Page 1

User’s Manual
©2018 Abbott ART38553-101 Rev. A 01/18
FreeStyle, Libre, and related brand marks are trademarks of Abbott Diabetes Care Inc.
in various jurisdictions. Other trademarks are the property of their respective owners.
Patent: https://www.abbott.com/patents
Distributed by:
Abbott Diabetes Care Inc.
1360 South Loop Road
Alameda, CA 94502 USA
Customer Service: 1-855-632-8658
Monday through Friday,
8AM to 8PM Eastern Standard Time
www.FreeStyleLibre.com
FLASH GLUCOSE MONITORING SYSTEM
Manufacturer:
CAUTION: Federal law restricts this device
to sale by or on the order of a physician.
.224”
.257”
4.6”4.6”
ART38553-101_rev-A_cover.indd 1-2 2/1/18 2:18 PM
Page 2
Your Name _________________________________________________
.224”
.257”
4.6” 4.6”
ART38553-101_rev-A_cover.indd 3-4 2/1/18 2:18 PM
Page 3
Contents
Important Safety Information........................... 1
Indications For Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Contraindications ........................................1
Warnings................................................. 2
Cautions and Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Reader Symbols ........................................10
Getting to Know Your System . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Reader Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Sensor Kit ...............................................14
FreeStyle Libre Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Setting up Your Reader for the First Time . . . . . . . . . . . . . . 18
Using Your Sensor ......................................21
Applying Your Sensor....................................22
Starting Your Sensor .....................................26
Checking Your Glucose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Making Treatment Decisions ...........................33
Making Treatment Decisions – Getting Started ...........34
Making Treatment Decisions – Advanced ................39
ART38553-101_rev-A_manual.indd 1 2/1/18 2:13 PM
Page 4
Adding Notes...........................................50
Reviewing Your History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Logbook . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Daily Graph .............................................55
Other History Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Removing Your Sensor .................................58
Replacing Your Sensor .................................59
Using Reminders . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Using the Reader’s Built-in Meter . . . . . . . . . . . . . . . . . . . . . . 62
Intended Use............................................62
Blood Glucose Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Control Solution Testing .................................71
Charging the Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Changing the Reader Settings .........................77
Living With Your FreeStyle Libre System . . . . . . . . . . . . . . . 80
Maintenance and Disposal .............................83
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
Reader Does Not Power On . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
Problems at the Sensor Application Site ..................89
Problems Starting Your Sensor or Receiving
Sensor Readings.........................................90
Blood Glucose Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . 93
Problems Checking Your Blood Glucose . . . . . . . . . . . . . . . . . . 97
Perform a Reader Test....................................99
Customer Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99
System Specications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .100
Labeling Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Performance Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . .107
Electromagnetic Compatibility (EMC) .................123
Limited Warranty......................................134
ART38553-101_rev-A_manual.indd 2-3 2/1/18 2:13 PM
Page 5
Important Safety Information
Indications For Use
The FreeStyle Libre Flash Glucose Monitoring System is a continuous
glucose monitoring (CGM) device indicated for the management of
diabetes in persons age 18 and older. It is designed to replace blood
glucose testing for diabetes treatment decisions.
The System detects trends and tracks patterns aiding in the detection of
episodes of hyperglycemia and hypoglycemia, facilitating both acute and
long-term therapy adjustments. Interpretation of the System readings
should be based on the glucose trends and several sequential readings
over time. The System is intended for single patient use and requires a
prescription.
Contraindications
MRI/CT/Diathermy: The FreeStyle Libre Flash Glucose Monitoring
System must be removed prior to Magnetic Resonance Imaging
(MRI), Computed Tomography (CT ) scan, or high-frequency
electrical heat (diathermy) treatment. The eect of MRI, CT scans, or
diathermy on the performance of the System has not been
evaluated. The exposure may damage the Sensor and may impact
proper function of the device which could cause incorrect readings.
WARNINGS
• Do not ignore symptoms that may be due to low or high blood
glucose: If you are experiencing symptoms that are not consistent
with your glucose readings, consult your health care professional.
• Checking Sensor glucose readings with a blood glucose meter:
Under the following conditions, Sensor glucose readings may not
be accurate and you should conduct a ngerstick test using a blood
glucose meter. You should not use Sensor glucose readings to make
a diabetes treatment decision:
• If you suspect that your reading may be inaccurate for any reason
• When you are experiencing symptoms that may be due to low or
high blood glucose
• When you are experiencing symptoms that do not match FreeStyle
Libre System readings
• During times of rapidly changing glucose (more than 2 mg/dL per
minute), when interstitial uid glucose levels as measured by the
Sensor may not accurately reect blood glucose levels
• When the Sensor glucose reading does not include a Current
Glucose number or Glucose Trend Arrow
• In order to conrm hypoglycemia or impending
hypoglycemia as reported by the Sensor
• When you see the symbol, you must check your
blood glucose with a blood glucose meter before
making any treatment decisions. Sensor readings may
not accurately reect blood glucose levels.
350
250
150
50
82
mg
dL
2pm 6pm 10pm
Glucose Going
Low
1 2
ART38553-101_rev-A_manual.indd 1-2 2/1/18 2:13 PM
Page 6
Cautions and Limitations
Below are important cautions an d limitations to keep in mind so you can
use the System safely. They are grouped into categories for e asy reference.
What to know about Alarms/Alerts:
• There are NO alarms or alerts unless you scan the Sensor.
What to know before using the System:
• Review all product information before use.
WARNINGS (cont.)
• Hypoglycemic unawareness: The FreeStyle Libre System has not
been evaluated for use in patients with hypoglycemic unawareness
and will not automatically alert you of a hypoglycemic event without
you scanning your Sensor.
• No alarms without a Sensor scan: The FreeStyle Libre System does
not have alarms that will automatically notify you when you are
having a severe low (hypoglycemic) or high (hyperglycemic) glucose
event unless you scan your Sensor. For example, the System does
not have an alarm that can alert or wake you when you are sleeping
in the case of low or high glucose.
• Choking hazard: The FreeStyle Libre System contains small parts
that may be dangerous if swallowed.
• Take standard precautions for transmission of blood borne pathogens
to avoid contamination.
Who should not use the System:
• Do not use the System in people less than 18 years of age. The
System is not approved for use in people under 18 years of age and
Sensor readings in this population may be inaccurate. In general,
continuous glucose monitoring systems are recognized to be less
accurate in children than in adults.
• Do not use the System in critically ill patients. The System is not
approved for use in these patients. It is not known how dierent
conditions or medications common to the critically ill population may
aect performance of the System. Sensor glucose readings may be
inaccurate in critically ill patients.
• Do not use the System in pregnant women or persons on dialysis.
The System is not approved for use in pregnant women or persons on
dialysis and has not been evaluated in these populations.
• Performance of the System when used with other implanted medical
devices, such as pacemakers, has not been evaluated.
What should you know about wearing a Sensor:
• After the 12 hour start-up period, the Sensor can be worn for up to 10
days.
• Some individuals may be sensitive to the adhesive that keeps the
Sensor attached to the skin. If you notice signicant skin irritation
3 4
ART38553-101_rev-A_manual.indd 3-4 2/1/18 2:13 PM
Page 7
around or under your Sensor, remove the Sensor and stop using the
FreeStyle Libre System. Contact your health care professional before
continuing to use the FreeStyle Libre System.
• Intense exercise may cause your Sensor to loosen due to sweat or
movement of the Sensor. Remove and replace your Sensor if it starts to
loosen and follow the instructions to select an appropriate application
site.
• Do not reuse Sensors. The Sensor and Sensor Applicator are designed
for single use. Reuse may result in no glucose readings and infection.
Not suitable for re-sterilization. Further exposure to irradiation may
cause inaccurate results.
• If a Sensor breaks inside your body, call your health care professional.
How to Store the Sensor Kit:
• Store the Sensor Kit between 39°F and 77°F. Storage outside of this
range may cause inaccurate Sensor glucose readings. While you don’t
need to keep your Sensor Kit in a refrigerator, you can as long as the
refrigerator is between 39°F and 77°F. Do not freeze.
• Store the Sensor Kit between 10-90% non-condensing humidity.
When not to use the System:
• Do NOT use if the Sensor Kit package, Sensor Pack, or Sensor
Applicator appear to be damaged or already opened due to risk of no
results and/or infection.
• Do NOT use if Sensor Kit contents are past expiration date.
• Do NOT use if the Reader appears to be damaged due to risk of electric
shock and/or no results.
What to know before you Apply the Sensor:
• The Sensor Pack and Sensor Applicator are
packaged as a set (separately from the Reader)
and have the same Sensor code. Check that the
Sensor codes match before using your Sensor
Pack and Sensor Applicator. Do not use Sensor
Packs and Sensor Applicators with dierent Sensor
codes together as this will result in incorrect
glucose readings.
• Clean the application site and ensure that it is dry prior to Sensor
insertion. This helps the Sensor stay attached to your body.
• Clean hands prior to Sensor handling/insertion to help prevent
infection.
• Change the application site for the next Sensor application to prevent
discomfort or skin irritation.
• Sensor placement is not approved for sites other than the back of the
arm. If placed in other areas, the Sensor may not function properly.
• Select an appropriate Sensor site to help the Sensor stay attached
to the body and prevent discomfort or skin irritation. Avoid areas
with scars, moles, stretch marks, or lumps. Select an area of skin
CODE
FreeStyle Libre
Sensor Pack
XXXXXXXXX
XXX
YYYY-MM-DD
5 6
ART38553-101_rev-A_manual.indd 5-6 2/1/18 2:13 PM
Page 8
that generally stays at during normal daily activities (no bending
or folding). Choose a site that is at least 1 inch away from an insulin
injection site.
When is Sensor Glucose dierent from Blood Glucose:
• Physiological dierences between the interstitial uid and capillary
blood may result in dierences in glucose readings between the
System and results from a ngerstick test using a blood glucose meter.
Dierences in glucose readings between interstitial uid and capillary
blood may be observed during times of rapid change in blood glucose,
such as after eating, dosing insulin, or exercising.
What to know about interfering substances such as Vitamin C
and Aspirin:
• Taking ascorbic acid (vitamin C) while wearing the Sensor may falsely
raise Sensor glucose readings. Taking salic ylic acid (used in some pain
relievers such as aspirin and some skin care products) may slightly
lower Sensor glucose readings. The level of inaccuracy depends on the
amount of the interfering substance active in the body.
• Test results did not indicate interference for methyldopa (used in some
drugs to treat high blood pressure) or tolbutamide (infrequently used
in some drugs to treat diabetes in the US) at maximum circulating
levels. However, concentrations of potential interferents in interstitial
uid are unknown compared to circulating blood.
What to know about X-Rays:
• The Sensor should be removed prior to exposing it to an X-ray
machine. The eect of X-rays on the performance of the System
has not been evaluated. The exposure may damage the Sensor and
may impact proper function of the device to detect trends and track
patterns in glucose values during the wear period.
When to remove the Sensor:
• If the Sensor is becoming loose or if the Sensor tip is coming out of
your skin, you may get no readings or unreliable readings, which may
not match how you feel. Check to make sure your Sensor has not come
loose. If it has come loose, remove it and apply a new one.
• If you believe your glucose readings are not correct or are inconsistent
with how you feel, perform a blood glucose test on your nger to
conrm your glucose. If the problem continues, remove the current
Sensor and apply a new one.
What to do if you are dehydrated:
• Severe dehydration and excessive water loss may cause inaccurate
Sensor glucose readings. If you believe you are suering from
dehydration, consult your health care professional immediately.
7 8
ART38553-101_rev-A_manual.indd 7-8 2/1/18 2:13 PM
Page 9
What to know about the Reader’s Built-in Meter:
• The FreeStyle Libre Flash Glucose Monitoring System has a built-in
blood glucose meter that is designed to be used only with FreeStyle
Precision Neo blood glucose test strips and MediSense Glucose and
Ketone Control Solution. Using other test strips with the Reader’s
built-in meter will produce an error or cause the Reader’s built-in
meter to not turn on or start a test. The Reader’s built-in meter does
not have ketone testing functionality.
• The Reader’s built-in meter is not for use on people who are
dehydrated, hypotensive, in shock, or for individuals in hyperglycemichyperosmolar state, with or without ketosis.
• The Reader’s built-in meter is not for use on neonates, in critically-ill
patients, or for diagnosis or screening of diabetes.
• See Using the Reader’s Built-in meter section for additional important
information on the use of the Reader’s built-in meter.
Where to charge your Reader:
• Be sure to select a location for charging that allows the power adapter
to be easily unplugged. Do NOT block access to the charger due to the
potential risk of electrical shock.
Reader Symbols
Symbol What It Means
Sensor may be inaccurate. Check blood glucose with
a test strip before making any treatment decisions
Active Sensor
Direction your glucose is going. See Checking Your
Glucose section for more information
Caution
View previous/next screen
Notes
+
Add more information to notes
Food note
Rapid-acting insulin note
9 10
ART38553-101_rev-A_manual.indd 9-10 2/1/18 2:13 PM
Page 10

