risk threat in 2000 (U.S. EPA, 1996). Thus, ketalization
reaction between glycerol and acetone where 2, 2-dimethyl1, 3-dioxolane-4- methanol known as solketal, is formed as
the condensation product over an acid catalyst is shown in
Figure 1. Solketal, an oxygenate fuel additives, could reduce
the particulate emission and improve the cold flow properties
of liquid transportation fuels (Pariente et al., 2008). It helps
to reduce the gum formation, improves the oxidation stability,
and enhances the octane number when added to gasoline
(Mota et al., 2010). Maksimov et al. (2011) reported its use
as a versatile solvent and a plasticizer in the polymer industry
and a solubilizing and suspending agent in pharmaceutical
preparations. More importantly, the aquatox fish test on the
toxicity of the solketal showed that solketal (with a LC50 for
fish to be as hig h as 3,162 ppm) has demonstrated much less
environmental toxicity than the common fuel additive, MTBE,
with a LC50 of 1,000 ppm (Nanda et al., 2014a).
Thus, the main objective of this review is to collect
information about the latest advances in glycerol conversion
to oxygenated fuel additives from biomass sources. In
addition, the review addresses the critical knowledge gaps for
enhancing conversion and selectivity in glycerol acetalization.
Fundamentals of reaction mechanisms for the acid-catalyzed
conversion of glycerol into solketal are presented. Some aspects
such as the influence of various reaction parameters, reactant
selection, reaction temperature, catalyst acidity, water removal,
and reactor design are exclusively summarized and discussed.
Finally, the application of crude-glycerol is discussed in batch
and continuous-flow processes.
CATALYTIC ACETALIZATION OF GLYCEROL
Glycerol is an organic compound which is a low toxicity alcohol
that consists of a three-carbon chain with a hydroxyl group
attached to each carbon. These groups made glycerol hygroscopic
and water-soluble. Glycerol has low volatility and low vapor
pressure and is nontoxic to both humans and the environment.
Physically, glycerol is a clear, colorless, odorless, viscous,
and sweet-tasting liquid. Table 2 lists the physico-chemical
characteristics of glycerol (
rst discovered by K.W. Scheele, a Swedish researcher, in 1779.
fi
He produced a material with a sweet taste by heating olive oil
with lead oxide. Three decades later, a French chemist, Michel
Rahmat et al., 2010). Glycerol was
Frontiers in Chemistry | www.frontiersin.org 2 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
9
)
(C + H
2
Carbon+
)
2
(CO + H
ROH
(Continued)
Hydrogen
Syngas
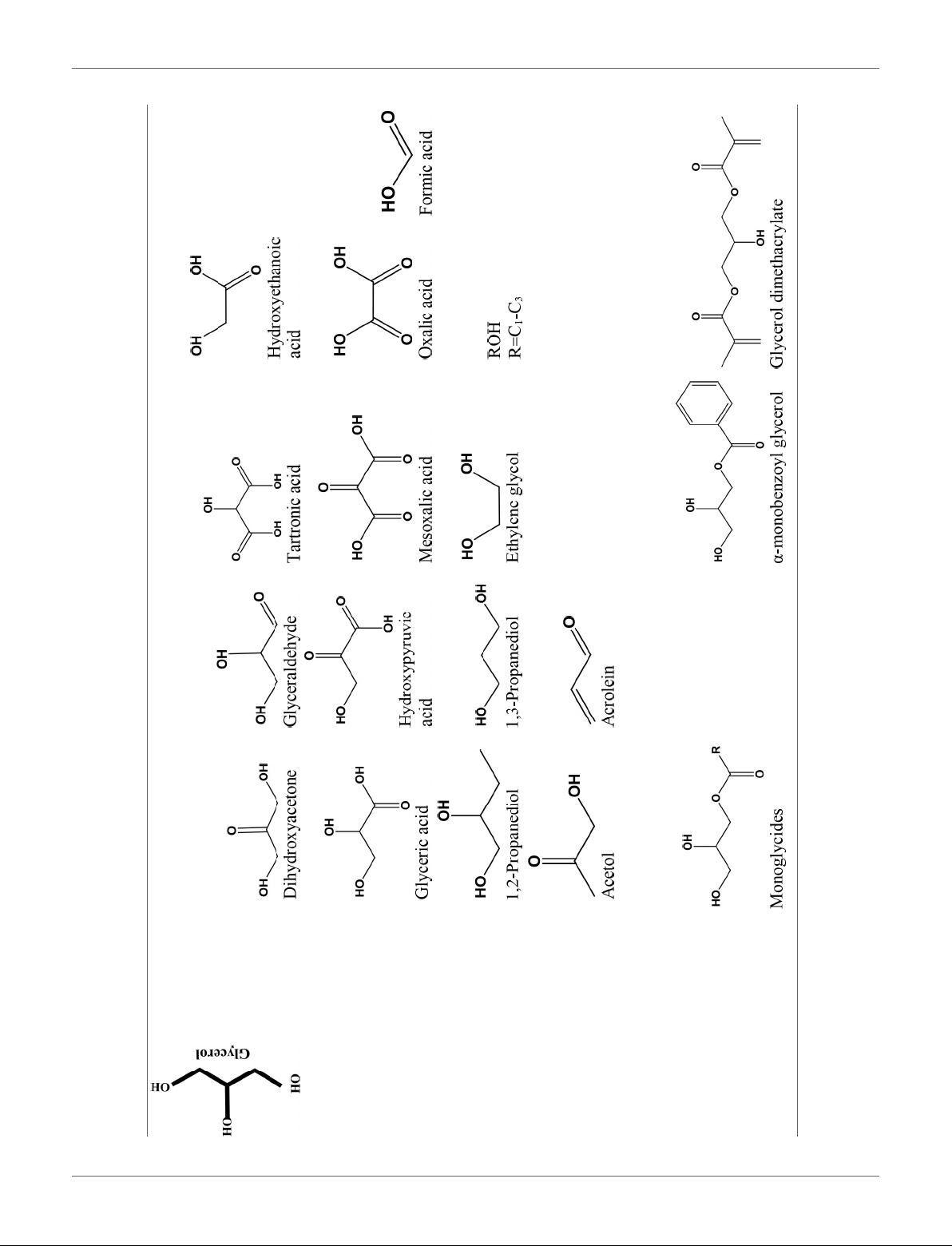
Alcohol
TABLE 1 | Catalytic conversion of glycerol into value-added chemicals by different processes.
2n
H
n
C
Olefin
2n+2
H
n
C
Alkane
Oxidation
Hydrogenolysis
Dehydration
Pyrolysis, Gasification
Trans- Esterification
Frontiers in Chemistry | www.frontiersin.org 3 November 2018 | Volume 6 | Article 573
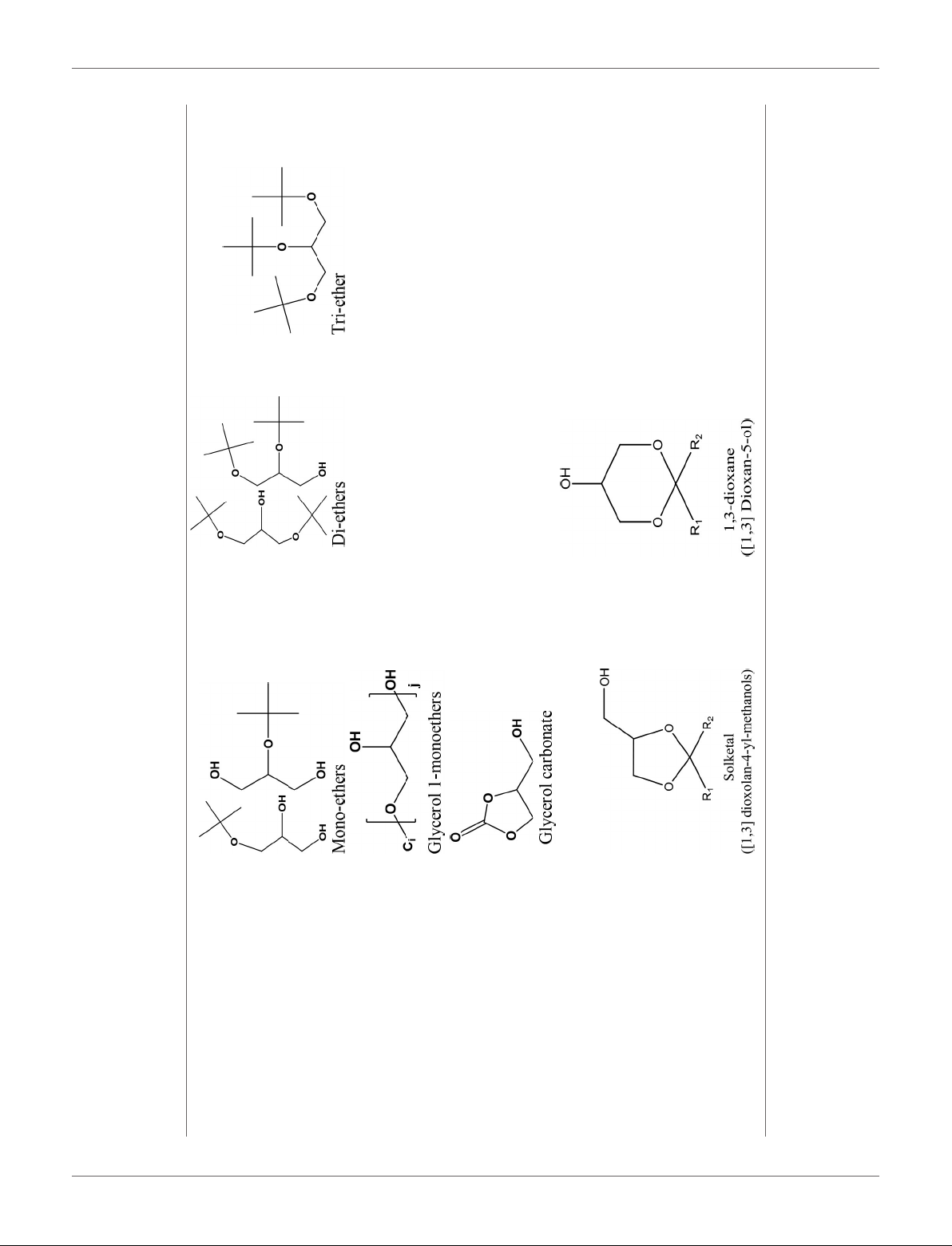
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
10
Etherification
TABLE 1 | Continued
Oligomerization, Polymerization Polyglycerol methacrylates
Carboxylation
Acetalization
Different catalytic conversion of glycerol into value-added chemicals.
Frontiers in Chemistry | www.frontiersin.org 4 November 2018 | Volume 6 | Article 573
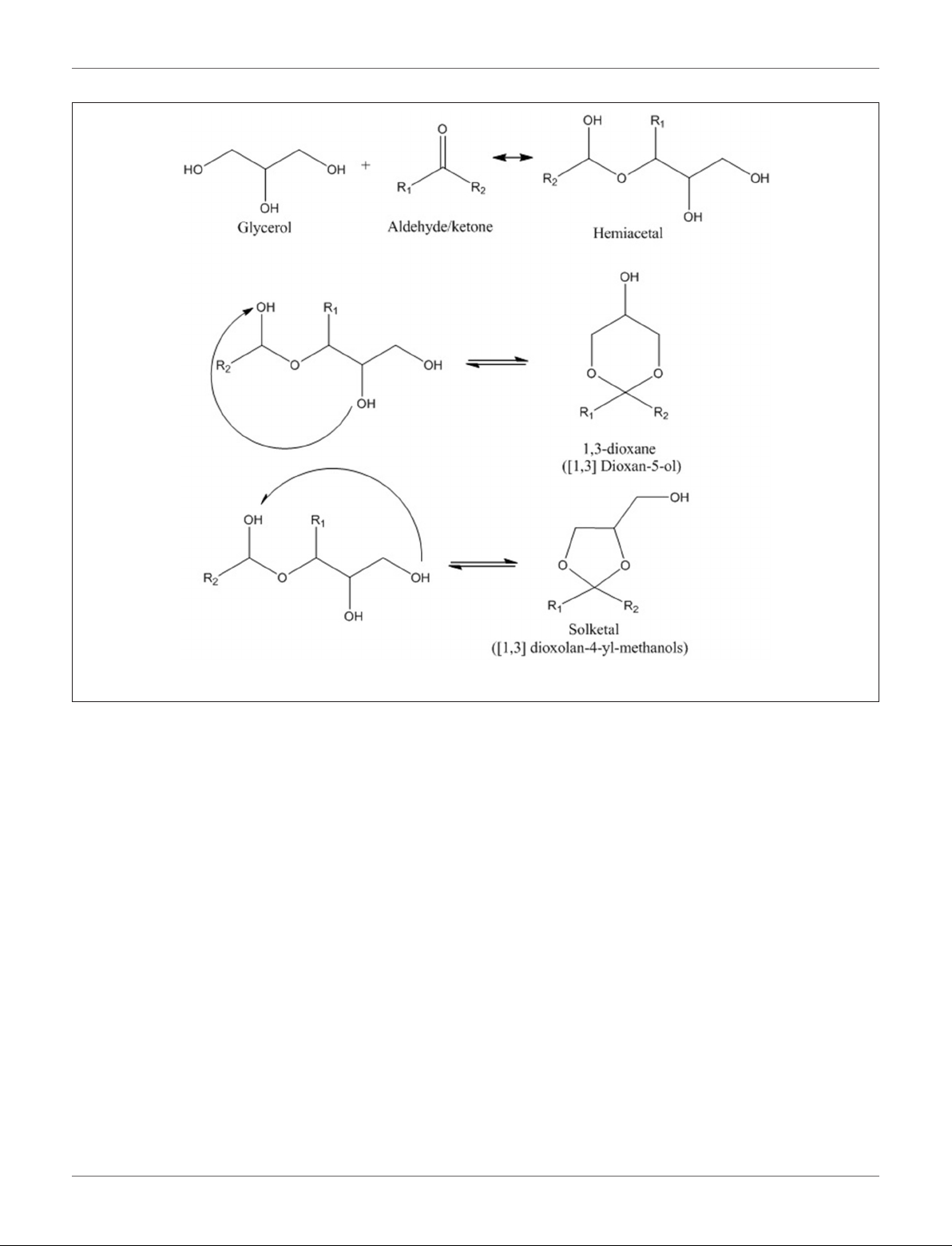
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
11
FIGURE 1 | Reaction mechanism of glycerol with aldehydes/ketones.
Eugene Chevrel, named it “glycerin.” He then proposed fatty
acids ethereous chemical formulas along with glycerin formulas
in vegetable oils and animal fats. Finally, his study on the
production of fatty acids (FA) from the reaction of fatty materials
with lime and alkali was reported, which was the first industrial
attempt in this field (Gesslein, 1999). It is an irrefutable fact that
the discovery of glycerol has brought significant breakthroughs
in the production different products. Re cent advances in catalyst
and bio-refinery industries have provided great opportunities
for industrialization of bio-based processes which could produce
food, fuel, and chemicals from glycerol.
Based on the literature in the last decades, catalytic
acetalization of glycerol process could be categorized by three
generations. In fact, the first generation of studies on the catalytic
acetalization of glycerol to fuel additives reported in the presence
of homogenous catalysts and a solvent. Fischer and co-workers
pioneered the synthesis of solketal from glycerol and acetone,
catalyzed by hydrogen chloride, in a batch reactor (Fischer,
1895). A few years later, a similar process by Fischer and Pfahler
(1920) was applied for the ketalizationof glycerol with anhydrous
sodium sulfate
(1945) reported the
and hydrogen chloride. Newman and Renoll
preparation of solketal in a three-neck flask
using reflux and mechanical stirrer in 1948. To obtain high
solketal yield, they used pTSA monohydrate as the catalyst and
petroleum ether as the reaction medium. After the reaction, the
products were separated by reducing the pressure and distilling.
The drawback of this system was its very long reaction time (21–
36 h). Generally, the reaction of glycerol with aldehydes/ketones
is conducted under homogenous Lewis catalysts (
010) or
2
mineral acids such as HF, HCl, H3PO4,H2SO4,and p-
Ruiz et al.,
toluenesulfonic acid (pTSA) to form solketal (1,2-isopropylidene
glycerol, 2,2-dimethyl-1, or 3-dioxolane-4-methanol) (Sato et al.,
2008; Coleman and Blankenship, 2010; Suriyapradilok and
Kitiyanan, 2011; Nanda et al., 2014a; Sun et al., 2017). The first
generation of studies was stopped more than half a century ago
due to the economic barriers. Indeed, availability of cheap fossil
fuels was the main obstacle for bio-based processes.