Symbol What It Means
Time changed on Reader
Reminders
Blood glucose test
Settings
Control solution test result
Low battery
Battery charging
Sensor too cold
Sensor too hot
Getting to Know Your System
The FreeStyle Libre Flash Glucose Monitoring System has two main
parts: a handheld Reader and a disposable Sensor that you wear on your
body. The Sensor does not need to be calibrated with blood glucose
values. You use the Reader to wirelessly scan the Sensor and get your
glucose readings. The Reader also has a built-in blood glucose meter,
which works with FreeStyle Precision Neo blood glucose test strips.
Your System comes in a Reader Kit and a Sensor Kit. When opening
your kits, check that the contents are undamaged and that you have
all parts listed. If any parts are missing or damaged, contact Customer
Service.
IMPORTANT:
• Before you use you r System , review all the
product instructions an d the Interactive
Tutorial. The Quick Reference Guide and
Interactive Tutorial give you quick access
to important aspect s and limitations of the
System. The User’s Manual includes all safet y
information and instructions for use.
• Talk to your health care professional about
how you should use your Sensor glucose
information to help manage your diabetes.
11 12
ART38553-101_rev-A_manual.indd 11-12 2/1/18 2:13 PM
Page 11

Reader Kit
The Reader Kit includes:
• FreeStyle Libre Reader
• USB Cable
• Interactive Tutorial on USB
• Power Adapter
• User’s Manual
• Quick Start Guide
• Quick Reference Guide
The Reader is used to get glucose readings from your Sensor. It can
store approximately 90-days of glucose history and notes you enter
about activities, such as taking insulin, eating food, or exercising. This
information can help you understand how these activities aect your
glucose.
USB Port
Used to charge the Reader
and connect it to a computer.
Test Strip Port
Insert a test strip here to use
the built-in meter.
Touchscreen
Home Button
Turns the Reader on/o
and takes you to the
Home screen from any
other screen.
Sensor Kit
The Sensor Kit includes:
• Sensor Pack
• Sensor Applicator
• Alcohol wipe
• Product insert
Sensor Pack
Used with the Sensor Applicator to
prepare the Sensor for use.
Sensor Applicator
Applies the Sensor to your body.
The Sensor measures and stores glucose readings when worn on your
body. It initially comes in two parts: one part is in the Sensor Pack and the
other part is in the Sensor Applicator. By following the instructions, you
prepare and apply the Sensor on the back of your
upper arm. The Sensor has a small, exible tip that
is inserted just under the skin. After the 12 hour
start-up period, the Sensor can be worn for up to
10 days.
Sensor
Measures your glucose while on your
body (only visible after applied).
13 14
ART38553-101_rev-A_manual.indd 13-14 2/1/18 2:13 PM
Page 12
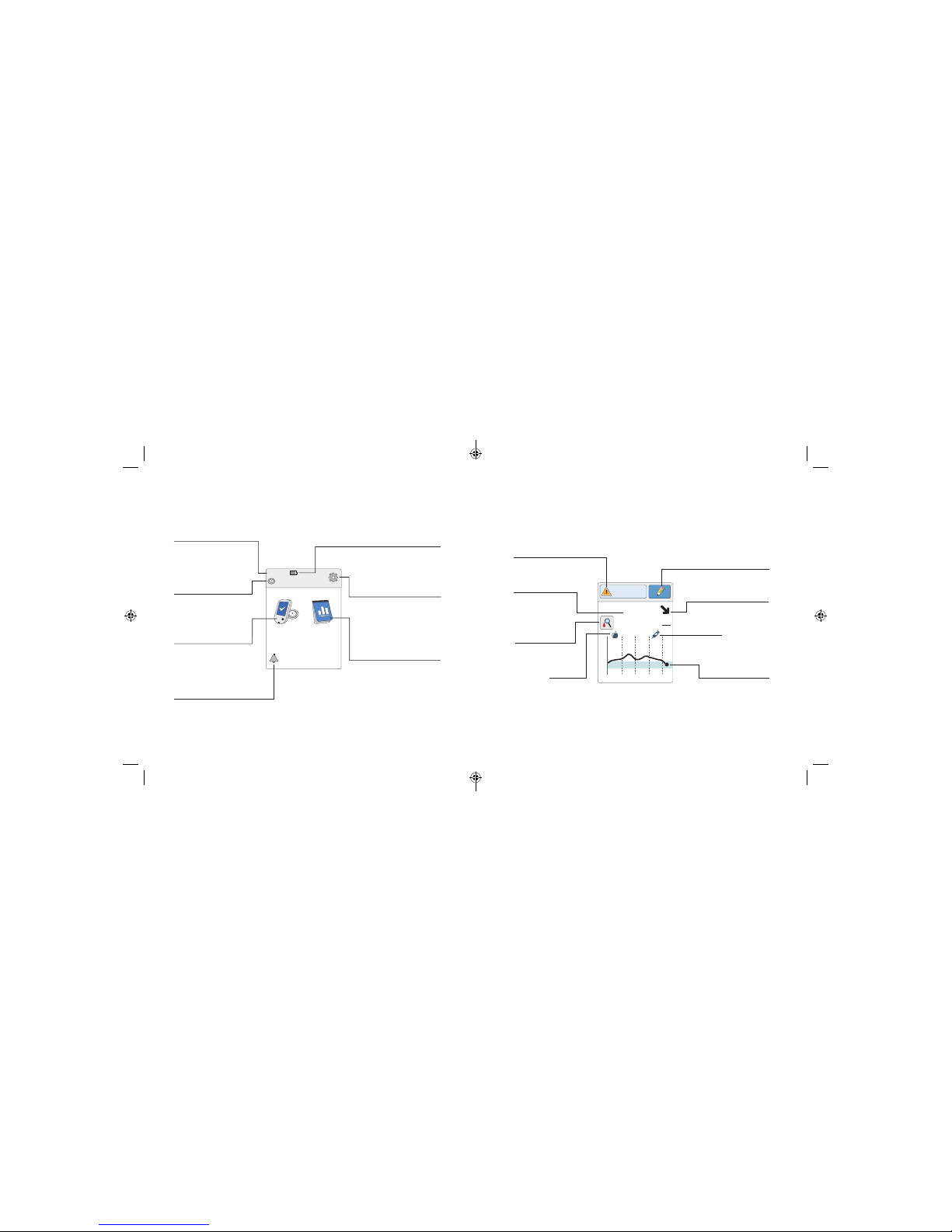
The Reader Home Screen provides access to information about your
glucose and the System. You can press the Home Button to get to the
Home Screen.
Home Screen
Ends in 10 days
10:23pm
Check
Glucose
Review
History
Time
Current time set on
the Reader.
Sensor Status
Information about your
current Sensor.
Battery Level
Battery charge remaining.
Settings
Touch to change the
Reader’s settings.
Review History
Touch to review information
about your past glucose
readings.
Check Glucose
Touch to check your
Sensor glucose.
Reminder
Touch to set or change
reminders.
The Sensor Glucose Readings screen appears after you use the Reader to
scan your Sensor. Your Reading includes your Current Glucose, a Glucose
Trend Arrow indicating which way your glucose is going, and a graph of
your current and stored glucose readings.
Sensor Glucose Readings
350
250
150
50
2pm 6pm 10pm
82
mg
dL
Glucose Going
Low
Rapid-Acting
Insulin Note
Message
Touch for more
information.
Current Glucose
Glucose from your
latest scan.
Add Notes
Touch to add notes to the
glucose reading.
Glucose Trend Arrow
Direction your
glucose is going.
Glucose Graph
Graph of your current
and stored glucose
readings
.
Food Note
Check Blood
Glucose symbol
Touch for more
information.
15 16
ART38553-101_rev-A_manual.indd 15-16 2/1/18 2:13 PM
Page 13
FreeStyle Libre Software
FreeStyle Libre software can be used to view reports and change
Reader settings. The software is compatible with most Windows
and Mac operating systems. Go to www.FreeStyleLibre.com and
follow onscreen instructions to download and install the software.
INTENDED USE
FreeStyle Libre software is intended for use by individuals and
health care professionals to aid in the review, analysis, and
evaluation of information such as Sensor glucose readings,
blood glucose test results, and other data uploaded from the
FreeStyle Libre Flash Glucose Monitoring System, in support of
an eective diabetes health management program.
FreeStyle Libre software is not intended for the diagnosis of
or screening for diabetes mellitus. Users should be aware that
FreeStyle Libre software is merely an information management
tool and it is therefore not intended to substitute for the
support of a health care professional. Individuals should always
consult their health care professional if they have any queries or
concerns about diabetes management.
Setting up Your Reader for the First Time
Before using the System for the rst time, the Reader must be set up.
Step Action
1
Press the Home Button to turn on the Reader.
2
If prompted, use the touchscreen to select your
preferred language for the Reader. Touch OK to
continue.
Note: Use the pad of your nger. Do NOT use
your ngernail or any other object on the screen.
3
Set the Current Date using the arrows on the
touchscreen. Touch next to continue.
14
back next
Current Date
June
2018
17 18
ART38553-101_rev-A_manual.indd 17-18 2/1/18 2:13 PM
Page 14
Step Action
4
Set the Current Time. Touch next to continue.
5
Set your Target Glucose Range. Work with your
health care professional to determine your Target
Glucose Range. Touch next to continue.
Note: Your Target Glucose Range is displayed
on glucose graphs on the Reader and used to
calculate your Time In Target.
12 am 00
back next
Current Time
CAUTIO N: It is very important to set the time
and date correctly. These values aect the
Reader data and settings.
mg
dL
80
to
140
back next
Target Glucose
Range
?
Step Action
6
The Reader now displays important information about key
topics to help you use the System:
• How to understand the Glucose Trend Arrow included on
the Glucose Reading screen.
• When to do a blood glucose test.
• Where to apply the Sensor.
• How to return to the Home Screen from any other screen.
Touch next to move
to the next topic.
At the end of the
Reader setup, touch
done to go to the
Home Screen.
Note: Charge the Reader if the bat tery level is low. Only use the USB cable
and power adapter included with the System.
nextback
When you scan your
Sensor an arrow will
indicate your recent
glucose trend:
Rising quickly
Rising
Changing slowly
Falling
Falling quickly
If you see this symbol, do
a blood glucose test before
making treatment
decisions.
back next
If the Sensor glucose
reading does not match
how you feel, do a blood
glucose test.
back next
nextback
The Sensor can only be
applied to the back of
your upper arm.
doneback
While using the Reader, press
the Home Button to return to
the Home Screen.
19 20
ART38553-101_rev-A_manual.indd 19-20 2/1/18 2:13 PM
Page 15
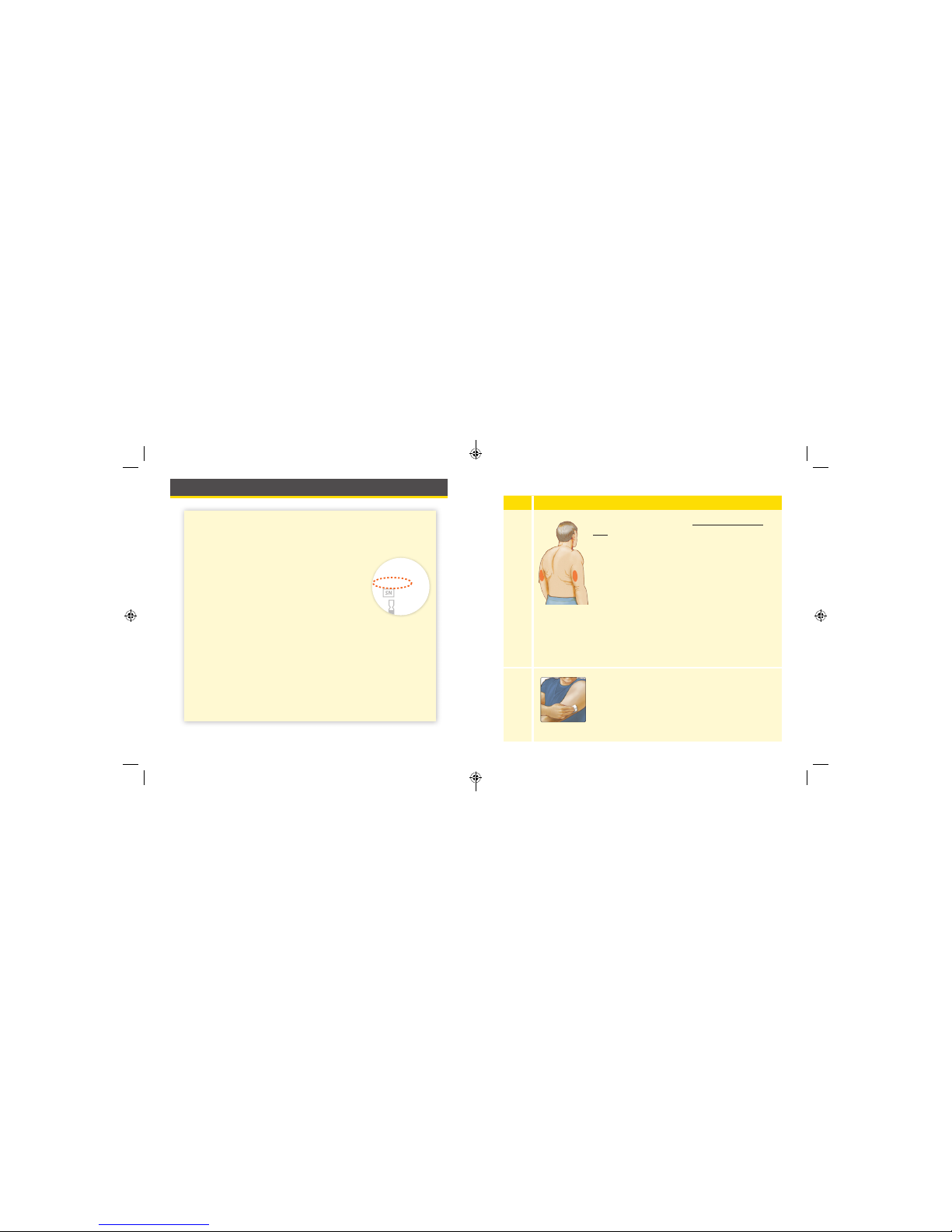
Using Your Sensor
CAUTIO NS:
• The Sensor Pack and Sensor Applicator are packaged as a
set (separately from the Reader) and have the same Sensor
code. Check that the Sensor codes match
before using your Sensor Pack and Sensor
Applicator. Do not use Sensor Packs and
Sensor Applicators with dierent Sensor
codes together as this will result in
incorrect glucose readings.
• Intense exercise may cause your Sensor to loosen due to
sweat or movement of the Sensor. Remove and replace
your Sensor if it starts to loosen and follow the instructions
to select an appropriate application site.
CODE
FreeStyle Libre
Sensor Pack
XXXXXXXXX
XXX
YYYY-MM-DD
Applying Your Sensor
Step
Action
1
Apply Sensors only on the back of your upper
arm. If placed in other areas, the Sensor may
not function properly and could give inaccurate
readings. The application of the Sensor is not
approved for other sites. Avoid areas with scars,
moles, stretch marks, or lumps.
Select an area of skin that generally stays at
during your normal daily activities (no bending
or folding). Choose a site that is at least 1 inch
(2.5 cm) away from an insulin injection site. To
prevent discomfort or skin irritation, you should
select a dierent site other than the one most
recently used.
2
Clean application site with an alcohol wipe and
allow site to dry before proceeding. This helps
the Sensor stay attached to your body.
Note: The area MUST be clean and dry, or the
Sensor may not stick to the site.
21 22
ART38553-101_rev-A_manual.indd 21-22 2/1/18 2:13 PM
Page 16
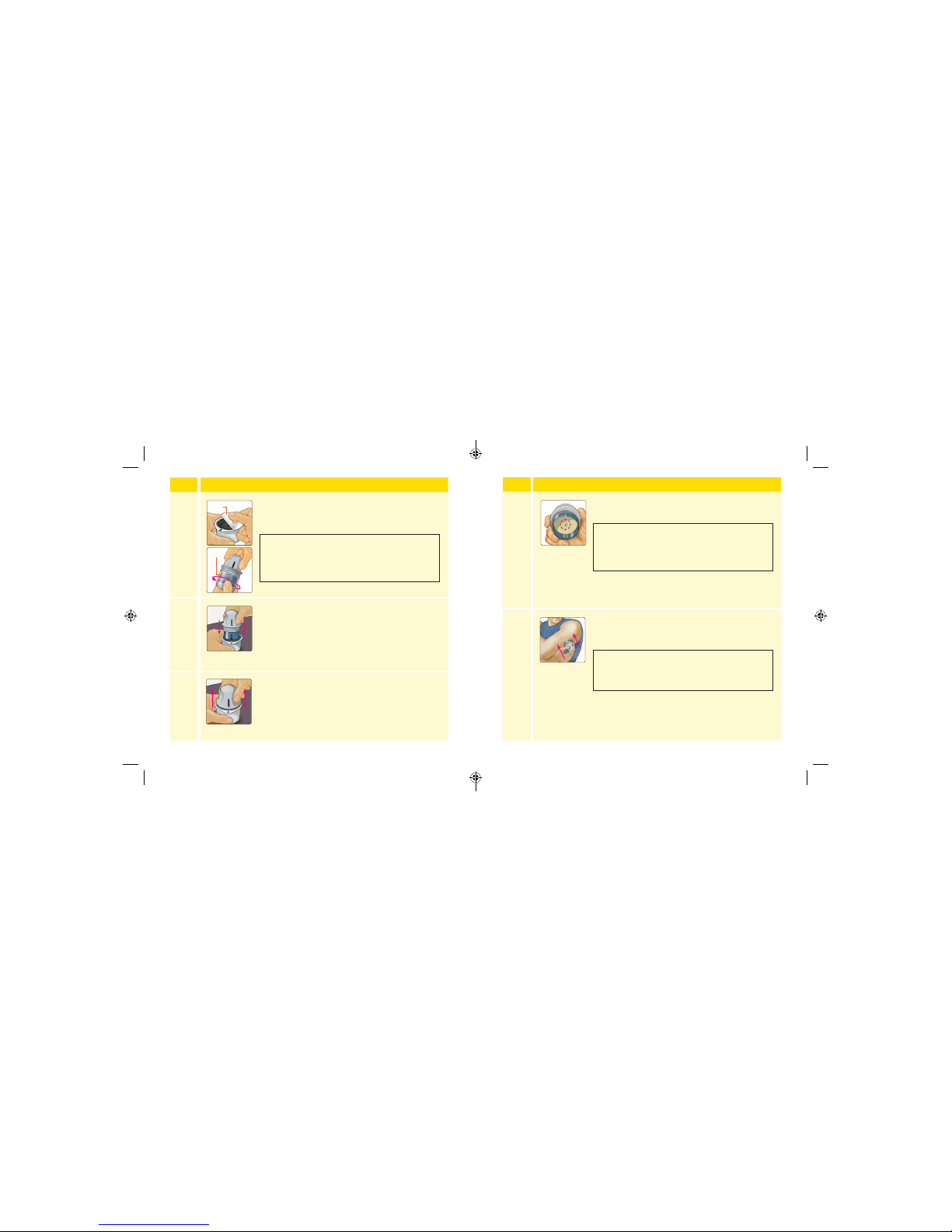
Step
Action
3
Open the Sensor Pack by peeling the lid o
completely. Unscrew the cap from the Sensor
Applicator and set the cap aside.
4
Line up the dark mark on the Sensor Applicator
with the dark mark on the Sensor Pack. On a
hard surface, press rmly down on the Sensor
Applicator until it comes to a stop.
5
Lift the Sensor Applicator out of the Sensor Pack.
Lid
Cap
CAUTIO N: Do NOT use if the Sensor Pack or
the Sensor Applicator seem to be damaged
or already opened. Do NOT use if past
expiration date.
Step
Action
6
The Sensor Applicator is prepared and ready to
apply the Sensor.
7
Place the Sensor Applicator over the prepared
site and push down rmly to apply the Sensor to
your body.
CAUTIO N: The Sensor Applicator now
contains a needle. Do NOT touch inside the
Sensor Applicator or put it back into the
Sensor Pack.
CAUTIO N: Do NOT push down on the Sensor
Applicator until placed over prepared site to
prevent unintended results or injury.
23 24
ART38553-101_rev-A_manual.indd 23-24 2/1/18 2:13 PM
Page 17
Step
Action
8
Gently pull the Sensor Applicator away from your
body. The Sensor should now be attached to your
skin.
Note: Applying the Sensor may cause bruising
or bleeding. If there is bleeding that does not
stop, remove the Sensor and contact your
health care professional.
9
Make sure the Sensor is secure after application.
Put the cap back on the Sensor Applicator.
Discard the used Sensor Pack and Sensor
Applicator according to local regulations.
Sensor
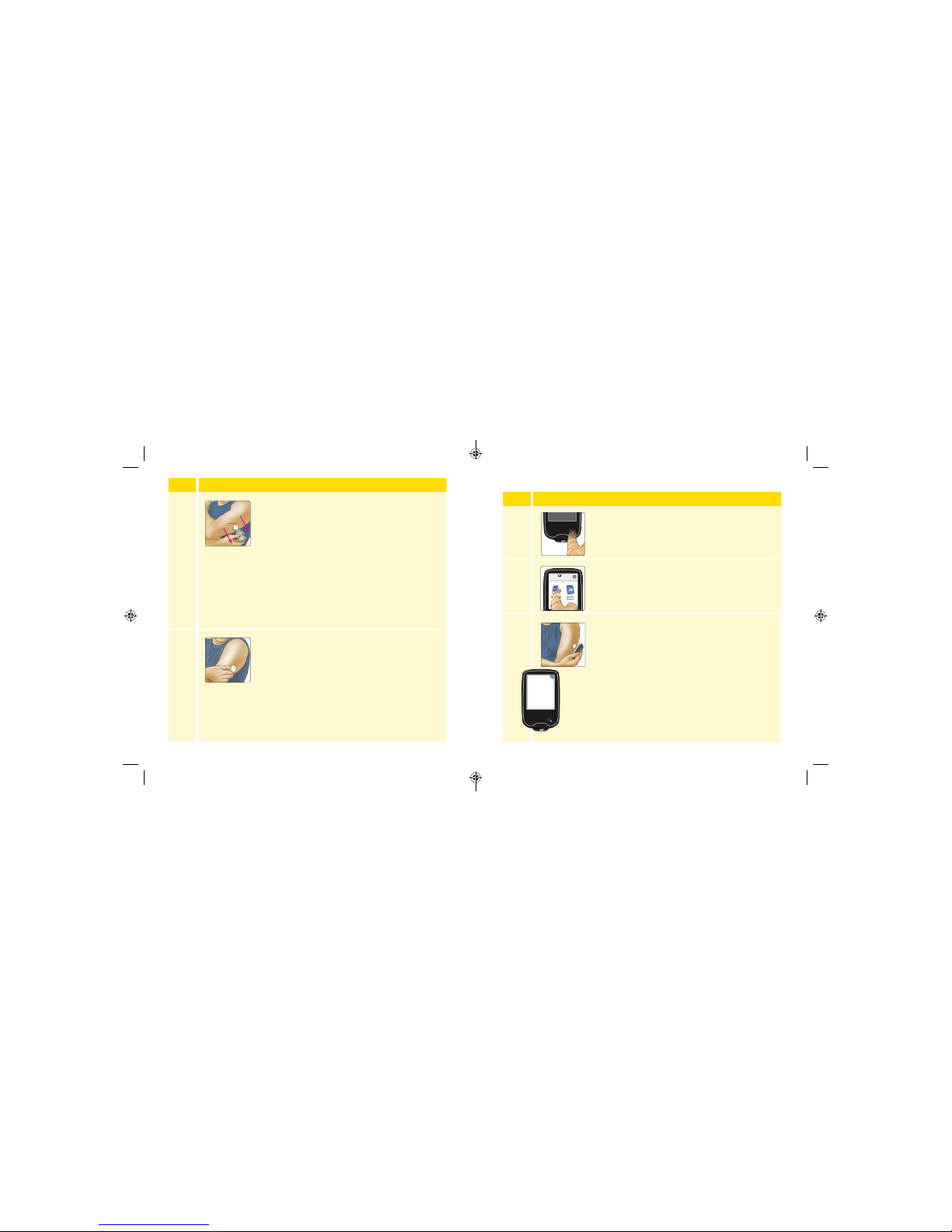
Starting Your Sensor
Step
Action
1
Press the Home Button to turn on the Reader.
2
Touch Start New Sensor.
3
Hold the Reader within 1.5 inches (4 cm) of the
Sensor to scan it. This starts your Sensor. If sounds
are turned on, the Reader beeps when the Sensor
has been successfully activated. The Sensor can
be used to check your glucose after 12 hours.
During the 12 hour period, you can scan the
Sensor to check its status.
Note: If the Sensor is not successfully scanned
within 15 seconds, the Reader displays a prompt
to scan the Sensor again. Touch OK to return to
the Home Screen and touch Start New Sensor
to scan your Sensor.
No Active Sensor
10:23pm
Start New
Sensor
Review
History
hours
OK
New Sensor
Starting Up
12
Sensor can be used in:
25 26
ART38553-101_rev-A_manual.indd 25-26 2/1/18 2:13 PM
Page 18
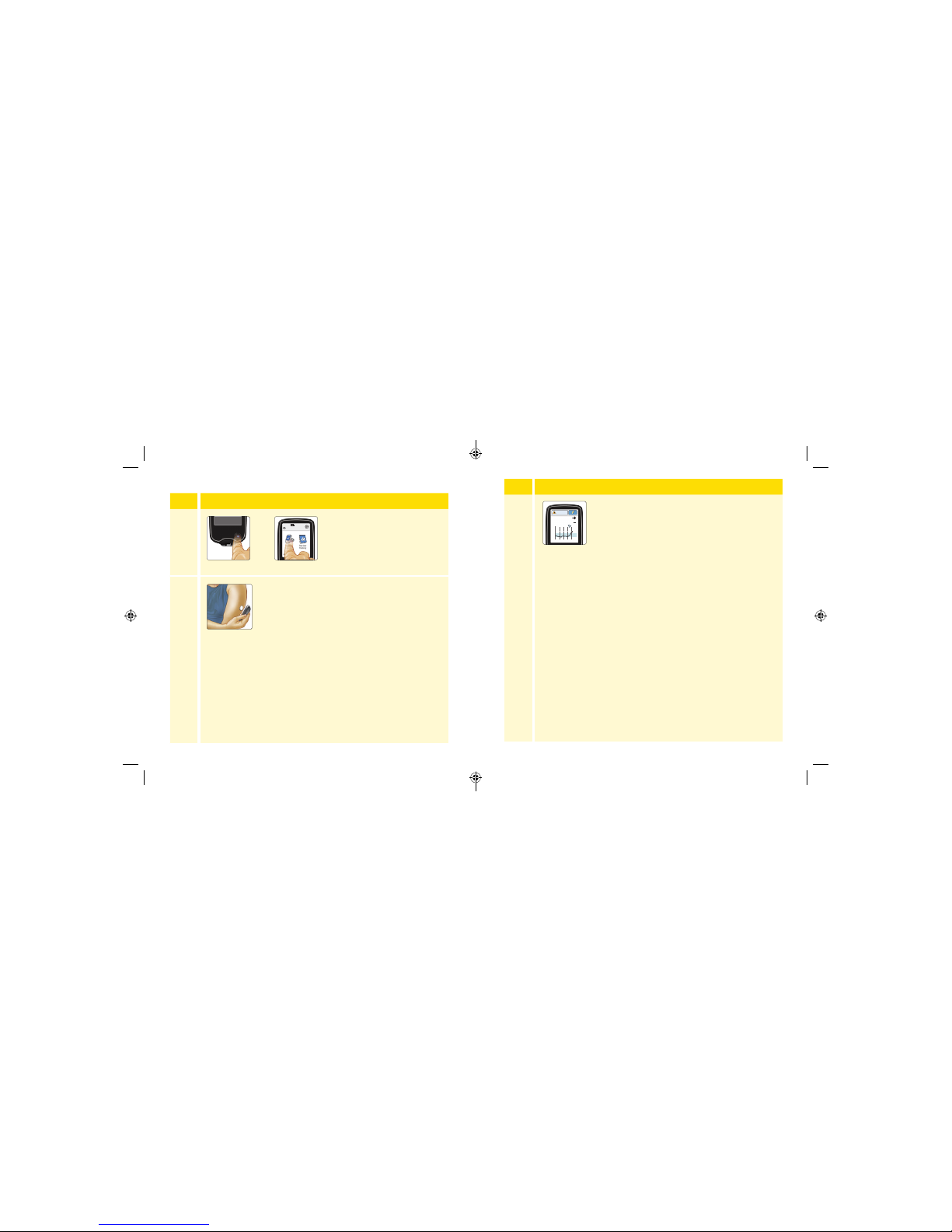
Checking Your G lucose
Step Action
1
Turn the Reader on by pressing
the Home Button or touch
Check Glucose from the Home
Screen.
2
Hold the Reader within 1.5 inches (4 cm) of your
Sensor to scan it. Your Sensor wirelessly sends
glucose readings to the Reader. If sounds are
turned on, the Reader beeps when the Sensor
has been successfully scanned.
Note: If the Sensor is not successfully scanned wi thin 15
seconds, the Reader displays a prompt to scan the Sensor
again. Touch OK to return to the Home Screen and touch
Check Glucose to scan your Sensor.
10:23pm
Check
Glucose
Review
History
Ends in 10 days
OR
Step Action
3
The Reader displays your current glucose reading
along with your glucose graph and an arrow
indicating the direction your glucose is goin g.
350
250
150
50
236
mg
dL
6am 10am 2pm
Glucose Going
High
27 28
ART38553-101_rev-A_manual.indd 27-28 2/1/18 2:13 PM
Page 19