The second generation of catalytic acetalization of glycerol
performed in the presence of heterogeneous catalysts and a
solvent as reaction medium. Indeed, this group of investigations
on bio-based glycerol acetalization to fuel additives started
after the introduction of large amount of inexpensive glycerol
from biodiesel industry at the end of the Twentieth century.
Science and technological advances in synthesis of heterogeneous
Frontiers in Chemistry | www.frontiersin.org 5 November 2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
12
TABLE 2 | Physico-chemical properties of glycerol.
Properties Values
Chemical formula CH2OH–CHOH–
Formula weight 92.09
Form and color Colorless and liquid
Specific gravity 1.260
Melting point 17.9◦C
Boiling point 290◦C
Solubility in 100 parts
Water Infinitely
Alcohol Infinitely
Ether Insoluble
Vapor pressure in 760 mmHg 290◦C
Heat of fusion at 18.07◦C 47.49 cal/g
Viscosity liquid glycerol
100%
50% 25 cP
Diffusivity in (DL×105sq.cm/s)
i-Amyl alcohol 0.12
Ethanol 0.56
Water 0.94
Specific heat glycerol in
aqueous solution (mol%)
2.12 0.961 0.960
4.66 0.929 0.924
11.5 0.851 0.841
22.7 0.765 0.758
43.9 0.670 0.672
100 0.555 0.576
15◦C (cal/g◦C) 30◦C(cal/g◦C)
CH2OH
50/4
10 cP
catalysts and their applications provide spectacular opportunities
for further investigations in this field. In fact, homogenous
catalysts like Lewis catalysts and strong mineral acids are
known to not only cause difficult purification and product
separation, but also environmental and corrosion problems.
Several studies were employed to solve the shortcomings
of homogenous catalysts using heterogeneous catalysts by
evaluating the most important characteristics of a catalyst
which are cost, accessibility, efficiency, easy removal, and good
activity at mild conditions. One of the earliest studies on the
use of heterogeneous acid catalyst was reported by Deutsch
et al. (2007). They applied Amberlyst-36 with various solvents
(dichloromethane, chloroform, toluene, and benzene) as organic
solvents to obtained >62% glycerol conversion in the presence
of three different reactants (acetone, benzene, and furfural) in
a batch reactor (Deutsch et al., 2007). Application of Hβ and
MMT-K10 zeolites were reported in catalytic acetalization of
glycerol to fuel additives in the presence of chloroform as solvent
and benzaldehyde as reactant. The results indicated that >95%
of solketal yield was obtained at glycerol to benzaldhyde molar
ratio of 1.1/1 and after 6 h of reaction time (Deutsch et al.,
2007). In addition,
in cat
alytic acetalization of glycerol by Umbarkar et al. (2009).
toluene is another solvent which was utilized
They reported about 72% glycerol conversion over MoO3/SiO
catalyst at optimum reaction of 1.1/1 molar ration of glycerol
to benzaldehyde, reaction temperature of 100◦C and in 8 h. As
mentioned earlier, simultaneous application of heterogeneous
catalysts and solvent is one of the old methods in catalytic
acetalization process and there are limited number of studies
in this field in the last decade. However,
Nanda et al. (2014b)
reported one of the successful studies on application of ethanol
s solvent in the presence of Amberlyst-35 as catalyst to reach
a
more than 74% solketal yield at 2/1 molar ratio of glycerol to
acetone and quite very low reaction temperature of 25–45◦C.
Indeed, they could significantly reduce the reaction temperature
by application of ethanol as solvent.
Finally, the third generation is solvent free glycerol
acetalization reaction by heterogeneous catalysts in batch
or continuous processes. In fact, new heterogeneous catalysts are
active enough to push the catalytic process to produce desired
products (solketal) at high reaction conversion even without
solvent (Chen et al., 2018a; Ferreira et al., 2018). In this regard,
different types of heterogeneous acid catalysts have been recently
applied in the acetalization of various carbonyl compounds with
glycerol such as activated carbons, montmorillonite (MMT),
zeolites, metal-based catalysts, ionic liquids, supported multiwalled carbon nano-tubes (MWCNTs) or, mesoporous silicates
with arylsulphonate group, heteropoly acids, rare-earth triflates,
and ion-exchange resins. Thus, the latest trend in catalytic
acetalization of glycerol to fuel-additives will be investigated in
the following sections. Table 3 summarizes some of the recent
studies related to glycerol acetalization with different aldehydes
and ketones in batch and continuous processes. All the reported
studies are organized into two main groups of homogenous and
heterogeneous catalysts. Also, the heterogeneous c atalysts are
divided into four categories of zeolite-, heteropoly acid-, metal-,
and polymers-based catalysts. As it can clearly be seen, the
highest catalytic activity (complete conversions) were observed
from the rare-earth triflate catalysts (Pierpont et al., 2015),
Ni-Zr/activated carbon catalyst (Khayoon and Hameed, 2013),
(L)Ru (II)@SBA-15 (Lazar et al., 2018), Amberlyst-47 (Guemez
et al., 2013), and [5%V] Si-ITQ-6 (Vieira et al., 2018). The detail
of each reaction process and optimum conditions reported in
Table 3.
Lack of research studies on different methods of catalyst
synthesize (e.g., sol-immobilization) is obvious, which has great
influence on the catalyst activity and selectivity. In fact, the
majority of reported heterogeneous catalysts synthesized through
simple impregnation approach. Also, the photo-catalytic process
is a promising strategy in various reaction processes and under
mild reaction conditions with altered selectivities, compared
with the conventional thermo-catalytic route. Unfortunately,
application of photo-catalytic acetalization of glycerol has rarely
been reported, while it definitively requires more attention in the
future.
In addition to investigating the synthesis of an effective
catalyst, the process engineering for economic evaluation was
also rarely investigated. The UNISimTMsoftware was used
2
Frontiers in Chemistry | www.frontiersin.org 6 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
13
TABLE 3 | Glycerol acetalization with different aldehydes and ketones in batch and continuous processes.
Type Catalyst Optimum condition Conc(%) Yd(%) Description References
Homogenous PTSA MRaGl/Fb= 1:1
T = 80◦C, t = 9 h
PTSA MR Gl/Ace= 1:4
t = 12 h
PTSA MR Gl/Benf= 1:2
T = 140◦C, t = 15 min
H2SO
4
MR Gl/F = 1.5/1
T = 100◦C, t = 4 h
Heterogeneous Zeolites Zeolite beta MR Gl/F = 1/1 T = 100◦C,
t = 2 h
Zeolite beta MR Gl/Ac = 1/2 T = 70◦C,
t = 1 h
H beta zeolite MR Gl/Ac = 1/2 T = 25◦C,
t = 2 h
Zeolite USY MR Gl/Bug= 1/2.5 T =
70◦C, t = 4 h
Zeolite BEA Con = 87
Zeolite ZSM Con = 28
MMT K10 MR Gl/Ben = 1/2 T =
140◦C, t = 15 min
Nb5-HUSY MR Gl/Ac = ½ T = 40◦C,
Cat = 2 wt%
MK-10S
MW
T = 40◦C, t = 2 h, Cat = 5
wt%, MR Gl/F = 1/1
[5%V]Si-ITQ-6 T = 60◦C, t = 120 min,
Ac/Gl = 3/1, Cat = 0.02 g
Zr-MO-KIT-6 T = 50◦C, t = 4 h, Ac/Gl =
8/1, Cat = 0.05 g
Immobilize sulfonic
T = 120◦C, t = 8 h Con = 78 Argon atmosphere in
acid on to silica
Zeolite beta
CP814E
MR Gl/Ac = 1/6 T = 35◦C,
t = 4 h
Zeolite beta
CP811T1
Zeolite HY Con = 37%
Heterogeneous Zeolites Hierarchical Zeolite
(H/BEA5)
T = 70◦C, t = 240 min, MR
G/F = 1/1.25, Cat = 10%
6.8v-MCM-41 T = 60◦C, t = 60 min,
Ac/Gl =6.5, Cat =20 mg
ITQ-2 T = 83◦C, HMF/Gl = 1/2,
Cat =20 wt%, Si/Al = 15
MCM-41 Con = 99
Heteropoly
acid
Cs
2.5H0.5PW12O40
T = 25◦C, MR Gl/Ac = 1/6,
Cat = 0.25 g/batch, t =
15 min
Cs
/KIT-6 T = 70◦C, MR Gl/F = 1/1.2,
2.5
Cs= 3.83 g/batch, t = 24 h
Y
= 80 – Ruiz et al., 2010
Acetal
Con = 82 – Suriyapradilok and
Kitiyanan, 2011
Con = 67 Microwave assisted,
Pawar et al., 2014
Power = 600 W; Con
= 95% without catalyst
Y
= 89 – Coleman and
Acetal
Y
= 25 – Ruiz et al., 2010
Acetal
Blankenship, 2010
Con = 90 – da Silva and Mota,
2011
Con = 86 – Manjunathan et al.,
2015
Con = 72 – Serafim et al., 2011
Con = 84 Microwave assisted,
Roldan et al., 2009
Power = 600 W
Con = 95 No catalyst
Con = 66
S
Solketal
Con = 68
S
Solketal
Con = 100
S
Solketal
= 98
= 66
= >95
– Ferreira et al., 2018
Microwave synthesis
enhanced reaction
Gutiérrez-Acebo et al.,
2018
conversion
Acetone washing could
Vieira et al., 2018
reduce the catalyst
deactivation after each
run
Con = 85.8
S
= 97.8
Solketal
– Li et al., 2018a
Adam et al., 2012
the presence of
Benzaldehyde
Con = 82% – Maksimov et al., 2011
Con = 85%
Con = 78
S
Solketal
Con = 92
S
Solketal
Con = 98
S
5R+6R
= 85
= 95
= 100
– Sonar et al., 2018
– Abreu et al., 2018
Products ratio 5R/6R =
Arias et al., 2018
2.8
Products ratio 5R/6R =
S
5R+6R
Con = 95
S
Solketal
Con = 95
=100
= 98
3.9
– Chen et al., 2018a
– Chen et al., 2018b
YGF= 60
(Continued)
Frontiers in Chemistry | www.frontiersin.org 7 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
14
TABLE 3 | Continued
Type Catalyst Optimum condition Conc(%) Yd(%) Description References
Acid
Nafion SAC 13 MR Gl/Ben = 1/2 T =
exchange
resins
Dowex MR Gl/Bu = 1/2.5 T =
Amberlyst 36 MR Gl/F =1/1, T = 100◦C,
Amberlyst 15 MR Gl/Ac = 1/2 T = 70◦C,
Amberlyst 15 MR Gl/Ac = 1/2 T = 50◦C,
Amberlyst 47 MR Gl/F = 2/1 T =
Amberlyst 47 MR Gl/Ac = 2/1 t =
Amberlyst 47 MR Gl/Buth= 3/1 T =
Amberlyst 15 MR Gl/F = 1/2 T = 75◦C, t
Heterogeneous Metal-based Ni-activated
carbon
Zr-activated
carbon
X%Ni-Y%Zr/
activated carbon
Ni-MWCNT
Pt-TNT T = 50◦C, t = 24 h, Ac/Gl
M-AlPO
4
M-ZnAlPO
M-CuAlPO
M-NiAlPO
M-CoAlPO
PTNT T = 50◦C, MR G/Ac = 1/1,
SO4/SnO
TiO2-SiO
2
MoX/TiO2-ZrO
Niobium
oxyhydroxyde
Nb2O
5
HC-SZ (SO
coated on
cordierite
honeycomb
monolith)
140◦C, t = 15 min
Con = 81 Microwave assisted,
Power = 600 W, Con =
Trifoi et al., 2016
95% without catalyst
Con = 66 – Serafim et al., 2011
70◦C, t = 4 h
Y = 55 – Ruiz et al., 2010
t = 4 h
Con = 95 –
t = 1 h
Con = 95 –
P = 8.0 bar t = 6 h
Con = 75 – Agirre et al., 2011
80–100◦C, t = 3 h
Con = 90
10–50◦C, t = 4 h
Con = 95 – Guemez et al., 2013
80◦C, t = 100 min
MR Gl/But = 0.5/1 T = 60
◦
C, t = 4 h
= 2 h
MR Gl/Ac = 1/8 T = 45◦C,
t = 3 h
Con = 100
Con = 100% Reactive distillation
process
Con = 98 3% reduction of
catalytic activity after
Hasabnis and
Mahajani, 2014
Khayoon and Hameed,
2013
the 4th run
Con = 67
Con = 100
i
MR Gl/Ac = 1/6 T = 40◦C,
t = 3 h
Con = 96 5% reduction of
catalytic activity after
Khayoon et al., 2014
4th run
= 1/1, Cat = 130 mg
MR Gl/Ac = 1/8 T = 80◦C,
4
4
4
4
t = 1 h
S
= 10
Solketal
Con = 75 80% reduction of
Con = 40
Con = 46.7
t = 6 h
j
2
Gl-Fur
MR Gl/Ac = 1/4 T =
S
= 20
Soketal
Con = 99 – Mallesham et al., 2014
Con = 98 Sel
40–90◦C, t = 3 h
MR Gl/Ben = 1/1 T =
2
60–100◦C, t = 90 min
MR Gl/Ac = 1/4 T = 40◦C,
Con = 74 – Sudarsanam et al.,
Con = 74 – Souza et al., 2014,
t = 1 h
MR Gl/Ac = 1/3 T = 70◦C,
Con = 80 Up to 4 time reusability Nair et al., 2012
– Gomes et al., 2018
Zhang et al., 2015
M-NiAlPO4activity
after the 5th run
– Gomes et al., 2018
k
5−memberedring
95%
=
Fan et al., 2012
2013
2015
t = 6 h
2
4
/ZrO
T = 60◦C, MR G/Ac = 1/3,
2
Cat = 0.2 g
Con = 96,
Y
Solketal
= 94
– Vasantha et al., 2018
(Continued)
Frontiers in Chemistry | www.frontiersin.org 8 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
15
TABLE 3 | Continued
Type Catalyst Optimum condition Conc(%) Yd(%) Description References
Meso-SnO2-350 T = 60◦C, t = 30 min, Ac/Gl
Heterogeneous Other
a
MR, Molar Ratio;bF, Formaldehyde;cCon, Conversion (%);dY, Yield (%);eAc, Acetone;fBen, Benzaldehyde;gBu, Butanal;hBut, Butiraldehyde;iMWCNTs, Multiwall carbon
nano-tubes;jFur, Furfural;kSel, Selectivity.