Sensor Glucose Readings
Notes:
• While Sensor glucose readings are gathered in the System range of
40-500 mg/dL, the graph display rang e is 0-350 mg/dL for eas e of
review on screen. Glucose readings above 350 mg/dL are displayed
at 350 mg/dL. For sequential readings above 350 mg/dL, a line is
displayed at 350 mg/dL.
• The symb ol may appear, indicating the Reader time was changed.
Gaps in the graph may result or glucose readings may be hidden .
350
250
150
50
2pm 6pm 10pm
82
mg
dL
Glucose Going
Low
Glucose Trend Arrow
Current Glucose
Target Glucose Range
Check Blood Glucose
When you see this symbol,
do a blood glucose test
before making treatment
decisions.
The Glucose Trend Arrow gives you an indication of the direction your
glucose is going.
Glucose is rising quickly
(more than 2 mg/dL per minute)
Glucose is rising
(between 1 and 2 mg/dL per minute)
Glucose is changing s lowly
(less than 1 mg/dL per minute)
Glucose is falling
(between 1 and 2 mg/dL per minute)
Glucose is falling quickly
(more than 2 mg/dL per minute)
Note: The Glucose Trend Arrow may not always appear with your readin g.
29 30
ART38553-101_rev-A_manual.indd 29-30 2/1/18 2:13 PM
Page 20

The following table shows messages you may see with your glucose
readings.
Display What To Do
350
250
150
50
2pm 6pm 10pm
mg
dL
Low Glucose
LO
350
250
150
50
2pm 6pm 10pm
mg
dL
High Glucose
HI
If LO appears on the Reader, your reading is lower
than 40 mg/dL. If HI appears on the Reader, your
reading is higher than 500 mg/dL. You can touch
the message button for more information. Check
your blood glucose on your nger with a test strip.
If you get a second LO or HI result, contact your
health care professional immediately.
350
250
150
50
2pm 6pm 10pm
mg
dL
63
Low Glucose
350
250
150
50
2pm 6pm 10pm
289
mg
dL
High Glucose
If your glucose is higher than 240 mg/dL or lower
than 70 mg/dL, you will see a message on the
screen. You can touch the message button for
more information and set a reminder to check
your glucose.
Display What To Do
350
250
150
50
2pm 6pm 10pm
mg
dL
72
Glucose Going
Low
350
250
150
50
2pm 6pm 10pm
237
mg
dL
Glucose Going
High
If your glucose is projected to be higher than
240 mg/dL or lower than 70 mg/dL within 15
minutes, you will see a message on the screen.
You can touch the message button for more
information and set a reminder to check your
glucose.
If your glucose reading is less than 70 mg/dL,
projected to be less than 70 mg/dL, rapidly
changing, or there is no number or trend arrow,
you will see this symbol
. You can touch the
symbol for more information. Check your blood
glucose on your nger with a test strip before
making treatment decisions.
Note: If you are not sure about a message o r reading, contact your
health care professional before you do anything.
350
250
150
50
2pm 6pm 10pm
mg
dL
63
Low Glucose
31 32
ART38553-101_rev-A_manual.indd 31-32 2/1/18 2:13 PM
Page 21

Making Treatment Decisions
Work with your health care professional to put together a plan for
managing your diabetes that includes when to use the FreeStyle Libre
System information for making treatment decisions.
WARNING: The FreeStyle Libre System can replace blood glucose testing
except in a few situations. These are the times when you need to do a
blood glucose test before deciding what to do or what treatment decision
to make as Sensor readings may not accurately reect blood glucose levels:
Do a blood glucose test if you see the Check
Blood Glucose
symbol. The symbol
means your Sensor glucose reading may not be
accurate. For example, there may be times when
you get a low glucose reading but you do not
actually have low glucose.
Do a blood glucose test if you think your
glucose readings are not correct or do not
match how you feel. Do not ignore symptoms
that may be due to low or high glucose.
Note: The
symbol will NOT appear in this
situation.
Making Treatment Decisions – Getting Started
Before you start using the FreeStyle Libre System for treatment decisions,
make sure you have a good understanding of how the System works for
your body. Continue to use your blood glucose meter for treatment
decisions until you are comfortable with the information you receive
from your FreeStyle Libre System. This includes understanding that:
Sensor performance can vary in between Sensors, within a Sensor wear,
and in dierent situations.
Getting familiar with the System could take days, weeks, or even months.
The more you check readings from the FreeStyle Libre System with a
blood glucose meter, the better you will understand how the System
works for you.
Work with your health care professional to put together a plan for
managing your diabetes that includes when to use the FreeStyle Libre
System information for making treatment decisions.
Helpful Tips
• Conrm your Sensor glucose readings with a blood glucose meter until
you understand:
• Sensor accuracy may vary between Sensors.
• Sensor accuracy may vary during a Sensor wear session.
• Sensor accuracy may vary in dierent situations (meals, exercise, rst
day of use, etc.).
33 34
ART38553-101_rev-A_manual.indd 33-34 2/1/18 2:13 PM
Page 22
• Scan your Sensor often to see how carbs, medication, exercise, illness, or
stress levels impact your Sensor glucose readings. The information you
get can help you gure out why your glucose sometimes goes too high
or too low, and how to prevent it from doing so in the future.
• Talk to your health care professional about how your insulin works. The
more you understand about your insulin, including how long it takes to
start working and how long it lasts in your body, the more likely you will
be to make better treatment decisions.
• Making a treatment decision doesn’t just mean taking insulin. Treatment
decisions can also include things like taking fast-acting carbs, eating, or
even doing nothing and scanning again later.
• Your health care professional can also help you to understand when
doing nothing and scanning again later is the right treatment decision.
For example, if your glucose is high and going up, your rst instinct may
be to take more insulin to lower your glucose, however depending on
when you last took insulin or your recent activity, the right treatment
decision may be to do nothing and scan again later. Avoid “insulin
stacking”.
When not to use Sensor Glucose readings for treatment decisions
Glucose is Falling Quickly or Rising Quickly
Sensor glucose values, which are based on interstitial uid glucose levels,
can be dierent from blood glucose levels (ngersticks), particularly
during times when your blood glucose is changing quickly. For example
after eating, taking insulin, or exercising. When glucose levels are falling
quickly, glucose readings from the Sensor may be higher than blood
glucose levels. On the other hand, when glucose levels are rising quickly,
glucose readings from the Sensor may be lower than blood glucose levels.
If glucose is rising quickly or falling quickly, you will see the
symbol.
Whenever you see the
symbol, do a blood glucose test and treat
based on that result.
Glucose
Rising
Quickly
Blood Glucose
100 mg/dL
Sensor Glucose
95 mg/dL
Glucose
Falling
Quickly
Sensor Glucose
80 mg/dL
Blood Glucose
70 mg/dL
Low Glucose or Glucose Going Low message
The System lets you know about hypoglycemia or impending
hypoglycemia with a Low Glucose or Glucose Going Low message. These
messages may not accurately reect blood glucose. When there is a Low
35 36
ART38553-101_rev-A_manual.indd 35-36 2/1/18 2:13 PM
Page 23

Glucose or Glucose Going Low message, you will also see the symbol.
Whenever you see the
symbol, do a blood glucose test and treat
based on that result.
No Glucose Trend Arrow
When there is no Glucose Trend Arrow, the System can’t tell if your
glucose is rising quickly or falling quickly and will display the
symbol.
Whenever you see the
symbol, you should do a blood glucose test
and treat based on that result.
No Current Glucose Number
When there is no Current Glucose number, such as when you receive an
error message or a LO or HI result, you don’t have enough information to
make a treatment decision. When there is no Current Glucose you will see
the
symbol. Whenever you see the symbol, do a blood glucose
test and treat based on that result.
Think Your Readings are Incorrect?
Don’t trust Sensor glucose readings that you think may be incorrect or
that don’t match what you would expect based on your recent activity.
For example, if you ate dinner but forgot to take insulin before eating, you
would expect your glucose to be high. If your glucose reading is low, then
it doesn’t match your recent activity, so don’t use it to make treatment
decisions. Don’t make treatment decisions if you think your Sensor glucose
readings are incorrect. Do a blood glucose test and treat based on that
result.
You Have Low or High Blood Glucose Symptoms
Don’t ignore symptoms that may be due to low or high blood glucose. Do
a blood glucose test and treat based on that result.
Symptoms Don’t Match Readings
There may be times when your symptoms don’t match your Sensor
glucose readings. For example, you are feeling shaky, sweaty, and dizzy –
symptoms you generally get when you have low glucose, but your glucose
reading is within your target range. When symptoms don’t match readings,
do a blood glucose test and treat based on that result. Don’t ignore
symptoms that may be due to low or high blood glucose.
If you’re the caregiver, pay attention to times when the symptoms of the
one you’re caring for don’t match their Sensor glucose readings. When
symptoms don’t match readings, do a blood glucose test and treat based
on that result.
Note: The
symbol will NOT display in these situations.
When to do Nothing and Scan Again Later
Your health care professional can help you understand when doing
nothing and scanning again later is the right treatment decision. For
example, if your glucose is high and going up, your rst instinct may be to
take more insulin to lower your glucose, however depending on when you
last took insulin or your recent activity, the right treatment decision may
be to do nothing and scan again later.
Don’t take a correction dose within 2 hours of your meal dose. This may
result in “insulin stacking” and low glucose.
37 38
ART38553-101_rev-A_manual.indd 37-38 2/1/18 2:13 PM
Page 24