catalysts
Rare earth triflate Gl-Ac T = 25◦C Con = 100 – Pierpont et al., 2015
Organic-inorganic
hybrid catalyst
(L)Ru(II)@SBA-15 T = 25◦C, t = 20 min, MR
80LS20PS450H
PrSO3H-SBA-15400
Carbon-based
catalyst
Co(II)
(Co(III)
Purolite PD206 T = 40.66◦C, P = 42.31
KU-2 MR Gl/Ac = 1/6 T = 60◦C,
Purolite PD 206 MR Gl/Ac = 5/1 T = 20◦C,
1.25
)Al
2−0.75)O4
= 1/1, Cat = 0.125 g
MR Gl/Ac = 1/6 T = 30◦C,
t = 3 h
Al/MeOH = 1/250
+
T = 40◦C, Cat = 5 wt%,
MR G/Ac = 1/6, t = 60 min
T = 90◦C, t = 8 h, F/Gl =
1.5/1, Cat = 0.2 g
T = 28◦C, t = 30 min, Ac/Gl
=4/1, Cat = 3 wt%
T = 130◦C, t = 3 h, 2 g Gl
and 12.72 g AC, Cat = 0.1 g
bar, MR Gl/Ac = 1/4.97,
Feed flow rate = 0.49
ml/min, Cat = 0.5 g
t = 4 h
P = 120 bar
Con = 51.3
S
= 98
Solketal
Con = 94 Water resistance Sandesh et al., 2015
Con = 100
S
= 100
Solketal
Con = 90 S
S
= 60 – Li et al., 2018b
Solektal
Con = >78
S
= 73
Solketal
Con = 69.2
S
= 98.6
Solketal
Normalized exergy
destraction =
6.18%, Universal
Exergetic
efficiency =
90.36%
Con = 85% – Maksimov et al., 2011
Con = 95% Acetone-solvent Shirani et al., 2014
Higher selectivity to the
solketal in the presence
of Acetone compared
to the Furfuraldehyde
and benzaldehyde
– Lazar et al., 2018
=51–53%
Soketal
obtained over Furfural
and Methyl levulinate
instead of acetone
– Mantovani et al., 2018
– Li et al., 2018c
Optimization and
modeling of continuous
acetalization process
with subcritical acetone
Manjunathan et al.,
2018
Konwar et al., 2017
Aghbashloa et al., 2018
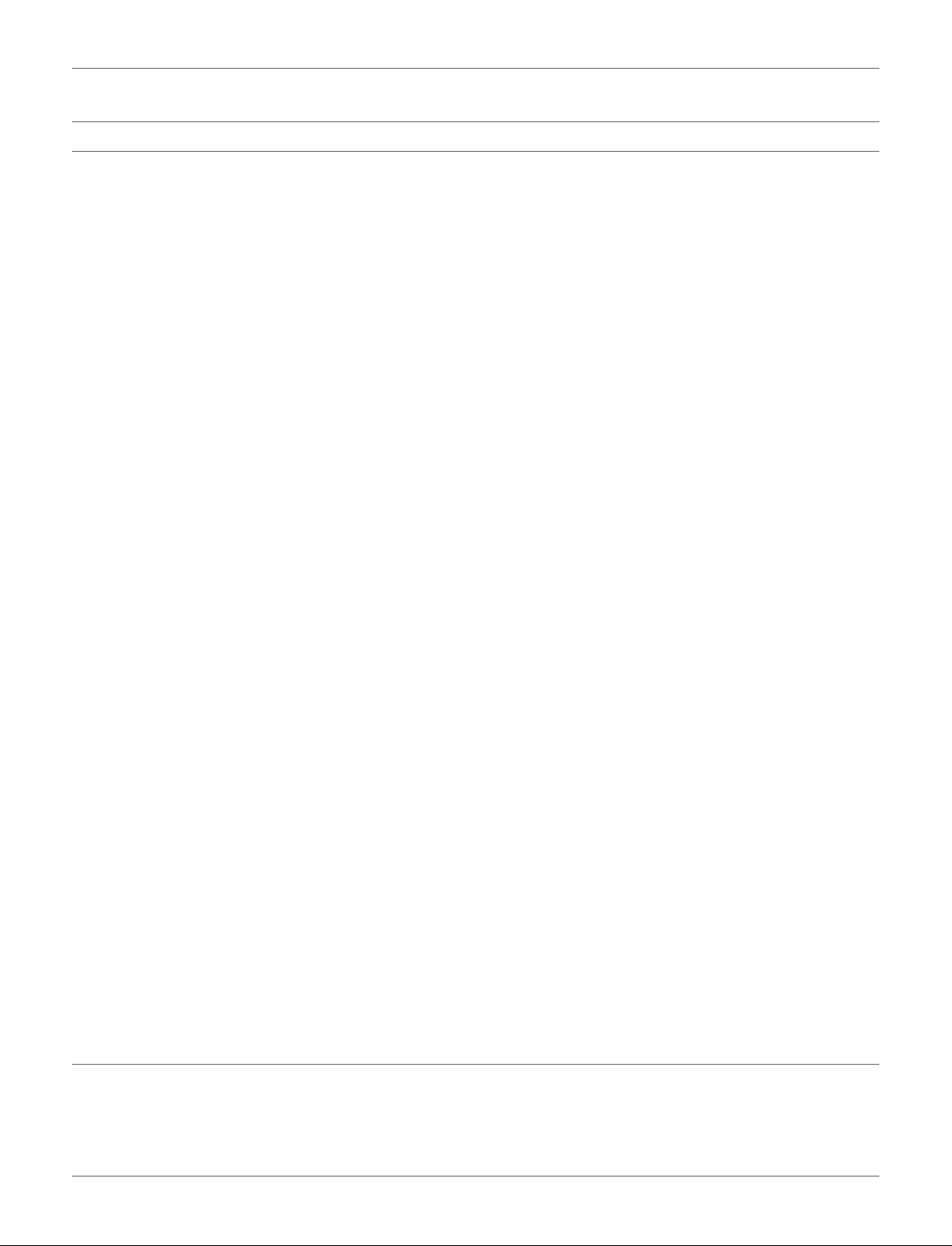
operation costs are shown in Figure 2. The results of this
study suggest t hat glycerol acetalization for the production
of solketal (fuel-additive) requires more attention and study.
Such simulation studies could provide valuable information
before large-scale industrialization of glycerol acetalization
process. Indeed, researchers could analyses and evaluate different
scenarios to evaluate the environmental and economic aspects of
this process such as material, energy and production cost.
Zeolite Based (Micro- and Mesoporous) Catalysts
Zeolite is a micro-porous, alumino-silicate mineral
conventionally used as commercial adsorbents due to its
unique porous characteristics (tunable pore size), acid sites,
FIGURE 2 | Annual operation costs.
for the material and energy balances. The proposed plant
could consume 432
t/y of glycerol and produce 620.9 t/y
of solketal. The solketal cost was 12.29US$/kg. The annual
Frontiers in Chemistry | www.frontiersin.org 9 November 2018 | Volume 6 | Article 573
and high thermal stability (Halgeri and Das, 1999). Zeolites
could be used
in various applications with a global market of
several million tons annually, which includes petrochemical,
water purification, gas separator, nuclear, and biogas industries.
Among different forms of zeolite, nano-crystalline zeolite Beta
and Y showed higher activity than micro-crystalline zeolites due

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
16
to their high surface area, lower diffusion path length and more
exposed active sites (Taufiqurrahmi et al., 2011).
Despite all the research studies which have been used
zeolite catalysts, some studies reported diffusion problems (mass
transfer resistance) by utilization of bulk zeolites due to the
presence of micro-porous network (Sharma et al., 2011). Thus,
researchers have decided to use different metal oxides, metals, or
metal nanoparticles as a support for zeolites to overcome these
limitations. However, the synthesis procedures are sometimes
laborious and require additional costs due to application of
noble metals or thermal treatment which in general consume
lots of time, energy, and cost. As a result, new concept of
“Hierarchical zeolites” have attracted much attention recently.
Based on our knowledge, application of supported hierarchical
zeolites acid catalysts is reported rarely in this field. Indeed,
hierarchical zeolites overcome the drawbacks related to hamper
mass transfer and limited accessibility of conventional zeolites
by the introduction of secondary, larger porosity within the
micro-porous framework (García-Martínez and Li, 2015). In
hierarchical meso-micro-porous zeolites, mesopores facilitate the
physical transport of reactant molecules, whereas micropores
act as nano-reactors to provide both active sites and shape
selectivity (Groen et al., 2007). Therefore, hierarchical zeolites
have recently been explored as catalysts for reactions t hat
involve bulky molecules and their outstanding activities have
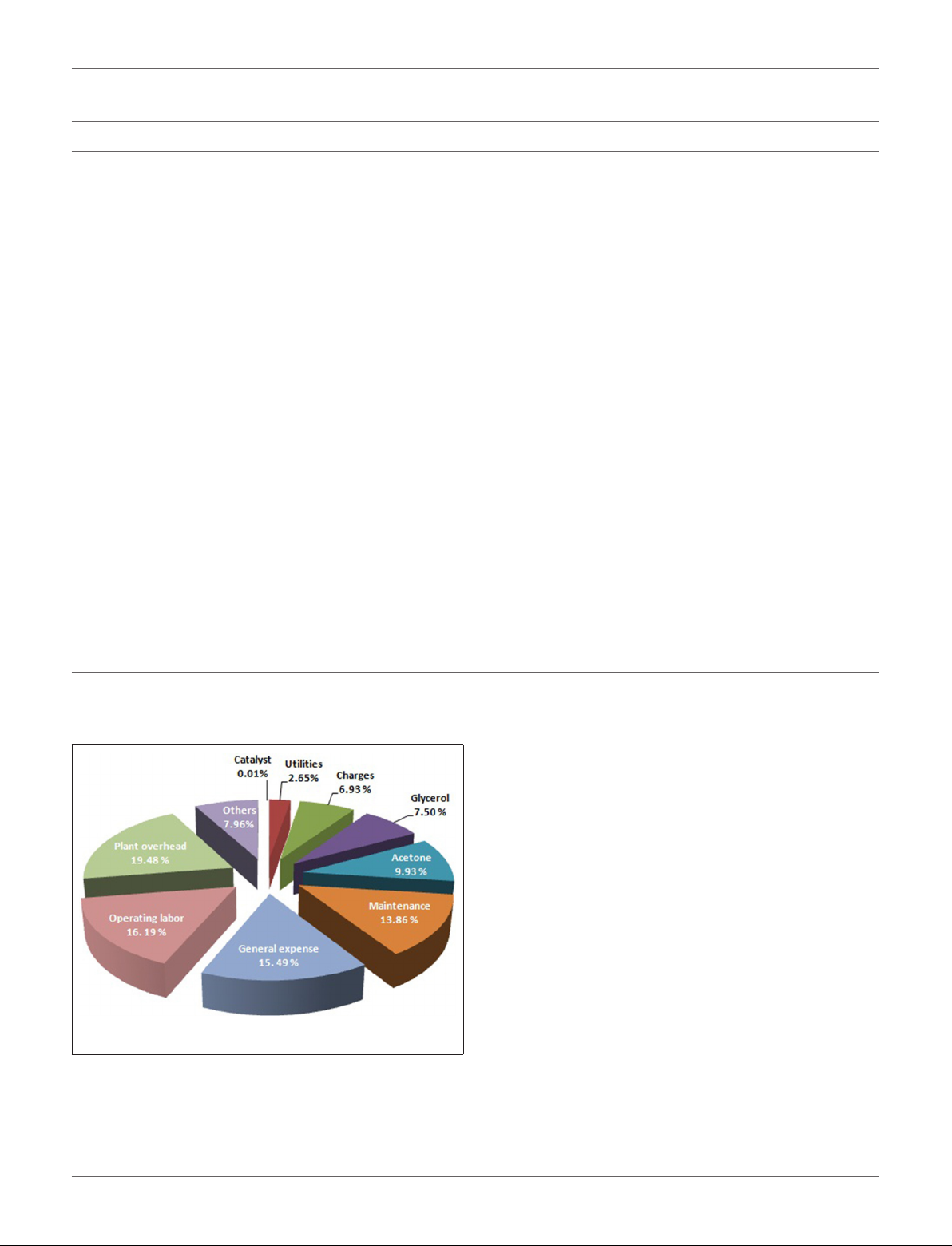
been reported (Zhou et al., 2010). There are two approaches
to introduce a hierarchical pore structure (connected pore
structure) in zeolites. In fact, the bottom up and the top-down
methods which hierarchical zeolites are synthesized directly from
a silica-alumina gel or by post-treatment of the existing zeolites,
respectively. Extra-crystalline, hard, templates such as carbon,
(Egeblad et al., 2008) starch, (Park et al., 2009) resins, (Tosheva
et al., 2000) and surfactants, (Choi et al., 2006) which are removed
by calcination after crystallization to create mesoporosity can be
used in the bottom-up approach (Fan et al., 2008). In the topdown approach to achieve hierarchical form, zeolites are posttreated after synthesis. The easiest way to introduce mesoporosity
is by dealumination, which can be achieved by steaming and
chemical treatments, such as acid leaching which remove the
resulting extra-framework alumina. The increased mesoporosity
may give rise to increasing rates in bimolecular and oligomeric
reaction pathways that require large transition states (Lupulescu
and Rimer, 2012). Another way of producing mesopores is
desilication which can be done by base leaching. Figure 3
illustrates bottom-up and top-down methods for synthesizing
hierarchical meso-porous zeolites (Vogt and Weckhuysen, 2015).
Undoubtedly, application of hierarchical zeolites as one of the
catalysts with high activity and selectivity to the desired product
should be more studied in the glycerol acetalization process due
to its characteristics and acceptable results in other chemical
processes particularly as a fluid catalytic cracking (FCC) catalyst
in petrochemical industry.
One of the early studies regarding application of zeolites, was
performed by da Silva et al. (2009) who investigated different
catalysts (K10 MMT, zeolite Beta, amberlyst 15, and p-toluene
sulfonic acid) for the conversion of glycerol to fuel-additives
in the presence of acetone or formaldehyde. Consequently, the
zeolite Beta (Si/Al = 16) reached conversion >95% in 1 h. In fact,
high content of Si/Al ratio led to the hydrophobic characteristic
of zeolite, which prevents the diffusion of water to inside
the pores and acid sites’ strength was preserved. Nevertheless,
with aqueous formaldehyde solution, the glycerol conversion
illustrated a drop to between 60 and 80% for different catalysts
(Amberlyst-15, K-10 montmorillonite, p-toluene-sulfonic acid).
Indeed the main reason was high amount of water in the reaction
medium, which shifts the equilibrium and weakens the acid sites.
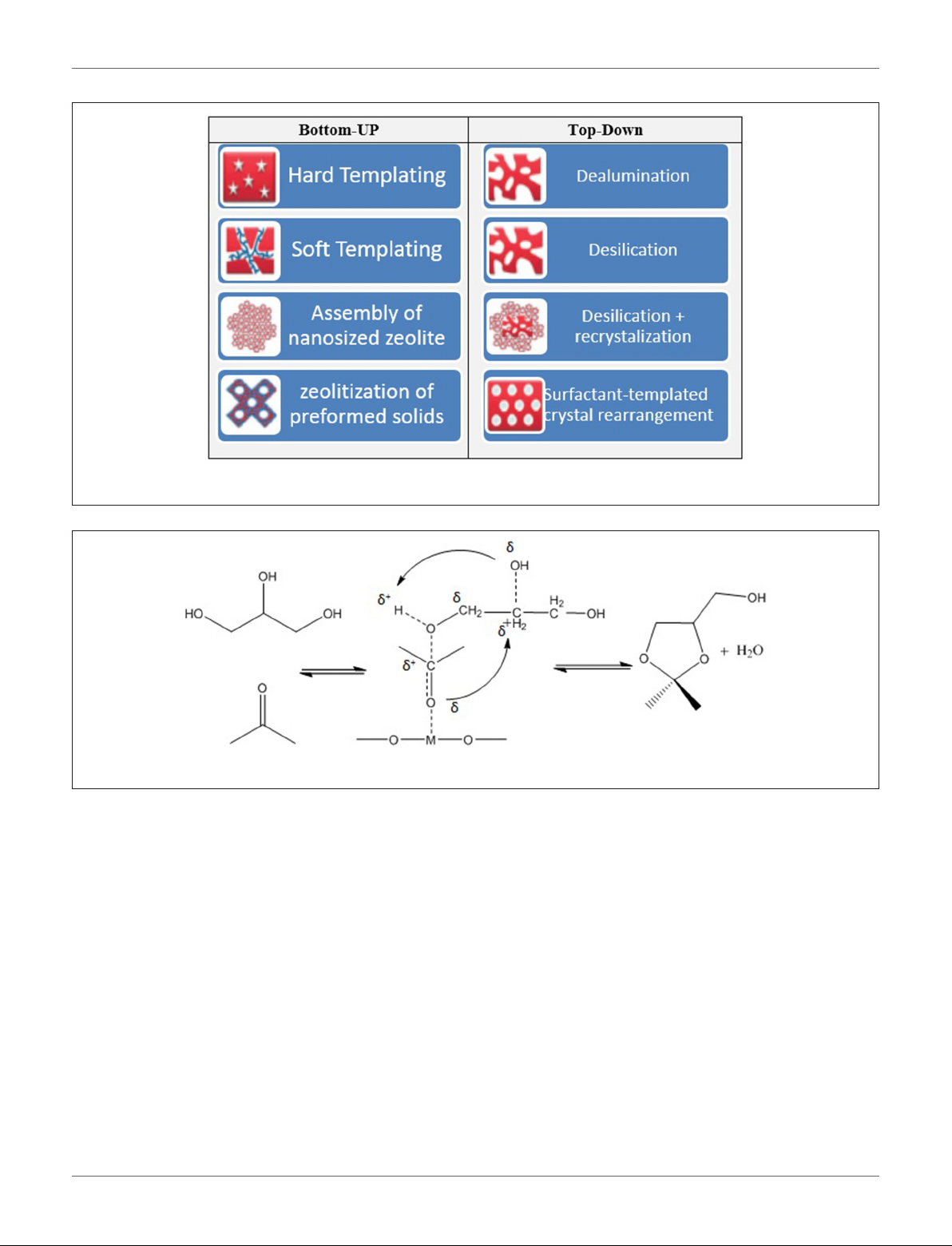
Li et al. (2012) reported that mesoporous Lewis acid catalysts
could be active in acetalization of glycerol with acetone to
produce solketal. A series of three-dimensional mesoporous
silicate catalysts (Hf-TUD-1, Zr-TUD-1, Al-TUD-1, and SnMCM-41) synthesized and two of these catalysts (Hf-TUD1 and Zr-TUD-1) showed excellent catalytic activities in
solketal production. Indeed, 65 and 64% glycerol conversion
obtained over Hf-TUD-1 and Zr-TUD-1 catalysts, respe ctively,
at optimum reaction condition of 2/1 molar ratio of acetone
to glycerol, 25 mg of catalyst weigh, at 80◦C in 6 h reaction
time. The main reasons for such high activity of synthesized
catalysts were wide pores (Hf-TUD-1 = 0.6 cm2/g, Zr-TUD1 = 0.8 cm2/g), large specific surface area (Hf-TUD-1 = 715
m2/g, Zr-TUD-1 = 651 m2/g), large pore size (Hf-TUD-1 =
4 nm, Zr-TUD-1 = 13.3 nm), the amount of accessible acid sites,
and a relatively hydrophobic surface of catalyst. In addition,
the active mesoporous materials didn’t suffer from leaching
and could be efficiently reused in consecutive catalytic cycles.
They also proposed a reaction mechanism for acetalization
reaction in the presence of Lewis acid catalysts. The Lewis acid
metal sites coordinate and activate acetone’s carbonyl group.
Then, the carbon atom of the carbonyl group is attacked by
the primary alcoholic group of glycerol accompanied by the
formation of a bond between the carbonyl oxygen atom and
the secondary carbon atom of glycerol. Finally, solketal forms
through the dehydration step. Figure 4 displays the detailed
reaction mechanism.
Jamil et al. (2017) used different tailored forms of zeolite
Beta in the condensation of bio-glycerol with acetone for
production of the Solketal. The zeolite Beta catalysts treated with
acids (hydrochloric acid, nitric acid, and oxalic acid) exhibited
enhanced catalytic activity, irrespective of the nature of the acid
used for the de-alumination. The nitric acid-treated beta zeolite
sample (AB-2) exhibited a higher conversion than the other acidtreated samples. At optimum conditions (1:6 glycerol to acetone
molar ratio, 4 h reaction time, 60◦C reaction temperature) the
bio-glycerol conversion and solketal yield were 94.26% and
94.21 wt%, respectively. The AB-2 sample was reusable for at
least 4 times without any significant loss in its activity with
approximately >80% glycerol conversion and >80% solketal
yield.
Kowalska-Kus et al. (2017) investigated the glycerol
acetalization reaction with acetone in the presence of hierarchical
zeolites comprising pores of different diameters (MFI, BEA,
and MOR) at 343 K and 1:1 glycerol to acetone molar ratio.
The best catalytic performance for glycerol acetalization, which
was 100% solketal selectivity at 80% reaction conversion, was
achieved over hierarchical (micro/mesoporous) MFI zeolites. A
Frontiers in Chemistry | www.frontiersin.org 10 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
17
FIGURE 3 | Bottom-up and top-down models for synthesizing hierarchical mesoporous zeolites.
FIGURE 4 | Proposed reaction mechanism for the acetalization of glycerol and acetone over Lewis acid catalyst.
significant increase in reaction conversion and solketal selectivity
in the studied reaction resulted from the easier accessibility of
the active sites to reagents due to the formation of mesopores by
means of desilication of the micro-porous zeolites.
Heteropoly Acid Based Catalysts
Application of heteropoly acid (HPA) has attracted much
attention due to its wide applic ations in biodiesel industry and
production of value-added chemical from glycerol. HPAs are
highly stable against humidity and air,low toxicity, high solubility
in polar solvents, production of less residues than mineral acids,
less corrosive, and highly safer than other catalysts (Martin
et al., 2012). Tungstophosphoric acid (HPW), silicotungstic
acid (HSiW), and Phosphomolybdic acid (HPMo) are three
commercially available HPAs. The HPW is a common HPA
catalyst which is widely used. HPA catalysts have high ability
for adjustment by modifying their central atoms with various
Frontiers in Chemistry | www.frontiersin.org 11 November 2018 | Volume 6 | Article 573
compounds. Researchers have attempted to increase the catalytic
activity and long-life stability of the catalysts to achieve the
highest fuel-additive yield. The Cs/HPW catalyst displayed one of
the highest potential catalyst in acetalization of glycerol with 98%
selectivity to solketal at about 95% glycerol conversion (
t al., 2018a). HPA’spossessed Keggin structure. It is the structural
e
Chen
form of α-Keggin anions, which have a general formula of
[XM12O40]n−, where X, M, and O represent the heteroatom, the
addenda atom, and oxygen, respectively (Figure 5). The structure
self-assembles in acidic aqueous solution and is the most stable
structure of polyoxometalate catalysts. Despite the enormous
applications of HPA catalysts as active components in various
heterogeneous catalytic processes [e.g., glycerol dehydration to
acrolein (Talebian-Kiakalaieh et al., 2014), glycerol oxidation to
glyceric acid (Talebian-Kiakalaieh et al., 2018)], application of
these type
s of catalysts are rarely reported in glycerol acetalization
reaction.

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
18
Metal Based Catalysts
Mixed oxides, phosphates, and pyrophosphates have been
used in glycerol acetalization to fuel-additives. Metal oxide
catalysts such as niobium oxide (Nb2O5), tungsten oxides
(WO3), silicon dioxide (SiO2) have been widely used in various
chemical processes. The most important factors about metalbased catalysts are their synthesis method (especially calcination
temperature) and their binary or tertiary combinations which
have detrimental impact on physicochemical characteristics
FIGURE 5 | HPA keggin structure.
The glycerol acetalization was studied using a series of
supported HPAs [HPW, HPMo, HSiW, and molybdosilisic
(SiMo)], immobilized in silica catalysts by sol–gel method
(Ferreira et al., 2010). As results, all catalysts exhibited high
solketal selectivities (near S
= 98%) at quite complete
Solketal
conversions at optimum reaction conditions of 70◦C reaction
temperature, 0.2 g catalyst weight, 6:1 molar ratio of acetone
to glycerol and after 4h reaction time. Also, the catalytic
activities decreased in the following order: HPW-S > SiW-S
>PMo-S > SiMo-S. All the catalysts exhibited high stability
even after the fourth consecutive run, having lost only 10–
13% of their initial activity. In another study, Narkhede and
Patel (2014) achieved high selectivity toward solketal using
supported SiW wit h MCM-41 catalysts (30% SiW11/MCM-41,
30% SiW12/MCM-41) in the presence of benzaldehyde. The
results indicated that the 30%-SiW11/MCM-41 could reach the
highest solketal selectivity of 82 at 85% glycerol conversion
at room temperature (30◦C), 1/1.2 molar ratio of glycerol to
benzaldehyde, 100 mg catalyst weight and in 1h. Also, tuning of
the acidity of the parent SiW led to an increase in the selectivity
toward solketal. High activity of t hese catalysts was attributed
to their strength of acidity, wide pores and large specific surface
area.
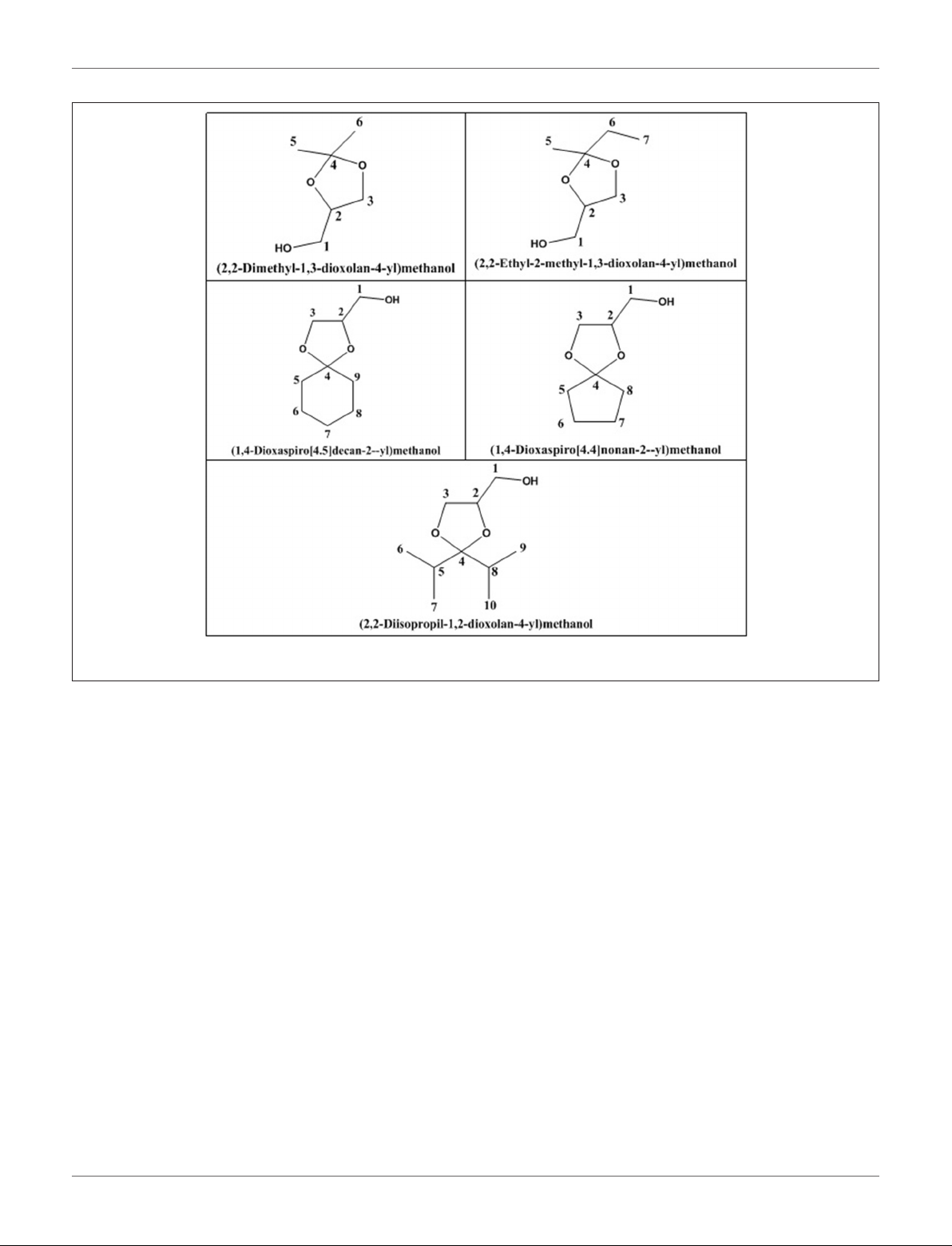
da Silva et al. (2015b) evaluated the activity of various
Brønsted acid catalysts e.g., HPW, H2SO4, p-toluene sulfonic
acid, PMo, or SiW on glycerol ketalization with different ketones
(e.g., propanone, butanone, cyclopentanone, and cyclohexanone)
at room temperature and in the absence of an auxiliary solvent.
The HPW sample exhibited the highest activity among the
Brønsted acid catalysts and exhibited high (> 85%) selectivity
toward five-membered (solketal) cyclic ketals. The highest (98%)
selectivity of solketal is obtained at 288 K reaction temperature,
1 mol% catalyst (HPW) loading, 1:30 glycerol to ketone
(propanone) molar ratio. The activity of different tested catalysts
was as follows HPW> p-toluene sulfonic acid > PMo > SiW
> H2SO4with 83% > 76% > 41% > 40% > 31%, respectively.
In addition, the results revealed that the application of various
ketones with Brønsted acid catalyst in absence of solvent for
ketalization of glycerol has significant influence on product
distribution. Figure 6 summarizes the possible products that can
be obtained as a result of different ketone application.
catalyst (
w
with two different methods of fusion and wet-impregnation.
XRD results suggested that solid solutions of nano-crystalline
SnO2were formed due to the incorporation of Mo and W
cations into the SnO2lattice. Textural characterization results
revealed that all the compounds showed smaller crystallite
size, large specific surface area (WO3-SnO2= 32 m2/g and
MoO3-SnO2= 56 m2/g), and high porosity. Moreover, Raman
measurements and TPR results confirmed the formation of
more oxygen vacancy defects in the doped catalysts along with
facile reduction of the doped SnO2, respectively. The positive
impact of Mo and W oxides on the acidic properties of the
SnO2was revealed by NH3-TPD. Total acidity of MoO3-SnO
and WO3-SnO2were 81.45 and 61.81 µmol/g, respectively.
The presence of larger number of Brønsted (B) acidic sites
vs. Lewis (L) sites (B/L = >95%) was confirmed by pyridineFTIR characterization. High selectivity to solketal (96%) at
approximately 70% glycerol conversion was achieved over the
MoO3-SnO2sample at the optimum reaction condition of 1:1
glycerol to acetone molar ratio, 5 wt% catalyst loading in 150 min.
In addition, this catalyst reached around 65% solketal selectivity
at almost complete glycerol conversion in the presence of
furfural (1:1 glycerol to furfural molar ratio) in 120 min. Finally,
applications of different mono-substituted furfural compounds
(e.g., 5-methylfurfural, 5-nitrofurfural, 5-chlorofurfural, and 5hydroxymethyl furfural) were evaluated for acetalization of
glycerol in the presence of MoO3/SnO2sample. The results
confirmed that all the substituted compounds reached lower
glycerol conversion than furfural. This observation confirms
the impact of steric hindrance induced with substitutes rather
than the electronic effects of the substituent (i.e., inductive,
resonance and hyper conjugation influences). In detail, the
acetalization reaction reached >61% solketal selectivity at >60%
glycerol conversion in the presence of different mono-substituted
furfural.
e
solventless/solvent-containing systems [Formalin (solvent-less),
Para-formaldehyde (water), Para-formaldehyde (solventless)] in
the presence of Zr-SBA-16 catalyst with three different Si/Zr
ratios (100, 50, and 25) for glycerol acetalization to glycerol
formal (GF). Results showed that the Zr–SBA-16(100) sample
exhibited 24 and 76% selectivity to the solketal and dioxane,
respectively, at 77% glycerol conversion at optimum reaction
conditions of 100◦C reaction temperature, 1:1 glycerol to paraformaldehyde molar ratio. In addition, the Zr–SBA-16(50)
Talebian-Kiakalaieh et al., 2014).
Mallesham et al. (2013) synthesized a series of supported SnO
ith molybdenum (Mo) and tungsten (W) solid acids catalysts
In another study,
Gonzalez-Arellano et al. (2014b) also
valuated the application of various formaldehyde sources and
2
2
Frontiers in Chemistry | www.frontiersin.org 12 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
19
FIGURE 6 | Products with application of various ketones.
sample could be successfully reused up to five times under
identical reaction conditions, without any noticeable decrease
in activity. The main reason for better stability of Zr-SBA-16
(50) compared to the Zr-SBA-16 (100) was acidity. Indeed, the
Zr-SBA-16(50) possessed higher amount of total acidity (116
µmol/g) with Lewis acidic nature (B/L = 36/80) compared to
the Zr-SBA-16 (100) with just 40 µmol/g total acidity and with
Bronsted acidic nature (B/L = 28/12).
Gonzalez-Arellano et al. (2014a) continued their study on
the acetalization of glycerol with different aldehyde sources
(para-formaldehyde, benzaldehyde, furfural, and acetone) in the
presence of another newly synthesized heterogeneous catalyst,
which was supported iron oxide nano-particle system of a
mesoporous alumino-silicate heterogeneous catalyst (Fe/Al-SBA-
15). The characterization results confirmed that Fe/Al-SBA-15
possessed high surface area (688 m2/g) with Brønsted acidic
nature (88 µmol−1g−1). Experimental results revealed that the
product distribution was totally dependent on the use of different
aldehyde sources. In fact, acetalization of glycerol with paraformaldehyde results in the production of dioxane (selectivity
66%) as the main product compared to the dioxolane with
just 34% selectivity at almost complete glycerol conversion. In
contrast, the use of other aldehyde sources led to production
of dioxolane (solketal) as the main product. The product’s
selectivities (dioxolane/dioxane) were 84%/16% at 70% glycerol
conversion, 60%/40% at >95% glycerol conversion, and 99%/1%
at 58% glycerol conversion in the presence of benzaldehyde,
furfural and acetone, respectively. Finally, the Fe/Al-SBA-15
showed the highest stability after five consecutive runs without
significant reduction in catalyst activity in the presence of acetone
as the aldehyde source.