Making Treatment Decisions – Advanced
After you scan your Sensor, use all of the information on the
screen when deciding what to do or what treatment decision
to make.
350
250
150
50
82
mg
dL
2pm 6pm 10pm
350
250
150
50
mg/dL
2pm 6pm 10pm
Current Glucose
Check Blood Glucose
When you see this symbol, do a blood glucose test
before making a treatment decision
Message
Glucose Graph
Graph of your current and stored glucose readings
Food Note
Target
Glucose
Range
Rapid-Acting
Insulin Note
Current
Glucose
Glucose Trend Arrow
Direction your glucose is going
Arrow What it means
Glucose rising quickly
Glucose rising
Glucose changing slowly
Glucose falling
Glucose falling quickly
Glucose
Trend
Arrow
Glucose Graph
Glucose Going
Low
Time
This table provides some information on how you can factor the Glucose
Trend Arrow into your treatment decisions. Remember that you should
never make a treatment decision based on the Glucose Trend Arrow alone.
Glucose
Trend
Arrow
Treatment Decision Considerations
Low Glucose
(< 70 mg/dL)
Glucose in
Target Range
High Glucose
(> 240 mg/dL)
No Arrow or
No Number
You will see the
symbol. Do not treat based on Sensor
glucose reading. Do a blood glucose test.
You will see the symbol. Do not treat based on Sensor
glucose reading. Do a blood glucose test.
You will see the
symbol. Do not
treat based on Sensor
glucose reading. Do a
blood glucose test.
If you are about to eat,
take insulin to cover
your meal. Consider
taking a little more
since glucose is rising.
If you have taken
insulin recently, do
nothing and scan again
later.
Avoid “insulin stacking”.
If you are about to eat,
take insulin to cover
your meal. Consider
taking a little more
since glucose is high
and rising.
If this is between
meals, consider taking
an insulin correction
dose, unless you have
taken insulin recently. If
you have taken insulin
recently, do nothing
and scan again later.
Avoid “insulin stacking”.
39 40
ART38553-101_rev-A_manual.indd 39-40 2/1/18 2:13 PM
Page 25

Glucose
Trend
Arrow
Treatment Decision Considerations
Low Glucose
(< 70 mg/dL)
Glucose in
Target Range
High Glucose
(> 240 mg/dL)
You will see the
symbol. Do not
treat based on Sensor
glucose reading. Do a
blood glucose test.
If you are about to eat,
take insulin to cover
your meal.
If this is between
meals, do nothing and
scan again later.
If you are about to eat,
take insulin to cover
your meal. Consider
taking a little more
since glucose is high.
If this is between
meals, consider taking
an insulin correction
dose, unless you have
taken insulin recently. If
you have taken insulin
recently, do nothing
and scan again later.
Avoid “insulin stacking”.
Glucose
Trend
Arrow
Treatment Decision Considerations
Low Glucose
(< 70 mg/dL)
Glucose in
Target Range
High Glucose
(> 240 mg/dL)
You will see the
symbol. Do not
treat based on Sensor
glucose reading. Do a
blood glucose test.
If you are about to eat,
take insulin to cover
your meal. Consider
taking a little less since
glucose is falling.
If this is between
meals, consider eating
a snack or fast-acting
carbohydrates to stay
within target and scan
again later.
If you are about to eat,
take insulin to cover
your meal. Consider
taking a little less since
glucose is falling.
If this is between
meals, consider doing
nothing and scan again
later.
Avoid “insulin stacking”.
You will see the symbol. Do not treat based on Sensor glucose reading.
Do a blood glucose test.
41 42
ART38553-101_rev-A_manual.indd 41-42 2/1/18 2:13 PM
Page 26

Example Scenarios
Next are some example scenarios to help you understand how to use
the information on your screen. Always use all of the information on the
screen before deciding what to do or what treatment decision to make. If
you are not sure about what to do, consult your health care professional.
What you see What it means
When you wake-up:
350
250
150
50
mg
dL
9pm 1am 5am
65
Low Glucose
When you wake up, your current glucose
is 65 mg/dL and the trend arrow shows
it is changing slowly
. There is also a
Low Glucose
message at the top of the screen
and the
symbol.
Anytime you see the
symbol, you should
do a blood glucose test before deciding what
to do.
What you see What it means
Before breakfast:
350
250
150
50
12am 4am 8am
mg
dL
115
Ends in 2 days
8:06am
Before breakfast, your current glucose is
115 mg/dL. The graph shows that your glucose
is going up and so does the trend arrow
.
Consider what might be causing your glucose
to go up and what you might do to prevent a
high glucose. For example:
• How much insulin should you take before
your meal?
• Since you see
, should you consider taking
a little more insulin?
After breakfast:
350
250
150
50
108
mg
dL
1am 5am 9am
Glucose Going
Low
After breakfast, your current glucose is
108 mg/dL. The trend arrow shows it is
going down quickly
. There is also a
Glucose Going
Low
message at the top of the screen
and the
symbol.
Anytime you see the
symbol, you should
do a blood glucose test before deciding what
to do.
43 44
ART38553-101_rev-A_manual.indd 43-44 2/1/18 2:13 PM
Page 27

What you see What it means
Before lunch:
350
250
150
50
4am 8am 12pm
Ends in 3 days
12:00pm
mg
dL
90
When you checked your glucose before lunch,
it was 90 mg/dL and rising. Before eating lunch,
you took enough insulin to cover the meal and
a little more since your trend arrow was
.
After lunch:
350
250
150
50
5am 9am 1pm
Ends in 3 days
1:30pm
mg
dL
225
90 minutes later, your current glucose is
225 mg/dL. The graph shows that your glucose is
still going up, and so does the trend arrow
.
Don’t take a correction dose within 2 hours
of your meal dose. This may result in “insulin
stacking” and low glucose.
Consider what might be causing your glucose
to go up and what you might do to prevent a
high glucose. For example:
• Has the insulin you took for your meal reached
its full eect?
• Scan your Sensor again later.
What you see What it means
After exercising:
350
250
150
50
9am 1pm 5pm
Ends in 9 days
5:47pm
mg
dL
204
After exercising, you are feeling shaky, sweaty,
and dizzy – symptoms you generally get when
you have low glucose. But, your current glucose
is 204 mg/dL.
Anytime you get a reading that doesn’t match
how you feel, do a blood glucose test.
Note: The Check Blood Glucose
symbol will
NOT appear in this situation.
Before dinner:
350
250
150
50
11am 3pm 7pm
mg
dL
134
Ends in 7 days
7:34pm
Before dinner, your current glucose is
134 mg/dL. The graph shows that your glucose
is going down and so does the trend arrow
.
Consider what might be causing your glucose
to go down and what you might do to prevent
a low glucose. For example:
• How much insulin should you take before
your meal?
• Since you see
, should you consider taking
a little less insulin?
45 46
ART38553-101_rev-A_manual.indd 45-46 2/1/18 2:13 PM
Page 28

What you see What it means
After dinner: After dinner, your current glucose is 215 mg/dL
but there is no trend arrow. There is also the
symbol on the screen.
Anytime you see the
symbol, you should
do a blood glucose test before deciding what
to do.
350
250
150
50
11am 3pm 7pm
7:49pm
215
mg
dL
Ends in 10 days
Other considerations
Deciding how much rapid-acting insulin to take for dierent meals and
situations can be dicult. Work with your health care professional to
discuss dierent situations and what might work best for you. Here are
some questions to consider:
Meal dosing
• What do you do if your before meal glucose is high?
• What do you do if your before meal glucose is low?
• How much time do you wait to eat after taking your meal insulin?
• Do you adjust the amount of meal insulin based on the number of carbs
or how much you are planning to eat?
• Do you adjust your meal insulin dose for high fat foods such as pizza?
• Do you know how to adjust your insulin doses when drinking alcoholic
beverages?
High glucose corrections
• Do you take extra insulin if your glucose is high?
• How do you decide how much insulin to take for a high glucose?
• How long do you wait between insulin doses to avoid insulin stacking?
47 48
ART38553-101_rev-A_manual.indd 47-48 2/1/18 2:13 PM
Page 29

Bedtime
• How often do you check your glucose before bed?
• What do you consider a safe bedtime glucose?
• What do you do if your bedtime glucose is high?
• What do you do if your bedtime glucose is low?
• When should you eat a bedtime snack?
• What do you do if your before meal glucose is high?
• What do you do if your before meal glucose is low?
Other factors
• How do you adjust your insulin dose based on the Glucose Trend Arrow?
• How do you adjust your insulin dose for dierent types of exercise or
activities?
• How do you adjust your insulin doses for stress?
• How do you adjust your insulin doses for illness?
Adding Notes
Notes can be saved with your glucose readings. You can add a note at the
time of your glucose reading or within 15 minutes after your reading was
obtained. You can track food, insulin, exercise, and any medication you
take.
Step Action
1
From the Glucose Reading screen, add notes
by touching the
symbol in the upper right
corner of the touchscreen. If you do not want to
add notes, you can press the Home Button to go
to the Home Screen or hold the Home Button to
turn the Reader o.
2
Select the checkbox next to the notes you would
like to add. Touch the down arrow to view other
note options.
350
250
150
50
236
mg
dL
6am 10am 2pm
Glucose Going
High
OK
Add Notes
1 / 4
Rapid-Acting
Insulin
Long-Acting
Insulin
Food
49 50
ART38553-101_rev-A_manual.indd 49-50 2/1/18 2:13 PM
Page 30