Gadamsetti et al. (2015) synthesized a series of supported SBA-
5 with molybdenum phosphate (MoPO 5–50 wt%) catalysts
1
for the acetalization of glycerol with acetone. Synthesized
catalysts were characterized and the XRD results revealed that
unsupported MoPO exhibits the formation of (MoO2)2P2O
phase and is dispersed well on the SBA-15 surface. Also,
Raman spectra characterization confirmed the existence of
MoPO species [(MoO2)2P2O7] in samples with more than 40
wt% MoPO supported on SBA-15. In addition the UV-DRS
results revealed the presence of both isolated tetrahedrally and
isolated octahedrally coordinated Mo centers in the supported
and unsupported MoPO. Finally, the NH3-TPD analysis shows
that the total acidity surged from 0.2 to almost 1 mmol/g with
MoPO loading from 5 to 40 wt%; however, total acidity dropped
by increasing MoPO loading beyond 40 wt%. In contrast, specific
7
Frontiers in Chemistry | www.frontiersin.org 13 November 2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
20
surface area of synthesized catalysts showed a downward trend
from 688 to 125 m2/g for 5 to 50 wt% of MoPO loading. Acidity
of catalysts had negative impact on catalytic performance. The
40 wt% MoPO/SBA-15 sample showed the best catalytic activity
with 98% Solketal selectivity at complete conversion (100%)
at optimal reaction condition of 3:1 molar ratio of acetone to
glycerol, 50 mg catalyst loading, room temperature, in 2h.
da Silva et al. (2017) used solid SnF2 catalyst for glycerol
etalization with propanone to solketal. The SnF2catalyst
k
reached 97% selectivity of solketal at 97% glycerol conversion at
optimum condition of glycerol (21.0 mmol), propanone (168.0
mmol) molar ratio (1:8), CH3CN (15mL), at room temperature
(298 K). Most importantly, this catalyst exhibited incredible
stability even after four times recycling and reuse with almost
constant reaction conversion and solketal selectivity.
Another recent study on a series of zirconia-based catalysts
for the acetalization of glycerol suggested that the activity
increased in the order of ZrO2< WOx/ZrO2< MoOx/ZrO
2−
<SO
/ZrO2. In particular, the use of a sulfated zirconia catalyst
4
led to ∼98% conversion of glycerol and ∼97% selectivity to
solketal. The surface acidity and crystalline state of ZrO2on
2−
the SO
/ZrO2catalyst were found to be very influential to the
4
catalytic performances (Reddy et al., 2011).
Kapkowski et al. (2017) synthesized a series of nano-silica
supported Re, Ru, Ir, Rh NPs along with different mixture of
the metal (Re, Ru, Ir, and Rh) catalysts for acetalization of
glycerol with acetone or butanone. It was found that nano-SiO
supported Re (1.0%Re/SiO2) was a highly efficient catalyst in
glycerol acetalization reaction for solketal production exhibiting
the highest activity (TOF = 620.7 h−1) with 94.1% selectivity
to solketal at 100% glycerol conversion. The addition of Ir
(1.0% Re.Ir (1:1)/SiO2) could also slightly improve the solketal
selectivity to 96% and catalyst activity to TOF = 630.5 h
−1
at complete conversion. Although 1.0%Re/SiO2favors fivemembered cycles, its substitution with Mo alters this selectivity
and both five- and six-membered products can be obtained. In
detail, the solketal selectivity and catalyst activity decreased to
about 78.9% and 336.9 h−1, respectively. Despite the addition of
Rh [1.0%RuRh(1:1)/Mo], the solketal selectivity did not increase
more than 93.4%.
Priya et al. (2017) used microwave irradiation as a heating
source in glycerol acetalization to fuel-additives over different
transition-metal-ion-promoted mordenite solid acid catalysts
which were synthesized by wet impregnation method. The
transition metal ions include Fe, Co, Ni, Cu, and Zn. This
approach is considered notably clean and green in this field. The
results from the microwave irradiation system were compared to
those from other processes that use conventional heating sources
to ascertain its efficiency and efficacy. The Cu-Mor catalyst
showed the highest activity because of the large number of acidic
sites and the synergetic effects of metal particles interacting
with mordenite. The activity of synthesized s amples is shown in
Figure 7A. Using Cu-Mor sample and 3:1 acetone/glycerol molar
ratio, 98% solketal selectivity at 95% glycerol conversion was
obtained in only 15 min. Figure 7B illustrates how the glycerol
conversion and solketal selectivity in the presence of the best
sample of Cu-Mor varies with microwave power. The reaction
mechanism of glycerol acetalization using microwave irradiation
and using Cu-Mor catalyst was proposed (Figure 8). Finally,
the Cu-Mor sample exhibited an excellent reusability of up
to four reaction cycles with only a marginal drop in reaction
conversion.
Timofeeva et al. (2017) investigated glycerol acetalization with
acetone using iso-structural MOFs of the families MIL-100(M)
and MIL-53(M) (M = V, Al, Fe, and Cr) and mixed MIL53(Al/V). The results revealed that the metal ion’s type in MIL100(M) and MIL-53(M) has significant impact on the rate of
reaction and selectivity of desired product. The zero point of
charge of the surface (pH
) values are revealed that the acidity
PZC
of MIL-100(M) dropped in the following order: MIL-100(V)
> MIL−100(Al) > MIL-100(Fe) > MIL-100(Cr). As a result,
glycerol conversion decreases in the following order V3+> Al
> Fe3+> Cr3+. Indeed, literature analysis revealed that isomer
selectivity depends on the length of the M-O bond in MIL-53(M)
and MIL-100(M). Thus, length of M-O bond in MIL-53(M) and
2
MIL-100(M) change in the following order: (Å): MIL-53(Cr)
[2.08 (Serre et al., 2002)] > MIL-53(Al) [1.82–2.00 (Loiseau
et al., 2004)] > MIL-47(V) [1.946–1.998 (Karin et al., 2004)]
and MIL-100(Cr) [2.18 (Férey et al., 2004)] > MIL-100(Fe)
[2.065 (Horcajada et al., 2007)] > MIL-100(Al) [1.831–1.995
(Volkringer et al., 2009)], respectively. The decrease in the length
of the M-O bond favors increased formation of solketal for both
samples. The solketal selectivities increased from approximately
80, 87, and almost 90% over MIL-100(Cr), MIL-100(Fe), and
2
MIL-100(Al). Similarly, it surged from about 80 to 90%, and
then around 97% for MIL-53(Cr), MIL-53(Al), and MIL-53(V),
respectively. Evaluation of mixed MIL-53(Al,V) showed that the
reaction rate and solketal selectivity rise from 90 to 97.5% with
increasing V3+content from 0 to 1% in MIL-53(Al,V). Also, the
efficiencies of MIL-100(V) (87 mol/mol) and MIL-47(V) (106.1
mol/mol), were higher than those of H2SO4, SnCl2and p-toluene
sulfonic acid with 50.9, 89.6, and 58.9 mol/mol, respe ctively at
25◦C. The MIL-100(V) catalyst exhibited four times recycling
and reusability with negligible reduction in glycerol conversion
(>80%).
de Carvalho et al. (2017) synthesized a series of titanatenano-
tubes (TNTs) by hydrothermal method (sodic and protonic
TNTs) to investigate the impact of the type of materials
and synthesis time. Physico-chemical characterization results
revealed that diversities in t he TNT tubular structures with inter
wall distances (1.07 to 1.11 nm) depend on the applied synthesis
time. TEM, SEM, and XRD characterization results confirmed
the enlargement of the layers in the protonic titanates unlike
the sodic ones. Sodic TNTs, with long synthesis time of up to
24 h, has the Na2Ti3O7phase, whereas the protonic TNTs has
H2Ti3O7ones. Although long hydrothermal treatment times
(72 h at 160◦C) exhibited a strong impact on the reduction
of the structural order of the TNTs, it does improve the
textural characteristics and acidities of the solids. Also, mild
reaction conditions were ineffective for conversion of glycerol
over most synthesized sodic TNTs. The best glycerol conversion
was obtained over the HTNT sample synthesized at 72 h, with
44.4% glycerol conversion and 83 and 15% selectivity to solketal
and acetal, respectively, and only 2% selectivity to by-products
3+
Frontiers in Chemistry | www.frontiersin.org 14 November 2018 | Volume 6 | Article 573
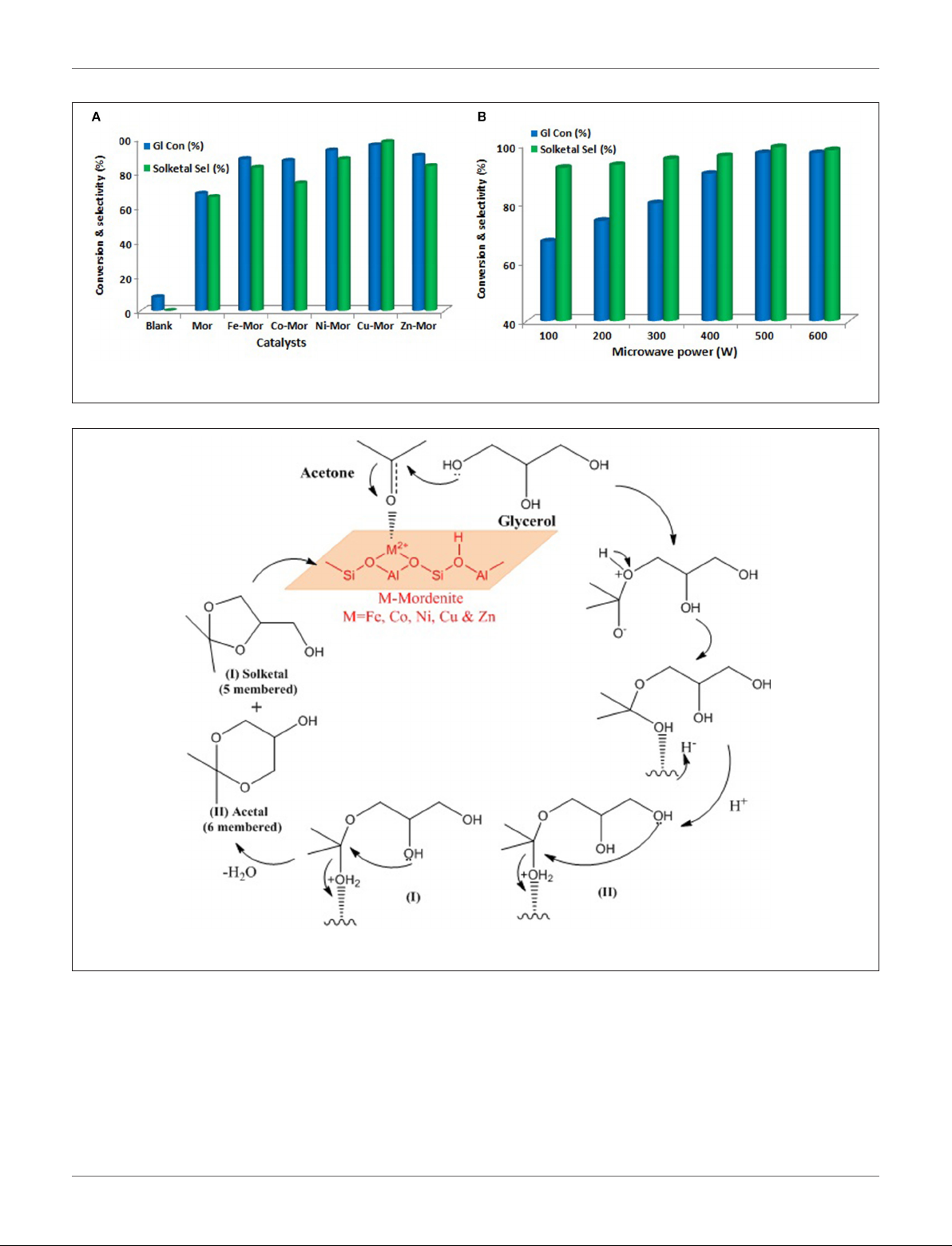
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
21
FIGURE 7 | (A) Glycerol acetalization using different mordenite catalysts. (B) Effect of microwave power on glycerol acetalization over Cu-Mor catalyst.
FIGURE 8 | Plausible reaction mechanism of glycerol acetalization over metal promoted mordenite catalysts.
(mostly cyclic products) at 50◦C and 1:1 acetone to glycerol molar
ratio.
Pawar et al. (2015) investigated the glycerol acetalization
to fuel-additives using an acid-activated clay catalyst of
6/BBnU/6 in the presence of different ketones (cyclohexanone,
Benzaldehyde, Ph-acetaldehyde, Furan aldehyde) in liquid phase.
Also, they evaluated the effect of various processes such as
Frontiers in Chemistry | www.frontiersin.org 15 November 2018 | Volume 6 | Article 573
solvent-free, conventional thermal activation, and nonconventional microwave/ultrasonic activation methods to
find the best operating conditions. Almost complete (99%)
selectivity to solketal at 45% conversion was obtained at 1:1
molar ratio of glycerol to cyclohexanone, at room temperature
in 3 h. The optimization results revealed that increasing the
reaction temperature to 60◦C, glycerol to cyclohexanone

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
22
molar ratio to 3:1 and reaction time to 20 h could significantly
increase the reaction conversion to more than 80%. In addition,
cyclohexanone showed the best effect on the acetalization
reaction among all the tested ketones. The eco-friendly process
involving a catalyst, microwave, or ultra-sonication were
successfully utilized to achieve a commercially valuable hyacinth
fragrance. About 98% selectivity to solketal could be achie ved
under microwave and ultrasonic processes at 92 and 89% ketone
conversion.
In some cases metal-based catalysts support with different
types of carbons [e.g., activated carbon (AC), carbon nano-tubes
(CNT), and multi wall carbon nano-tube (MWCNT)] (Khayoon
and Hameed, 2013; Khayoon et al., 2014). Carbon materials
have been proven to be a good catalytic support in liquid
phase reactions due to acid and base resistance, porosity, high
surface area, excellent electronic properties, surface chemistry
control, and the possibility of metal support (Demirel et al.,
2007). For instance, CNT’s external surface area led metals to
be highly exposed and accessible to reactants, which improves
the efficiency. All these spectacular characteristics provide more
opportunities for further investigation of carbon materials in
glycerol acetalization reaction. Application of other types of
metal-based catalysts particularly MOFs in this field were rarely
reported. These types of catalysts have showed great results in
other research areas such as production of biodiesel (Rafiei et al.,
2018), due to their physico-chemical characteristics (e.g., large
surface area and porosity), and their applications should be
accelerated in the glycerol acetalization reaction.
Polymer Based Catalysts
Environmentally friendly chemistry plays an important role
in design of a cheap and novel catalyst by utilizing cheaper
materials (Kobayashi and Miyamura, 2010). In this regard,
different approaches such as micro-encapsulation has been
recently recognized as a useful technique to immobilize metal
catalysts onto polymers (Akiyama and Kobayashi, 2009). The
micro-encapsulation refers to the incorporation of an active
substance in a shell or a matrix of a carrier component. The
catalysts could be separated from a reaction mixture by simple
filtration and recycled, making them suitable for green chemistry
processes (Ley et al., 2002). Despite all the recent efforts in
this field, the described methods constantly suffers from various
issues (e.g., complex systems, tedious processes, difficult control
of particles’ size and shape, and low activity) (Akiyama and
Kobayashi, 2009).
A new and environmentally friendly method was introduced
by Konwar et al. (2017) for synthesizing a strong solid acidic
meso/macro-porous carbon catalyst from Na-lignosulfonate
(LS), which is a byproduct from sulfite pulping. Ice-templated
LS was altered to macro/mesoporous solid protonic acid
at mild pyrolysis temperature (350–450◦C) and through
ion/H+exchanging approach (Figure 9). According to the
characterization results, the LS-derived components that
were synthesized contained heteroatom-doped (O, S) carbon
structures that are macro/mesoporous and highly functionalized,
as well as a large number of surface -OH, -COOH, and -SO3H
groups, making them similar to sulfonated carbon materials.
In addition, these carbon components exhibited very good
activity as solid acid catalysts in glycerol acetalization with
various bio-based aldehydes and ketones, easily outperforming
the commercial acid exchange resins (AmberliterIR120 and
Amberlystr70). The highest ≥99.5, 53, and 51% selectivities of
solketal were obtained at almost complete glycerol conversion
in the presence of acetone, methyl levulinate and furfural,
respectively in batch processes. In addition, the optimum LS
catalyst (80LS20PS450H+) exhibited a large specific surface
area (122 m2/g) and stable -SO3H sites (1.21 mmol/g) revealing
excellent potential for continuous production of solketal
(reaction condition: 100◦C reaction temperature, 60 min time,
50 ml/min N2flow), maintaining its activity (50% selectivity to
solketal at ≥91%conversion of glycerol) even after 90 h reaction
time.