Step Action
3
After you check the box for food and
insulin notes, the
+
symbol appears
to the right of the note. You can touch
it to add more specic information to
your note. Then touch OK.
• Insulin notes: Enter the number of
units taken.
• Food notes: Enter grams or serving
information.
Note: Food
and rapid-acting insulin notes are shown
on your glucose graphs and in your Logbook as symbols.
4
Touch OK to save your notes.
You can review your notes from the Logbook. See Reviewing Your History
section for more information.
+
+
+
OK
Add Notes
1 / 4
Rapid-Acting
Insulin
Long-Acting
Insulin
Food
units
Enter Rapid-Acting
Insulin
OK
+
+
+
OK
Add Notes
1 / 4
Rapid-Acting
Insulin
Long-Acting
Insulin
Food
Reviewing Your History
Reviewing and understanding your glucose history can be an important
tool for improving your glucose control. The Reader stores about 90 days
of information and has several ways to review your past glucose readings,
notes, and other information.
Step Action
1
Press the Home Button to turn on
the Reader. Press the Home Button
again to go to the Home Screen.
2
Touch the Review History icon.
Check Glucose
Scan Sensor to check
glucose.
10:23pm
Check
Glucose
Review
History
Ends in 10 days
51 52
ART38553-101_rev-A_manual.indd 51-52 2/1/18 2:13 PM
Page 31

Step Action
3
Use the arrows to view the available options.
Logbook
Daily Graph
Average Glucose
Review History
1 / 2
Daily Patterns
Time In Target
Low Glucose Events
Sensor Usage
IMPORTANT: Work with your health care professional
to understand your glucose history.
The Logbook and Daily Graph show detailed information, while other
history options show summaries of information over a number of days.
Logbook
Entries for each time you scanned your Sensor or
performed a blood glucose test. If you entered
Notes with a glucose reading, the
symbol
appears in that row. For more information about
the symbols, see Reader Symbols section.
Touch the entry to review the detailed information,
including any Notes you entered. You can add or
edit (change) Notes for the most recent Logbook
entry, provided your glucose reading was within
the last 15 minutes and you have not used
FreeStyle Libre software to create reports.
OK
Logbook
23 Feb
10:23am
23 Feb
6:37am
22 Feb
11:09pm
143
98
108
mg/dL
53 54
ART38553-101_rev-A_manual.indd 53-54 2/1/18 2:13 PM
Page 32

Daily Graph
A graph of your Sensor glucose readings by day.
The graph shows your Target Glucose Range and
symbols for food or rapi d-acting insulin notes you
have entered.
Notes:
• While Sensor glucose readings are gathered in
the System range of 40-500 mg/dL, the Daily
Graph display range is 0-350 mg/dL for ease
of review on screen. Glucose readings above
350 mg/dL are displayed at 350 mg/dL. For
sequential readings above 350 mg/dL, a line
is displayed at 350 mg/dL.
• You might see gaps in the graph during times
when you have not scanned at least once in
8 hours.
• The symb ol may appear indicating the
Reader time was changed. Gaps in the gra ph
may result or glucose readings may be hidden.
12am6am12pm6pm12
am
350
250
150
50
OK
Daily Graph
(mg/dL)
22 Feb
Wednesday
Other History Options
Use the arrows to view information about your last 7, 14, 30, or 90 days.
Average Glucose
Information about the average of your Sensor
glucose readings. The overall average for the time
is displayed above the graph. The average is also
shown for four dierent 6-h our periods of the day.
Readings above or below your Target Glucose
Range are orange, while readings in range are blu e.
Daily Patterns
A graph showing the pattern and variability of your
Sensor glucose over a typical day. The thick black
line shows the median (midpoint) of your glucose
readings. The gray shading represents a range
(10-90 percentiles) of your Sensor readings.
Note: Daily Patterns needs at least 5 days of
glucose data.
OK
Last 7 Days
12
am am ampm pm
6 12 6 12
121
152
134
Average:
119 mg/dL
69
Average Glucose
12am6am12pm6pm12
am
350
250
150
50
OK
Daily Patterns
(mg/dL)
Last 7 Days
55 56
ART38553-101_rev-A_manual.indd 55-56 2/1/18 2:13 PM
Page 33

Time In Target
A graph showing the percentage of time your
Sensor glucose readings were above, below, or
within your Target Glucose Range.
Low Glucose Events
Information about the number of low glucose
events measured by your Sensor. A low glucose
event is recorded when your Sensor glucose
reading is lower than 70 mg/dL for 15 minutes or
longer. The total number of events is displayed
above the graph. The bar graph displays the low
glucose events in four dierent 6-ho ur periods of
the day.
Sensor Usage
Information about how often you s can your Sensor.
The Reader report s an average of how many
times you scanned your Sensor each day, and the
percentage of possible Sensor data the R eader
recorded from your scans.
OK
34%
54%
12%
Time In Target
Above
In Target
Below
Target Range
80-140 mg/dL
Last 7 Days
OK
Last 7 Days
12
am am ampm pm
6 12 6 12
1
0
Total Events:
10
3
6
Low Glucose
Events
OK
100%
5
Scans Per
Day
Sensor Data
Captured
Sensor Usage
Last 7 Days
Removing Your Sensor
Step Action
1
Pull up the edge of the adhesive that keeps your
Sensor attached to your skin. Slowly peel away
from your skin in one motion.
Note: Any remaining adhesive residue on the
skin can be removed with warm soapy water or
isopropyl alcohol.
2
Discard the used Sensor following directions from your health
care professional. See Maintenance and Disposal section.
When you are ready to apply a new Sensor, follow the
instructions in the Applying Your Sensor and Starting Your Sensor
sections. If you removed your last Sensor before it ended, you
will be prompted to conrm that you would like to start a new
Sensor when you rst scan it.
57 58
ART38553-101_rev-A_manual.indd 57-58 2/1/18 2:13 PM
Page 34

Replacing Your Sensor
Your Sensor automatically stops working after 10 days of data and
must be replaced. You should also replace your Sensor if you notice any
irritation or discomfort at the application site or if the Reader reports a
problem with the Sensor currently in use. Taking action ear ly can keep
small problems from turning into larger ones.
CAUTIO N: If the Sensor is becoming loose or if the Sensor tip is
coming out of your skin, you may get no readings or unreliable
readings, which may not match how you feel. Check to make
sure your Sensor has not come loose. If it has come loose,
remove it and apply a new one.
Using Reminders
You can use Reminders to help you remember to check your glucose, take
insulin, or as a general alarm.
Step
Action
1
From the Home Screen, touch the symbol.
2
Touch to select which Type of reminder you want
to set: Check Glucose, Take Insulin, or Alarm.
3
Touch to select how often you want the reminder to Repeat:
Once, Daily, or Timer.
Note: You can set the reminders for a specic time (e.g. 8:30 am)
or as a timer (e.g. 3 hours from the current time).
Ends in 10 days
10:23pm
Check
Glucose
Review
History
cancel save
Set Reminder
Alarm
Daily
XX:XX
Type
Repeat
Time
59 60
ART38553-101_rev-A_manual.indd 59-60 2/1/18 2:13 PM
Page 35

Step
Action
4
Set the reminder Time using the arrows on the touchscreen.
Touch save.
5
From the Reminders screen, you can turn the
reminder On/O or add new reminders.
Touch done to return to the Home Screen.
When reminders are On, the next reminder time
appears next to the reminder symbol on the
Home Screen.
For example,
8:30am
Your reminder comes on even if the Reader is
turned o. Touch OK to dismiss your reminder or
snooze to be reminded again in 15 minutes.
Note: Reminders will not appear if the Reader is
connected to a computer.
add new done
Reminders
8:30am
12:30pm
00:00:00
On
On
Off
8:30am
Alarm
Reminder
snooze
15 min
OK
Using the Reader’s Built-in Meter
The Reader has a built-in meter that can be used to test your blood
glucose, or to test the meter and strips with control solution.
Intended Use
The FreeStyle Libre Reader’s built-in meter is for use outside the body
only (in vitro diagnostic use) in the quantitative measurement of glucose
in fresh whole blood for self testing by lay users from the ngers. It is
not intended to be used for testing neonatal blood samples or for the
diagnosis or screening of diabetes.
The FreeStyle Libre Reader’s built-in meter is indicated for the home (lay)
user in the management of patients with diabetes. It is intended to be
used by a single person and should not be shared.
The FreeStyle Precision Neo Blood Glucose Test Strips are for use with the
FreeStyle Libre Reader’s built-in meter to quantitatively measure glucose
(sugar) in fresh capillary whole blood samples drawn from the ngertips.
WARNING: Do NOT use the built-in meter while the
Reader is connected to an electrical outlet or a computer
due to the potential risk of electrical shock.
61 62
ART38553-101_rev-A_manual.indd 61-62 2/1/18 2:13 PM
Page 36

IMPORTANT:
• Use only FreeStyle Precision Neo test strips. Other test strips may
produce inaccurate results.
• Read all the instructions in this section. Failure to follow instructions
may cause incorrect blood glucose results. Practice the testing
procedures before using the Reader’s built-in meter.
• Read the test strip instructions for use before performing your rst
blood glucose test as they contain important information. They also
let you know how to store and handle the test strips and give you
information about sample types.
• The Reader’s built-in meter is not for use on people who
are dehydrated, hypotensive, in shock, or for individuals in
hyperglycemic-hyperosmolar state, with or without ketosis.
• The Reader’s built-in meter is not for use on neonates, in critically-ill
patients, or for diagnosis or screening of diabetes.
• Follow your health care professional’s advice when testing blood
glucose levels.
• Observe caution when using around children. Small parts may
constitute a choking hazard.
• You should clean and disinfect the Reader once per week. The
Reader should also be cleaned and disinfected prior to being
handled by any person providing testing assistance to the user.
IMPORTANT: (con t.)
• The Reader is for use by a single person. It must not be used on
more than one person including other family members due to the
risk of spreading infection. All parts of the Reader are considered
biohazardous and can potentially transmit infectious diseases, even
after performing the cleaning and disinfection procedure.
1, 2
• Use the Reader’s built-in meter within the test strip operating
temperature range or you will see Error Message E-1.
• Use a test strip immediately after removing from its foil packet.
• Only use a test strip once.
• Do not put urine on the test strip.
• Do not use expired test strips as they may cause inaccurate results.
• Do not use a wet, bent, scratched, or damaged test strip.
• Do not use the test strip if the foil packet has a hole or is torn.
• Results from the built-in meter are shown only in your Logbook and
not in other history options.
• Refer to your lancing device instructions for use for how to use your
lancing device.
63 64
ART38553-101_rev-A_manual.indd 63-64 2/1/18 2:13 PM
Page 37