For the first time, Qing et al. (2017) used a catalytic
active membrane synthesized by immersion phase inversion to
accelerate the glycerol conversion in an acetalization reaction
by continuous removal of water. Indeed, a highly porous
“sponge-like” catalytic layer was immobilized with catalyst
Zr(SO4)2−4H2O and coated on a polyvinyl alcohol/polyether
sulfone per-evaporation membrane. They compared the catalytic
activities in batch reactor, catalytically active membrane reactor,
and inert membrane reactor. The results revealed the absence
of any type of equilibrium limitations for glycerol conversion in
the catalytically active membrane reactor and inert membrane
reactor. The impact of different operational conditions on
synthesis performance in the catalytically active membrane
reactor were evaluated, illustrating that higher feed volume (A/V)
ratio and temperature enhanced glycerol conversion due to the
enhancement of water removal rate. The highest 93% glycerol
conversion was achieved at the optimum condition of 5 wt%
catalyst concentration, membrane area to A/V ratio of 50/108,
1.2:1 cyclohexanone to glycerol molar ratio, at 75◦C in 25 h
reaction time.
Organometallic complex, such as cationic oxorhenium (V)
oxazoline complex and [2-(2′-hydroxyphenyl)-2-oxazolinato (-
2)] oxorhenium (v), were recently tested for a reaction of
glycerol with furfural. As a result, solketal selectivity of 70
at 80% glycerol conversion was obtained at 100◦C in 4 h
(
Wegenhart and Abu-Omar, 2010). Also, Crotti et al. (2010)
investigated the organo-iridium derivatives [Cp∗I
pentamethylcyclopentadienyl) and [Cp∗Ir(Bu2-NHC)Cl2], (Bu2NHC = 1,3-di-nbutylimid azolylidene) as the catalysts for the
acetalization and transacetalization of glycerol with ketones and
aldehydes. Solketal was major product in all those catalytic
reactions.
rCl2]2(Cp∗=
IMPACTS OF VARIOUS REACTION PARAMETERS ON CATALYTIC PERFORMANCE
Reaction Temperature
Serafim et al. (2011) evaluated the influence of reaction
temperature on t
raising the temperature from 30◦C to 70◦C, the conversion of
he glycerol conversion. It was found that by
Frontiers in Chemistry | www.frontiersin.org 16 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
23
FIGURE 9 | Method for preparing sulfonic acid functionalized carbon materials from LS.
the reaction with butanal was greatly improved from 40 to 87%.
Improvement was also observed in the selectivity to solketal.
With a similar concept, Khayoon and Hameed (2013) reported
a moderate increase in reaction temperature led to increase of
glycerol conversion and the reaction successfully formed solketal.
Reaction between butyraldehyde and glycerol using Amberlyst
47 catalyst and stoichiometric feed ratio in the temperature
range of 50–80◦C was performed by Guemez et al. (2013).
The total reaction rate increased with temperature although
this growth did not affect the final equilibrium conversion. The
same results were demonstrated for the re action of glycerol with
formaldehyde and acetaldehyde (Agirre et al., 2011).
In contrast with the aforementioned results and statements,
some researchers revealed that product selectivity could be
reduced with increasing reaction temperature. Nanda et al.
(2014a) showed that a higher temperature lowered the product
yield for exothermic reactions. More specifically, the increase in
reaction temperature only affected the initial reaction rate. In
addition to the mentioned studies, Shirani et al. (2014) reported
reduction of solketal yield, by increment of the acetone to glycerol
molar ratio as well as the temperature. As the temperature
increased, the efficiency of the liquid glycerol molecules with
the interaction of the gaseous acetone molecules on the catalyst
surface decreases, which caused a lower conversion, yield, and
solketal production. Indeed, acetone was vaporized and glycerol
was still in the liquid phase while temperature was increased.
To enhance the quantity of six-membered cyclic acetal in the
mixture, a mild reaction temperature was found to be favorable
for the catalytic condensation of glycerol with para-formaldehyde
using Amberlyst−36 catalyst (Deutsch et al., 2007).
Application of Various Reactants
Many studies have confirmed that the reactant design has a
significant impact on reaction conversion and product selectivity
in the glycerol acetalization reaction. Agirre et al. (2011)
examined various mole ratios of glycerol and formaldehyde,
using Amberlyst 47
catalyst. The study was performed by altering
the molar ratio of glycerol: formaldehyde from 1:1; 1:2 and
1:3, at 353 K. The equilibrium conversion of the formaldehyde
increased with rising molar ratio of glycerol. They further applied
in excess glycerol for the glycerol and acetaldehyde reaction,
which led to 100% conversion of in all cases (Agirre et al.,
2013). T
his finding was in line with Nanda et al. (2014a) on
the condensation of glycerol with acetone whereby the glycerol
and acetone molar ratio significantly affected the kinetics and
thermodynamics of the reaction. When the molar ratios of
acetone to glycerol were 1.48:1 and 2.46:1, the solketal yields
were 68 and 74%, respectively. The influence of ethanol as a
solvent that enhances solubility in acetone was also studied and
showed insignificant effect. In another study, the effect of acetone
in excess on glycerol conversion was investigated by Ferreira
et al. (2010). The glycerol conversion improved with increasing
glycerol to acetone molar ratio (from 1:3 to 1:12), while the
selectivity to solketal remained constant.
Khayoon and Hameed (2013) stated that in the presence
of 5%Ni−1%Zr/AC, the glycerol conversion and the formation
of six-membered cyclic ketals were improved by raising the
glycerol to acetone molar ratio from 1:4 to 1:8. The same
results were reported by Guemez et al. (2013) for glycerol and
n-butyraldehyde. When the initial glycerol to butyraldehyde
ratio increased from 1:1 to 3:1, the n-butyraldehyde conversion
at 80◦C and 100 min of reaction time increased from 88 to
98%. When the glycerol to butyraldehyde molar ratio was
0.2, the glycerol conversion reached 100% after 40 min. In
another research, the effect of glycerol/butanal molar ratio on
the glycerol conversion was investigated (
n the presence of BEA zeolite catalyst at 80◦C, the glycerol
I
Serafim et al., 2011).
conversion after 4 h reaction for glycerol/butanal molar ratio
of 1:1 was 71%, whereas the conversion reached 88% in the
molar ratio of 1:2.5. However, the further increase of molar
ratio (1:6) did not affect the conversion. Applying Amberlyst
15 as a catalyst, Faria et al. (2013) investigated the effect of
Frontiers in Chemistry | www.frontiersin.org 17 November 2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
24
various solvents on the production of glycerol ethyl acetalin a
simulated moving bed reactor via acetalization of glycerol with
acetaldehyde. Compared with acetonitrile, and N,N dimethyl
formamide, dimethylsulfoxide solvent exhibited better results
owing to its capacity toward the catalyst adsorbents, inertness,
and miscibility with the reaction medium. da Silva et al. (2015a)
used an available, environmentally friendly, efficient and simple
tin-based catalyst for the ketalization of glycerol with different
ketones at 25◦C. The ketones conversions were 40 and 98% for
4-methyl 2 pentanone and cyclohexanone, respectively.
Water Removal
The acetalization reaction is reported to have a low equilibrium
constant (Garcia et al., 2008). Thus, shifting the equilibrium
to the product (solketal) side would lead to a higher glycerol
conversion. This could be executed by removing the water
continuously generated during the reaction or by feeding
excess acetone into the reactor. However, the former approach
is reported to be the more effective method to break the
thermodynamic barriers.
Entrainers have been used in different processes for the
continuous elimination of the water from a reaction mixture (Ag,
1998). Benzene, chloroform, and petroleum ethers are some of
the entrainers that can be used in this process. The effectiveness
of these entrainers is not excellent since their boiling points
are higher than acetone. Co-distillation of acetone leads to
low efficiency in azeotropic water removal. This obstacle was
observed when petroleum ether was used as an entrainer (Chen
et al., 2005). The application of phosphorous pentoxide and
sodium sulfate as catalyst and desiccant to remove water from
the reaction environment has also been reported (Ag, 1998).
However, the high amount of catalyst consumption in these
cases raises operation costs (He et al., 1992). The aforementioned
obstacles could be solved by enhancement of acetone utilization,
which not only acts as a reactant but also acts as an entrainer.
More importantly, the excess acetone could be recycled and
reused in the same process, or even other processes.
Roldan et al. (2009) used membrane batch reactor rather than
conventional batch reactor to eliminate water from the reaction
environment. Also, Vicente et al. (2010) investigated a two-step
batch mode operation for continuous removal of water from the
reaction environment. The reaction mixture comprising glycerol,
acetone and catalyst was stirred under reflux in a 100 mL flask
at 70◦C (first step), followed by the removal of produced water
as well as acetone by vaporization under vacuum at 70◦C. Fresh
acetone was added to maintain the liquid level to start a new
cycle (second step). After three consecutive steps, 90% solket al
yield was obtained at the optimum reaction conditions of 70◦C
reaction temperature, 5 wt% loading of ArSBA- 15 catalyst, and
30 min reaction time for each step.
Application of Different Types of Processes
Batch reactors commonly encounter drawbacks when scaling up
the process. Solketal production in a continuous-flow re actor and
in the presence of heterogeneous catalyst is an effective solution
because it leads to higher heat and mass transfer efficiency,
easy scaling-up of the process along with more environmental
TABLE 4 | Effect of glycerol ether additives on the antiwear properties of heavy
cycle oil (ASTM D 2266-01 test method).
Run Additivies
Type Amount
1 Additive-free cycle oil 0.94 –
2 Solketal 460 0.71 25
3 980 0.61 35
4 22,470 0.54 43
5 Mixture of di-GTBEs 5,250 0.76 19
6 1,200 0.84 11
7 440 0.88 6
8 STBE/solketal, 70/30 490 0.87 7
9 1,242 0.82 13
10 5,039 0.61 35
a
Wear spot diameter;bRelative change to additive-free cycle oil WSD.
(ppm)
Average
WSDa(mm)
1WSDb(%)
and economic advantages (Noël and Buchwald, 2011). Initial
attempts were not successful. For example, Clarkson et al. (2001)
used a semi-batch reactor while acetone was fed continuously
while the glycerol amount was constant. The main obstacle for
a continuous process was glycerol’s high viscosity particularly at
low temperatures. In another attempt, Monbaliu et al. (2011)
designed a continuous process using homogeneous H2SO4as
catalyst. However, application of H2SO4made the process not
environmentally friendly due to the corrosion and waste disposal
problems.
Cablewski et al. (1994) reported on the application of
a continuous microwave reactor (CMR) in the production
of solketal. A solution of glycerol, acetone and catalyst
(pTSA) was pumped into the microwave cavity at a desired
temperature, resulting in 84% solketal yield at 13.5 molar
ratio of acetone/glycerol, 132◦C reaction temperature, 1,175
kPa pressure, 1.2 min residence time and 20 mL/min feed flow
rate. However, this process was not appropriate for use with
heterogeneous catalysts and low reaction temperatures or for
reactants that are incompatible with microwave energy.
For the first time, Samoilov et al. (2016) studied the concurrent
ketalisation–alkylation processes in a continuous flow fixedbed reactor. The results revealed that a continuous single-step
process could be effective for glycerol conversion to a mixture
of ethers at mild reaction temperature (40–70◦C) over a zeolite
BEA catalyst. The STBE could be produced at 30% molar
yield at optimum reaction conditions of 1:3.4:10 glycerol: TBA:
acetone molar ratio and at 45◦C. The data in Table 4 show that
solketal has significant influence on the anti-wear characteristics.
Application of the glycerol derivatives (e.g., 0.046–2.25 wt%)
into hydrocarbon oil can enhance the anti-we ar characteristics
by 42%. The substitution of the solketal hydroxyl group by
TBA produces large hydrocarbon radicals, which could slow
down the adsorption of the molecule on metal surfaces by steric
hindrance along with a change in the polarity and molecules’
surface activity. Moreover, the spatial configuration of the
Frontiers in Chemistry | www.frontiersin.org 18 November 2018 | Volume 6 | Article 573
Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
25
FIGURE 10 | Possible pathways of STBE formation.
FIGURE 11 | Proposed reaction mechanism for glycerol and acetone acetalization over acid catalyst.
ketal molecule may have a positive impact on the adsorption.
Figure 10 illustrates the different steps of STBE formation. The
first route (A) includes glycerol ketalisation (A1) followed by
solketal tert-butylation with TBA (A2). The second route (B)
includes formation of 1-mono-GTBE (B1) and then ketalisation
of ether to STBE (B2).
Recently, Nanda et al. (2014b) developed a continuous-flow
reactor based on the “Novel Process Windows” concept with
respect to temperature, pressure and/or reactant concentration
to enhance the intrinsic kinetics of the reaction for an optimum
Frontiers in Chemistry | www.frontiersin.org 19 November 2018 | Volume 6 | Article 573
yield. Results indicated that they could achieve more than 97%
selectivity to solketal at >80% glycerol conversion using different
catalysts (H-β zeolite, Amberlyst 36 wet, Amberlyst 35, ZrSO4) at
40◦C reaction temperature, 600 psi reaction pressure, 6:1 molar
ratio of acetone to glycerol in 15 min reaction time. On the other
hand, this system suffered from the catalyst clogging the reactor.
The glycerol acetalization reaction mechanism in the presence
of an acid catalyst that leads to the formation of both fiveand six-membered rings (ketals) is illustrated in Figure 11
(Voleva et al., 2012). Nanda et al. (2014a) have also reported

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
26
a two-step processes for glycerol acetalization reaction. The
surface reaction between glycerol and the adsorbed acetone on
the catalyst surface form the hemi-acetal (Step 1), then the
carbonyl carbon turns into a c ar bocation by the removal of a
water molecule and deprotonization to form solketal and 1,3dioxane. The 1,3-dioxane (six membered ring ketal) is the less
favorable product because one of the methyl groups is in the
axial position of the chaircon formation (Maksimov et al., 2011).
Thus, in the majority of cases the resulting product has higher
ratio (even up to 99:1) of five-membered ring (solketal) to sixmembered ring (5-hydroxy-2,2-dimethyl-1,3-dioxane).
Perreia and Rodrigues (2013) creatively used process
intensification for acetal production along with advanced
technologies, applying Simulated Moving Bed Membrane
Reactor (PermSMBR), and Simulated Moving Bed Reactor
(SMBR), for the acetalization of2-butanol and ethanol with
acetaldehyde. These reactors achieved high conversion and
productivity at low temperatures (10–50◦C), while requiring
additional operational costs and capitals. This method could be
used for the acetalization of glycerol. The reactive distillation
with approximately 100% glycerol conversion is the best reactor
design since they feature low capital costs and no reactor
clogging with the catalyst. Performing the distillation and
reaction simultaneously also saves on operational cost.
Gorji and and Ghaziaskar (2016) synthesized mono-acetin by
reacting glycerol with acetic acid (first step) followed by solketal
production from the reaction of mono-acetin with acetone over
Purolite PD 206 catalyst (second step). This method was reported
as an economical and easy to scale up process for glycerol
conversion to fuel-additives. Results show 69% yield of solketal
was achieved at almost complete glycerol conversion at optimal
reaction conditions of 5:1 acetone to mono-acetin molar ratio, 0.2
mL/min feed flow rate, and 2.0 g catalyst at ambient temperature
of 20◦C and 45 b ar pressure in the second step.
Application of Crude Glycerol
The key factor for industrial applications of glycerol is its purity
level. The crude glycerol obtained from biodiesel production
is normally considered as waste by-product in the biodiesel
industry since it contains impurities, namely esters, methanol,
fatty acids, water, and inorganic salts (matter organic nonglycerol, MONG) (
conomically, it is feasible for large-scale biodiesel firms to purify
E
the crude glycerol for further utilizations. Small companies on
the other hand cannot afford it; therefore, they have to pay
for glycerol disposal or burn it as a waste stream (Wilson,
2002; McCoy, 2006). Heterogeneous catalysts and non-edible
oils have been used in the vast majority of studies to produce
higher quality biodiesel and glycerol. For example, high quality
biodiesel (98.3%) and glycerol (98%) were produced over the
Zn-Al heterogeneous solid catalyst (Bournay et al., 2005). Their
synthesized catalyst could avoid all the costly purification steps
in the direct utilization of crude glycerol. Thus, application of
crude-glycerol is another factor that could significantly impact
the catalyst activity. Although there have been plenty of studies
on the bio-based production of value-added chemicals from
Liang et al., 2010; Rosas et al., 2017).
refined glycerol, t he same cannot be said regarding the utilization
of crude glycerol as feedstock.
da Silva and Mota (2011) evaluated the impact of impurities
on the formation of solketal in a batch reactor to enable the
utilization of crude glycerol rather than purified glycerol. They
investigated the effect of common impurities (e.g., 1% methanol,
10% water, and 15% NaCl) in the acetalization of crude glycerol
with various heterogeneous catalysts (e.g., H-beta zeolite and
Amberlyst-15). When crude glycerol was used in place of refined
glycerol, a dramatic drop in reaction conversion was observed,
down to 47 and 50% for Amberlyst-15 and H-beta zeolite
catalysts, respectively, compared to 95% reaction conversion for
refined glycerol with similar catalysts. In fact, they revealed that
methanol had less effect than water and NaCl.
Vicente et al. (2010) reported the application of acid-
unctionalized SBA-15 catalysts for acetalization of crude glycerol
f
(85.8 wt.%). They obtained 81% conversion of glycerol. However,
high Na+content in crude glycerol deactivated significantly the
sulfonic acid sites due to a cation exchange reaction between Na
and H+.
In another study, Nanda (2015) continued their studies
by developing a modified continuous-flow reactor including
guard reactors that allow online elimination of impurities from
the glycerol feedstock and on-line regeneration of deactivated
catalysts. A maximum 78% yield of solketal was achieved using
crude glycerol and the modified continuous-flow reactor in only
1 h of on-stream time. They also conducted on-line regeneration
of the deactivated catalyst in the guard reactor concurrently with
the ketalization experiment using purified (96%) crude-glycerol
as feedstock. They found that the catalyst (Amberlyst-36 wet)
could be effectively regenerated for four consecutive times even
up to 96 h of reaction time with only 11% fall (92 to 81%) in
solketal yield (Nanda, 2015). For the catalyst regeneration, a 0.5M
H2SO4solution was passed through the guard reactor, followed
by a methanol washing of the regenerated catalyst and finally
drying the bed with nitrogen for 5 h.
CONCLUDING REMARKS
This review comprehensively summarizes various approaches
and strategies for the glycerol conversion to cyclic acetals
and ketals processes and recent progress in obtaining higher
conversion and selectivity of the desired products. Both
homogeneous and heterogeneous acid c atalysts can be used
in the conversion of glycerol to solketal. The vast majority
of studies are conducted over heterogeneous catalysts, which
can be easily separated from the system by filtration. Various
reaction parameters (e.g., reactor design, temperature, reactants,
and nature of catalyst) have significant impact on the catalytic
activity in this process.
Recent breakthroughs in catalyst synthesis and
characterization lead to unprecedented achievements in this
field. Despite various advances, there are still many challenges
for increasing selectivity and yield. Indeed, there are spectacular
opportunities in catalysis and nano-materials to synthesize
a highly active catalyst for glycerol acetalization to specific
+
Frontiers in Chemistry | www.frontiersin.org 20 November 2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
27
useful products. Application of more environmentally friendly
processes and materials for catalyst synt hesis will be the main
objective in the future. For instance, application of microwave
radiations could enlarge the specific surface area and increased
pore volume.
Investigation on the effect of different reaction parameters
on catalytic activity revealed that one of the major obstacles in
acetalization reaction is its very low equilibrium constant. As a
result, the best remedy for this problem is to shift the equilibrium
to the product side (e.g., solketal), by either feeding excess
reactant (e.g., acetone) or by removing water generated during
the reaction. Also, compared to operation in a batch reactor,
similar or even higher product selectivity and relatively shorter
reaction time could be achieved with the use of continuous flow
reactors. Definitely, more investigation on continuous glycerol
acetalization process could be one of the major steps toward
industrialization and commercialization of this process in the
near future.
The reviewed studies confirm that the acetalization of glycerol
is a promising process that could bring significant economic
prosperity. In addition to the economic benefits, it also has
tangible benefits on the environment by shifting the production
of industrial chemicals away from petroleum-based toward
bio-based processes. Indeed, fuel additive could improve the
cleanliness of different parts of the engine, promote complete
combustion, reduce fuel gelling and choking of nozzle, as
well as reduce corrosion impact on different parts of the
engine.
AUTHOR CONTRIBUTIONS
This research is based on AT-K’s postdoctoral fellowship
research project. NN assisted in collecting information, the
revision process, and manuscript improvement. NA was the
corresponding author and AT-K’s main supervisor during his
Postdoctoral fellowship. ST was AT-K’s co-supervisor during his
Postdoctoral fellowship.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to
the Universiti Teknologi Malaysia (UTM), for supporting the
project under PDRU grant no. 03E49. Also, authors would
like to express their sincere gratitude to Iran’s National Elites
Foundation and Iran Polymer and Petrochemical Institute (IPPI)
for their supports.
REFERENCES
Abad, S., and Turon, X. (2012). Valorization of biodiesel derived glycerol as a
carbon source to obtain added-value metabolites: focus on polyunsaturated
fatty acids. Biotechnol. Adv. 30, 733–741. doi: 10.1016/j.biotechadv.2012.01.002
Abreu, A. V., Meyer, C. I., Padro, C., and Martins, L. (2018). Acidic V-MCM-41
catalysts for the liquid-phase ketalization of glycerol with acetone. Miro. Meso.
Matt. 273, 219–2 2 5. doi: 10.1016/j.micromeso.2018.07.006
Adam, F., Batagarawa, M. S., Hello, K. M., and Al-Juaid, S. S. (2012). One-step
synthesis of solid sulfonic acid catalyst and its application in the acetalization
of glycerol: crystalstructure of−5-hydroxy-2-phenyl-1,3-dioxane trimer. Chem.
Papers 66, 1048–1058. doi: 10.2478/s11696-012-0203-x
Ag, B. (1998). Cyclic Acetal or Ketal Preparation From Polyol and Aldehyde or
Ketone. Patent DE19648960 A1.
Aghbashloa, M., Tabatabaei, M., Hosseinpoura, S., Rastegarid, H., and Ghaziaskar,
H. S. (2018). Multi-objective exergy-based optimization of continuous glycerol
ketalization to synthesize solketal as a biodiesel additive in subcritical acetone.
Energ. Conv. Manage. 160, 251–261. doi: 10.1016/j.enconman.2018.01.044
Agirre, I., Garcia, I., Requies, J., Barrio, V. L., Guemez, M. B., and
Cambra, J. F. (2011). Glycerolacetals, kinetic study of the reaction
between glycerol and formaldehyde. Biomass Bioenergy 35, 3636–3642.
doi: 10.1016/j.biombioe.2011.05.008
Agirre, I., Guemez, M. B., Uguarte, A., Requies, J., Barrio, V. L., and Cambra,
J. F. (2013). Glycerolacetals as diesel additives: kinetic study of the reaction
between glycerol andacetaldehyde. Fuel Process. Technol. 116, 182–188.
doi: 10.1016/j.fuproc.2013.05.014
Akiyama, R., and Kobayashi, S. (2009). “Microencapsulated” and related catalysts
for organic chemistry and organic synt hesis. Chem Rev. 109, 594–642.
doi: 10.1021/cr800529d
Arias, K. S., Garcia-Ortiz, A., Climent, M. J., Corma, A., and Iborra, S. (2018).
Mutual valorization of 5-hydroxymethylfurfural and glycerol into valuable diol
monomers with solid acid catalysts. ACS. Sust. Chem. Eng. 6, 4239–4245.
doi: 10.1021/acssuschemeng.7b04685
Ayoub, M., and Abdullah, A. Z. (2012). Critical review on the current scenario
and significance of crude glycerol resulting from biodiesel industry towards
more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 16,
2671–2686. doi: 10.1016/j.rser.2012.01.054
Bournay, L., Casanave,D., Delfort, B., Hillion, G., and Chodorge, J. A. (2005). New
heterogeneous process for biodiesel production: a way to improve the quality
and the value of the crude glycerin produced by biodiesel plants. Catal. Today
106, 190–192. doi: 10.1016/j.cattod.2005.07.181
Cablewski, T., Faux, A. F., and Strauss, C. R. (1994). Development and application
of a continuous microwave reactor for organic synthesis. J. Org. Chem. 59,
3408–3412. doi: 10.1021/jo00091a033
Chen, L., Liang, J., Lin, H., Weng, W., Wan, H., and Védrine, J. C.
(2005). MCM41 and silica supported MoVTe mixed oxide catalysts for
direct oxidation of propane to acrolein. Appl. Catal. A 293, 49–55.
doi: 10.1016/j.apcata.2005.06.029
Chen, L., Nohair, B., Zhao, D., and Kaliaguine, S. (2018a). Highly efficient glycerol
acetalization over supported heteropolyacid catalysts. Chem. Cat. Chem. 10,
1918–1925. doi: 10.1002/cctc.201701656
Chen, L., Nohair,B., Zhao, D., and Kaliaguine, S. (2018b). Glycerol acetalization
with formaldehyde using heteropolyacid salts supported on mesostructured
silica. Appl. Catal. A Gen. 549, 207–215. doi: 10.1016/j.apcata.2017.09.027
Choi, M., Cho, H. S., Srivastava, R., Venkatesan, C., Choi, D. H., and Ryoo, R.
(2006). Amphiphilic organosilane-directed synthesis of crystalline zeolite with
tunable mesoporosity. Nat. Mater. 5, 718–723. doi: 10.1038/nmat1705
Clarkson, J. S., Walker, A. J., and Wood, M. A. (2001). Continuous reactor
technology for ketal formation: an improved synthesis of solketal. Org. Process.
Res. Dev. 5, 630–635. doi: 10.1021/op000135p
Clomburg, J. M., and Gonzalez, R. (2013). Anaerobic fermentation of glycerol:
a platform for renewable fuels and chemicals. Trends. Biotechnol. 31, 20–28.
doi: 10.1016/j.tibtech.2012.10.006
Coleman, T., and Blankenship, A. (2010). Process for the Preparation of Glycerol
Formal. US Patent 0094027.
Crotti, C., Farnetti, E., and Guidolin, N. (2010). Alternative intermediates for
glycerol valorization: iridium-catalyzed formation of acetals and ketals. Green.
Chem. 12, 2225–2231. doi: 10.1039/c0gc00096e
da Silva, C. X. A., Goncalves, V. L. C., and Mota, C. J. A. (2009). Water tolerant
zeolite catalyst for the acetalisation of glycerol. Green. Chem. 11, 38–41.
doi: 10.1039/B813564A
da Silva, C. X. A., and Mota, C. J. A. (2011). The influence of impurities on the acid-
catalyzed reaction of glycerol with acetone. Biomass Bioenerg. 35, 3547–3551.
doi: 10.1016/j.biombioe.2011.05.004
Frontiers in Chemistry | www.frontiersin.org 21 November
2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
28
da Silva, M. J., De Oliveira, G. M., and Julio, A. A. (2015a). A highly regioselective
and solvent-freeSn(II)-catalyzed glycerol ketals synthesis at room temperature.
Catal. Lett.145, 769–776. doi: 10.1007/s10562-015-1477-8
da Silva, M. J., Julio, A. A., and Dorgetto, F. C. S. (2015b). Solvent-free
heteropolyacid-catalyzed glycerol ketalization at room temperature. RSC
Advance 5, 44499–44506. doi: 10.1039/C4RA17090C
da Silva, M. J., Rodrigues, F. D. A., and Julio, A. A. (2017). SnF2-catalyzed glycerol
ketalization: a friendly environmentally process to synthesize solketal at room
temperature over on solid and reusable Lewis acid. Chem. Eng. J. 307, 828–835.
doi: 10.1016/j.cej.2016.09.002
de Carvalho, D. C., Oliveira, A. C., Ferreira, O. P., Filho, J. M., Tehuacanero-
Cuapa, S., and Oliveira, A. C. (2017). Titanate nanotubes as acid catalysts for
acetalization of glycerol with acetone: influence of the synthesis time and the
role of structure on the catalytic performance. Chem. Eng. J. 313, 1454–1467.
doi: 10.1016/j.cej.2016.11.047
De Torres, M., Jimenez-oses, G., Mayoral, J. A., and Pires, E . (2011). Fatty acid
derivates and their use as CFFP additives in biodiesel. Bioresour. Technol. 102,
2590–2594. doi: 10.1016/j.biortech.2010.10.004
Demirel, S., Kern, P., Lucas, M., and Claus, P. (2007). Oxidation of mono-and
polyalcohols with gold: comparison of carbon and ceria supported catalysts.
Catal. Today 122, 292–300. doi: 10.1016/j.cattod.2006.12.002
Deutsch, J., Martin, A., and Lieske, H. (2007). Investigations on heterogeneously
catalyzed condensations of glycerol to cyclic acetals. J. Catal. 245, 428–435.
doi: 10.1016/j.jcat.2006.11.006
Egeblad, K., Christensen, C. H., and Kustova, M. (2008). Templating mesoporous
zeolites. Chem. Mater. 20, 946–960. doi: 10.1021/cm702224p
Fan, C. N., Xu, C. H., Liu, C. Q., Huang, Z. Y., Liu, J. Y., and Ye, Z.
X. (2012). Catalytic acetalization ofbiomass glycerol with acetone over
TiO2-SiO2mixed oxides. React. Kin. Mech. Cat. Lett. 107, 189–202.
doi: 10.1007/s11144-012-0456-y
Fan, W., Snyder, M. A., Kumar, S., Lee, P. S., Yoo, W. S., McCormick, A. V.,
et al. (2008). Hierarchical nanofabrication of microporous crystals with ordered
mesoporosity. Nat. Mater. 7, 984–991. doi: 10.1038/nmat2302
Faria, R. P. V., Pereira, C. S. M., Siva, V. M. T. M., Loureiro, J. M., and Rodrigues,
A. E. (2013). Glycerolvalorization as biofuels: selection of a suitable solvent for
an innovative processfor the synthesis of GEA. Chem. Eng. J. 233, 159–167.
doi: 10.1016/j.cej.2013.08.035
Fazal, M. A., Haseeb, A. S. M. A., and Masjuki, H. H. (2011). Biodiesel
feasibility study: an evaluation of material compatibility; performance;
emission and engine durability. Ren. Sus. Energ. Rev. 15, 1314–1324.
doi: 10.1016/j.rser.2010.10.004
Férey, G., Serre, C., Mellot-Draznieks, C., Millange, F., Surblé, S., Dutour, J., et al.
(2004). A hybrid solid with giant pores prepared by a combination of targeted
chemistry, simulation, and powder diffraction. Angew. Chem. 116, 6456–6461.
doi: 10.1002/ange.200460592
Ferreira, C., Araujo, A., Calvino-Casilda, V., Cutrufello, M. G., Rombi, E.,
Fonseca, A. M., et al. (2018). Y zeolite-supported niobium pentoxide catalysts
for the glycerol acetalization reaction. Micro. Meso. Mat. 271, 243–251.
doi: 10.1016/j.micromeso.2018.06.010
Ferreira, P., Fonseca, I. M., Ramos, A. M., Vital, J., and Castanheiro, J.
E. (2010). Valorisationofglycerol by condensation with acetone over
silica-included heteropolyacids. Appl. Catal. B Environ. 98, 94–99.
doi: 10.1016/j.apcatb.2010.05.018
Fischer, E. (1895). Ueber die verbindungen der zuckermit den alkoholen und
ketonen. Eur. J. Inorg. Chem. 28, 1145–1167.
Fischer, E., and Pfahler, E. (1920). Uber glycerin-aceton und
seine verwendbarkeitzurreindarstellung von α-glyceriden: uber
einephosphorsaureverbindungglykois. Eur. J. Inorg. Chem. 53, 1606–1621.
Forester, D. R., Robert, S. D. C., Manka, J. S., and Malik, B. B. (2003). Jet Fuel
Additive Concentrate Composition and Fuel Composition and Methods Thereof.
US Patent WO2003106595.
Franklin, P. M., Koshland, C. P., Lucas, D., and Sawyer, R. F. (2000). Clearing the
air: using scientific information to regulate reformulated fuels. Environ. Sci.
Technol. 34, 3857–3863. doi: 10.1021/es0010103
Gadamsetti, S., Rajan, N. P., Rao, G. S., and Chary, K. V. R. (2015).
Acetalization of glycerol with acetone to bio fuel additives over supported
molybdenum phosphate catalysts. J. Mol. Cat. A Chem. 410, 49–57.
doi: 10.1016/j.molcata.2015.09.006
Garcia, E., Laca, M., Pe, E., and Garrido, A. (2008). New class of acetal derived
from glycerin as abiodiesel fuel component. Energ. Fuel. 22, 4274–4280.
doi: 10.1021/ef800477m
García-Martínez, J., Li, K. (eds) (2015). Mesoporous Zeolites: Preparation
Characterization and Application (Weinheim: Wiley-VCH Verlag GmbH & Co
KGaA), 1–574.
Gesslein, B. W. (1999). “Humectants in personal care formulation: a practical
guide,” in Conditioning Agents for Hair and Skin, eds R. Schueller, P.
Romanowski (New York, NY; Basel: Marcel Dekker, Inc.), 95–6.
Gomes, I. S., de Carvalho, D. C., Oliveira, A. C., Rodriguez-Castellon,
E., Tehuacanero-Cuapa, S., Freire, P. T. C., et al. (2018). On the
reasons for deactivation of titanate nanotubes with metals catalysts in
the acetalization of glycerol with acetone. Chem. Eng. J. 334, 1927–1942.
doi: 10.1016/j.cej.2017.11.112
Gonzalez-Arellano, C., De, S., and Luque, R. (2014a). Selective glycerol
transformations to high value-added products catalysed by alumino-silicatesupported iron oxide nanoparticles. Catal.Sci. Technol. 4, 4242–4249.
doi: 10.1039/C4CY00714J
Gonzalez-Arellano, C., Parra-Rodriguez, L., and Luque, R. (2014b). Mesoporous
Zr–SBA-16 catalysts for glycerol valorization processes: towards bio-renewable
formulations. Catal. Sci. Technol. 4, 2287–2292. doi: 10.1039/C4CY00230J
Gorji, Y. M., and and Ghaziaskar, H. S. (2016). Optimization of solketalacetin
synthesis as a green fuel additive from ketalization of monoacetin with acetone.
Ind. Eng. Chem. Res. 55, 6904–6910. doi: 10.1021/acs.iecr.6b00929
Groen, J. C., Zhu, W., Brouwer, S., Huynink, S. F., Kapteijn, F., Moulijn, J. A.,
et al. (2007). Direct demonstration of enhanced diffusion in mesoporous ZSM5 zeolite obtained via controlled desilication. J. Am. Chem. Soc. 129, 355–360.
doi: 10.1021/ja065737o
Guemez, M. B., Requies, J., Agirre, I., Arias, P. L., Bario, L., and Cambra, J.
F. (2013). Acetalizationreaction between glycerol and n-butyraldehyde using
an acidic ion exchangeresin. Kinet. Model. Chem. Eng. J. 228, 300–307.
doi: 10.1016/j.cej.2013.04.107
Gutiérrez-Acebo, E., Guerrero-Ruiz, F., Centenero, M., Martínez, J. S., Salagre, P.,
Cesteros, Y. (2018). Effect of using microwaves for catalysts preparation on the
catalytic acetalization of glycerol with furfural to obtain fuel additives. Open
Chem. 16, 386–392. doi: 10.1515/chem-2018-0047
Halgeri, A. B., and Das, J. (1999). Novel catalytic aspects of beta zeolite
for alkyl aromatics transformation. Appl. Catal. A 181, 347–354.
doi: 10.1016/S0926-860X(98)0039 5 - 0
Hasabnis, A., and Mahajani, S. (2014). Acetalization of glycerol with
formaldehyde byreactive distillation. Ind. Eng. Chem. Res. 53, 12279–12287.
doi: 10.1021/ie501577q
He, D. Y., Li, Z. J., Li, Z. J., Liu, Y. Q., Qiu, D. X., and Cai, M. S. (1992). Studies
on carbohydrates X. A newmethod for the preparation of isopropylidene
saccharides. Synth. Com. 22, 2653–2658. doi: 10.1080/003979192080
21665
Horcajada, P., Surblé, S., Serre, C., Hong, D. Y., Seo, Y. K., Chang, J. S., et al. (2007).
Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with
large pores. Chem. Commun. 27, 2820–2822. doi: 10.1039/B704325B
Jamil, F., Saxena, S. K., Al-Muhtaseb, A. H., Baawain, M., Al-Abri, M.,
Viswanandham, N., et al. (2017). Valorization of waste “date seeds” bio-glycerol
for synthesizing oxidative green fuel additive. J. Clean. Prod. 165, 1090–1096.
doi: 10.1016/j.jclepro.2017.07.216
Kapkowski, M., Ambrozkiewicz, W., Siudyga, T., Sitko, R., Szade, J., limontko,
J., et al. (2017). Nano silica and molybdenum supported Re, Rh, Ru or
Irnanoparticlesfor selective solvent-free glycerol conversion to cyclic acetals
with propanone and butanone under mild conditions. Appl. Catal. B Env. 202,
335–345. doi: 10.1016/j.apcatb.2016.09.032
Karin, B., Marrot, J., Riou, D., and Férey, G. (2004). A breathing
hybrid organic–inorganic solid with very large pores and
high magnetic characteristics. Angew. Chem. 114, 291–294.
doi: 10.1002/1521-3757(200201 1 8 )1 1 4 :2 <2 9 1 ::AID- ANGE 2 9 1 >3 .0 .CO;2 - I
Khayoon, M. S., Abbas, A., Hameed, B. H., Triwahyono, S., Jalil, A. A., and
Harris, A. T. (2014). Selective acetalization of glycerol with acetone over nickel
nanoparticlessupported on multi-walled carbon nano-tubes. Catal. Lett. 144,
1009–1015. doi: 10.1007/s10562-014-1221-9
Khayoon, M. S., and Hameed, B. H. (2013). Solventless acetalization
of glycerol with acetone tofuel oxygenates over Ni–Zi supported
Frontiers in Chemistry | www.frontiersin.org 22 November 2018 | Volume 6 | Article 573

Talebian-Kiakalaieh et al. Glycerol Acetalization to Value-Added Chemicals
29
mesoporous carbon catalyst. Appl. Catal. A Gen. 464–465, 191–199.
doi: 10.1016/j.apcata.2013.05.035
Kobayashi, S., and Miyamura, H. (2010). Polymer-incarcerated metal (0) cluster
catalysts. Chem Rec. 10, 271–290. doi: 10.1002/tcr.201000026
Konwar, L. J., Samikannu, A., Maki-Arvela, P., Bostrom, D., and Mikkola,
J. (2017). Lignosulfonate-based macro/mesoporous solid protonic acids for
acetalization of glycerol to bio-additives. Appl. Catal. B Environ. 220, 314–323.
doi: 10.1016/j.apcatb.2017.08.061
Kowalska-Kus, J., Held, A., Frankowski, M., and Nowinska, K. (2017).
Solketal formation from glycerol and acetone over hierarchical zeolites
of different structure as catalysts. J. Mol. Catal. A Chem. 426, 205–212.
doi: 10.1016/j.molcata.2016.11.018
Lazar, A., Betsy, K. J., Vinod, C. P., and Singh, A. P. (2018). Ru(II)-functionalized
SBA-15 as highly chemoselective, acid free and sustainable heterogeneous
catalyst for acetalization of aldehydes and ketones. Cat. Comm. 104, 62–66.
doi: 10.1016/j.catcom.2017.10.016
Ley, S. V., Ramarao, C., Gordon, R. S. G., Holmes, A. B., Morrison, A. J., and
McConvey, I. F. (2002). D, Polyurea-encapsulated palladium (II) acetate: a
robust and recyclable catalyst for use in conventional and supercritical media.
Chem. Commun. 10, 1134–1135. doi: 10.1039/b200677b
Li, L., Korányi, T. I., Sels, B. F., and Pescarmona, P. P. (2012). Highly-efficient
conversion of glycerol to solketal over heterogeneous Lewis acid catalysts.
Green. Chem. 14, 1611–1619. doi: 10.1039/C2GC16619D
Li, R., Song, H., and Chen, J. (2018b). Propylsulfonic acid functionalized SBA-15
mesoporous silica as efficient catalysts for the acetalization of glycerol. Catalysts
8:297. doi: 10.3390/catal8080297
Li, X., Zheng, L., and Hou, Z. (2018c). Acetalization of glycerol with acetone over
Co[II](Co[III]xAl2–x)O4 derived from layered double hydroxide. Fuel 233,
565–571. doi: 10.1016/j.fuel.2018.06.096
Li, Z., Miao, Z., Wang,X.,Zhao, J., Zhou, J., Si, W., et al. (2018a). One-pot synthesis
of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to
solketal. Fuel 233, 377–387. doi: 10.1016/j.fuel.2018.06.081
Liang, Y., Cui, Y., Trushenski, J., and Blackburn, J. W. (2010). Converting crude
glycerol derived from yellow g rease to lipids through yeast fermentation.
Bioresour. Technol. 101, 7581–7586. doi: 10.1016/j.biortech.2010.04.061
Lin, C. Y., and Chen, W. C. (2006). Effects of potassium sulfide content
in marine diesel fuel oil on emission characteristics of marine furnaces
under varying humidity of inlet air. Ocean Eng. 33, 1260–1270.
doi: 10.1016/j.oceaneng.2005.06.009
Lin, Y. C., and Huber, G. W. (2009). The critical role of heterogeneous
catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2, 68–80.
doi: 10.1039/B814955K
Loiseau, T., Serre, C., Huguenard, C., Fink, G., Taulelle, F., Henry, M., et al. (2004).
A rationale for the large breathing of the porous aluminum terephthalate (MIL-
53) upon hydration. Chem. Eur. J. 10, 1373–1382. doi: 10.1002/chem.200305413
Lupulescu, A. I., and Rimer, J. D. (2012). Tailoring silicalite-1 crystal
morphology with molecular modifiers. Angew. Chem. Int. Ed. 51, 3345–3349.
doi: 10.1002/anie.201107725
Maksimov, L., Nekhaeva, I., Ramazanov, D. N., Arinicheva, Y. A., Dzyubenko,
A., and Khadzhiev, S. N. (2011). Preparation of high-octane oxygenate
fuel components fromplant-derived polyols. Pet. Chem. 51, 61–69.
doi: 10.1134/S096554411101011 7
Mallesham, B., Sudarsanam, P., Raju, G., and Reddy, B. M. (2013). Design of
highly efficient Mo and W-promoted SnO2solid acids for heterogeneous
catalysis: acetalization of bio-glycerol. Green. Chem. 15, 478–489.
doi: 10.1039/C2GC36152C
Mallesham, B., Sudarsanam, P., and Reddy, B. M. (2014). Eco-friendly synthesis of
bio-additivefuels from renewable glycerol using nano-crystalline SnO2- based
solid acids. Catal. Sci. Technol. 4, 803–813. doi: 10.1039/c3cy00825h
Manjunathan, P., Maradur, S. P., Halgeri, A. B., and Shanbhag, V. (2015). R oom
temperaturesynthesis of solketal from acetalization of glycerol with acetone:
effect ofcristallite size and the role of acidity of beta zeolite. J. Mol. Catal. A
Chem. 396, 47–54. doi: 10.1016/j.molcata.2014 .0 9 .0 2 8
Manjunathan, P., Marakatti, V. S., Chandra, P., Kulal, A. B., Umbarkar, S. B.,
Ravishankar, R., et al. (2018). Mesoporous tin oxide: an efficient catalyst with
versatile applications in acid and oxidation catalysis. Catal. Today 309, 61 –7 6.
doi: 10.1016/j.cattod.2017.10.009
Mantovani, M., Mandello, D., Goncalves, M., and Carvalho, W. A. (2018). Fructose
dehydration promoted by acidic catalysts obtained from biodiesel waste. Chem.
Eng. J. 348, 860–869. doi: 10.1016/j.cej.2018.05.059
Martin, A., Armbruster, U., and Atia, H. (2012). Recent developments in
dehydration of glycerol toward acrolein over heteropoly acids. Eur. J. Lipid Sci.
Technol. 114, 10–23. doi: 10.1002/ejlt.201100047
McCoy, M. (2006). Glycerin surplus. Chem. Eng. News 8 4 , 7–8.
doi: 10.1021/cen-v084n006.p007a
Menchavez, R. N., Morra, M. J., and He, B. B. (2017). Co-Production of
ethanol and 1,2-propanediol viaGlycerol hydrogenolysis using Ni/Ce–Mg
catalysts: effects of catalyst preparation and reaction conditions. Catalysts 7:290.
doi: 10.3390/catal7100290
Monbaliu, J. C. M., Winter, M., Chevalier, B., Schmidt, F., Jiang, Y., and
Hoogendoorn, R. (2011). Effective production of the biodiesel additive
STBE by a continuous flowprocess. Bioresour. Technol. 102, 9304–9307.
doi: 10.1016/j.biortech.2011.07.007
Mota, C. J., da Silva, C. X., Rosenbach, N., Costa, J., and da Silva, F. (2010). Glycerin
derivatives as fuel additives: the addition of glycerol/acetone ketal (solketal) in
gasolines. Energ. Fuel. 24, 2733–2736. doi: 10.1021/ef9015735
Nair, G. S., Adrijanto, E., Alsalme, A., Kozhevnikov, I. V., Cooke, D. J., Brown, D.
R., et al. (2012). Glycerol utilization over niobia catalysts. Catal. Sci. Technol. 2,
1173–1179. doi: 10.1039/c2cy00335j
Nanda, M. R. (2015). Catalytic Conversion of Glycerol to Value-Added Chemical
Products. Electronic Thesis and Dissertation Repository, Western University,
London, Canada, Paper 3215.
Nanda, M. R., Yuan, Z., Qin, W., Ghaziaskar, H. R., Poirer, M. A., and Xu,
C. C. (2014b). Thermodynamic and kinetic studies of a catalytic process to
convert glycerol into solketal as an oxygenated fuel additive. Fuel 117, 470–477.
doi: 10.1016/j.fuel.2013.09.066
Nanda, M. R., Yuan, Z., Qin, W., Ghaziaskar, H. S., Poirier, M. A., and Xu, C.
(2014a). A new continuous-flow process for catalytic conversion of glycerol
to oxygenated fuel additive: catalyst screening. Appl. Energ. 123, 75–81.
doi: 10.1016/j.apenergy.2014.02.055
Nanda, M. R., Yuan, Z., Shui, H., and Xu, C. (2017). Selective hydrogenolysis
of glycerol and crudeglycerol (a by-product orwaste stream from thebiodiesel
industry) to 1,2-propanediol over B2O3Promoted Cu/Al2O3Catalysts.
Catalysts 7:196. doi: 10.3390/catal7070196
Narkhede, N., and Patel, A. (2014). Room temperature acetalization of glycerol to
cyclicacetals over anchored silicotungstates under solvent free conditions. R.
Soc. Chem. Adv. 4, 19294–19301. doi: 10.1039/C4RA01851F
Newman, M. S., and Renoll, M. (1945). Improved preparation of isopropyledene
glycerol. J. Am. Chem. Soc. 67, 1621–1621. doi: 10.1021/ja01225a511
Nguyen, R., Galy, N., Singh, A. K., Paulus, F., Stobener, D., Schlesener, C., et al.
(2017). A simple and efficient process for large scaleglycerol oligomerization by
microwave Irradiation. Catalysts 7:123. doi: 10.3390/c atal7040123
Noël, T., and Buchwald, S. L. (2011). Cross-coupling in flow. Chem. Soc. Rev. 40,
5010–5029. doi: 10.1039/c1cs15075h
Pariente, S., Tanchoux, N., and Fajula, F. (2008). Etherification of glycerol
with ethanol over solid acid catalysts. Green Chem. 11, 1256–1261.
doi: 10.1039/B905405G
Park, D. H., Kim, S. S., Wang, H., Pinnavaia, T. J., Papapetrou, M. C.,
Lappas, A. A., et al. (2009). Selective petroleum refining over a zeolite
catalyst with small intracrystal mesopores. Angew. Chem. 121, 7781–7784.
doi: 10.1002/ange.200901551
Pawar, R. R., Gosai, K. A., Bhatt, A. S., Kumaresan, S., Lee, S. M., and Bajaj, H. C.
(2015). Clay catalysedrapid valorization of glycerol towards cyclic acetals and
ketals. RSC Adv. 5, 83985–83996. doi: 10.1039/C5RA15817F
Pawar, R. R., Jadhav, S. V., and Bajaj, H. C. (2014). Microwave-assisted rapid
valorization of glycerol towards acetals and ketals. Chem. Eng. J. 235, 61–66.
doi: 10.1016/j.cej.2013.09.018
Perreia, C. S. M., and Rodrigues, A. E. (2013). Process intensification: new
technologies (SMBRandPermSMBR) for the synthesis of acetals. Catal. Today.
218–219, 148–152. doi: 10.1016/j.cattod.2013.04.014
Pierpont, A. W., B atista, E. R., Martin, R. L., Chen, W., Kim, J. K., and Hoyt,
C. B. (2015). Origins ofthe region-selectivity in the lutetium triflate catalyzed
ketalization of acetonewith glycerol: a DFT study. ACS Catal. 5, 1013–1019.
doi: 10.1021/cs5010932
Frontiers in Chemistry | www.frontiersin.org 23 November 2018 | Volume 6 | Article 573
 Loading...
Loading...