Blood Glucose Testing
You can use the built-in meter to check your blood glucose, whether you
are wearing a Sensor or not. Be sure to read the test strip instructions for
use prior to using the built-in meter.
Step Action
1
Wash your hands with warm soapy water for
accurate results. Thoroughly dry your hands.
To warm the site, apply a warm dry pad or rub
vigorously for a few seconds.
Note: Do not use lotion or cream on the test
site. Avoid moles, veins, bones, and tendons.
Bruising may occur at the test site. If you get a
bruise, consider selecting another site.
CAUTIO N: Test on your ngers in accordance
with the Intended Use in this section.
Step Action
2
Check the test strip expiration date. Do not use expired test
strips as they may give inaccurate results.
3
Open the foil test strip packet at the notch and
tear down to remove the test strip. Use the test
strip immediately after removing from the foil
packet.
4
Insert the test strip with the three black lines at
the end facing up. Push the strip in until it stops.
Note: The Reader’s built-in meter turns o after
2 minutes of inactivity. Remove and reinsert the
unused test strip to restart the built-in meter.
Abbott Diabetes Care Ltd.
Range Road
Witney, Oxon, OX29 0YL
Made in UK.
Blood Glucose
Sensor Electrode
Abbott Diabetes Care Ltd.
Range Road
Witney, Oxon, OX29 0YL
Made in UK.
4˚C
65 66
ART38553-101_rev-A_manual.indd 65-66 2/1/18 2:13 PM
Page 38

Step Action
5
Use your lancing device to obtain a blood drop
and apply blood to the white area at the end
of the test strip. Refer to your lancing device
instructions for use if you need help using your
lancing device.
If sounds are turned on, the Reader beeps once
to let you know you have applied enough blood.
You will see a buttery on the screen while you
wait for your result. Do not remove the test strip
while the buttery is on the screen. If sounds are
turned on, the Reader beeps once when your
result is ready.
If the buttery does not appear, you may not
have applied enough blood to the test strip.
Apply a second drop of blood to the test strip
within 5 seconds of the rst drop. If the buttery
still does not appear or if more than 5 seconds
have passed, discard the test strip. Turn o the
Reader and repeat the steps in this section with a
new test strip.
Apply Blood
Step Action
5
(cont.)
Note:
• E-3 means the blood drop is too small, or incorrect test
procedure, or there may be a problem with the test strip.
• E-4 means the blood glucose level may be too high to be read
by the system or there may be a problem with the test strip.
See Troubleshooting section for more information.
6
After reviewing your result, remove and discard the used test
strip according to local regulations.
Your Blood Glucose Results
Blood glucose results are marked on the
results screen and in the Logbook with the
symbol.
Note: Contact your health care professional
if you have symptoms that do not match your
test results.
IMPORTANT: After performing a blood glucose test,
wash your hands with soap and water and thoroughly
dry them.
143
mg
dL
10:23pm
Example Screen Only
67 68
ART38553-101_rev-A_manual.indd 67-68 2/1/18 2:13 PM
Page 39

The expected glucose range for a non-diabetic, non-pregnant fasting
adult is under 100 mg/dL. Two hours after meals, levels should be less than
140 mg/dL.
3
Consult your healthcare professional to determine the range
that is appropriate for you.
Display What To Do
mg
dL
Low Glucose
LO
mg
dL
High Glucose
HI
If LO appears on the Reader, your result is lower
than 20 mg/dL. If HI appears on the Reader, your
result is higher than 500 mg/dL. You can touch the
message button for more information. Check your
blood glucose again with a test strip. If you get a
second LO or HI result, contact your health care
professional immediately.
IMPORTANT: The built-in meter displays results from 20 - 500 mg/dL .
Low or high blood glucose results can indicate a potentially ser ious
medical condition.
Display What To Do
63
mg
dL
Low Glucose
289
mg
dL
High Glucose
If your glucose is higher than 240 mg/dL or lower
than 70 mg/dL, you will see a message on the
screen. You can touch the message button for
more information and set a reminder to check
your glucose.
After you get your blood glucose result, you can add Notes by touching
the
symbol. If you do not want to add a Note, press the Home Button
to go to the Home Screen or hold the Home Button to turn the Reader o.
IMPORTANT: You should clean and disinfect your Reader once
per week. Refer to the Maintenance and Disposal section for
instructions.
69 70
ART38553-101_rev-A_manual.indd 69-70 2/1/18 2:13 PM
Page 40

Control Solution Testing
You should do a control solution test when you are not sure of your test
strip results and want to check that your Reader’s built-in meter and test
strips are working properly.
IMPORTANT:
• Control solution results should fall within the control solution range
printed on the test strip instructions for use.
• Do NOT use control solution past the expiration date. Discard
control solution 3 months after opening or on the expiration date
printed on the bottle, whichever comes rst. (Example: open April
15, discard July 15; write the discard date on the side of the bottle.).
• The control solution range is a target range for control solution
only, not for your blood glucose results.
• The control solution test does not reect your blood glucose level.
• Use only MediSense (low, medium or high) Glucose and Ketone
Control Solution with the Reader’s built-in meter.
• Check that the LOT number printed on the test strip foil packet and
instructions for use match.
• Replace the cap securely on the bottle immediately after use.
• Do NOT add water or other liquid to the control solution.
• Contact Customer Service (1-855-632-8658) for information on how
to obtain control solution.
Step
Action
1
From the Home Screen, touch the Settings
symbol
. Touch Control Solution Test to do
a control solution test.
2
Check the test strip expiration date.
3
Open the foil test strip packet at the notch and
tear down to remove the test strip.
Ends in 10 days
10:23pm
Check
Glucose
Review
History
Sounds
Target Range
Control Solution Test
Settings
1 / 3
Abbott Diabetes Care Ltd.
Range Road
Witney, Oxon, OX29 0YL
Made in UK.
Blood Glucose
Sensor Electrode
Abbott Diabetes Care Ltd.
Range Road
Witney, Oxon, OX29 0YL
Made in UK.
4˚C
71 72
ART38553-101_rev-A_manual.indd 71-72 2/1/18 2:13 PM
Page 41

Step
Action
4
Insert the test strip with the three black lines
facing up. Push the strip until it stops.
Note: The Reader’s built-in meter turns o after
2 minutes of inactivity. Remove and reinsert the
unused test strip to restart the built-in meter.
5
Apply Control Solution
Shake the control solution bottle to mix the
solution. Apply a drop of control solution to the
white area at the end of the test strip.
If sounds are turned on, the Reader beeps once
to let you know that you have applied enough
control solution.
Step
Action
5
(cont.)
You will see a buttery on the screen while you
wait for the result. Do not remove the test strip
while the buttery is on the screen. If sounds are
turned on, the Reader beeps once when the result
is ready.
If the buttery does not appear, you may not have
applied enough control solution to the test strip.
Apply a second drop of control solution to the
test strip within 5 seconds of the rst drop. If the
buttery still does not appear or if more than 5
seconds have passed, discard the test strip. Turn
o the Reader and repeat the steps in this section
with a new test strip.
73 74
ART38553-101_rev-A_manual.indd 73-74 2/1/18 2:13 PM
Page 42

Control Solution Results
Compare the control solution result to the
range printed on the test strip instructions
for use. The result on your screen should be
in this range.
Control solution results are marked on
the results screen and in the Logbook
with a
symbol.
Note: Repeat the control solutio n test if the results are
outside of the range printed on the test str ip instructions
for use. Stop using the built-in meter if the control solution
results are repeatedly outside of the printed ra nge. Contact
Customer Service.
100
mg
dL
10:23pm
Control Solution Test
Example Screen Only
Charging the Reader
A fully charged Reader battery should last up to 7 days. Your battery
life may vary depending on your usage. A Low Battery message
accompanies your result when you have enough charge remaining for
about one day of use.
Plug the included USB cable into an electrical
outlet using the included power adapter. Then,
plug the other end of the USB cable into the USB
port on the Reader.
Notes:
• You must charge the Reader when the battery
is low
to keep using the Reader.
• To fully charge the battery, charge the Reader
for at least 3 hours.
• Only use the USB cable and power adapter
included with the system.
• Fully charge your Reader before storing it for
more than 3 months.
OK
Please charge the Reader to
continue using it.
Low Battery
Charging
CAUTIO N: Be sure to select a location for
charging that allows the power adapter to be
easily unplugged. Don’t block access to the
charger due to the potential risk of electrical
shock.
75 76
ART38553-101_rev-A_manual.indd 75-76 2/1/18 2:13 PM
Page 43

Changing the Reader Settings
You can go to the Settings menu to change many settings on the Reader,
like Time & Date or Sounds. The Settings menu is also where you go to do
a Control Solution Test or to check the System Status.
Step
Action
1
To get to the Settings menu, touch the Settings
symbol
on the Home Screen.
Ends in 10 days
10:23pm
Check
Glucose
Review
History
Sounds
Target Range
Control Solution Test
Settings
1 / 3
Step
Action
2
Touch the setting you want to change:
Sounds – Set tones and vibrations
Target Range – Set range displayed on Reader
glucose graphs
Control Solution Test – Perform a Control Solution test
Time & Date – Change the Time or Date
Language – Change the language on the Reader
System Status – Check Reader information and performance
• View System Information: The Reader will display information
about your System including:
- Current Sensor end date and time
- Reader serial number and version number
- Serial numbers of most recent Sensors (up to three)
- Sensor version for most recent Sensor
- Number of Sensors that have been used with Reader
- Number of tests that have been performed using test strips
77 78
ART38553-101_rev-A_manual.indd 77-78 2/1/18 2:13 PM
Page 44

Step
Action
2
(cont.)
• View Event Logs: A list of events recorded by the
Reader, which may be used by Customer Service to help
troubleshoot your System
• Perform a Reader Test: The Reader Test will perform internal
diagnostics and allow you to check that the Display is
showing all pixels, Sounds (including both tones and
vibrations) are working, and the Touchscreen is responding
when touched
Reader Basics – Review the information screens shown
during the Reader setup
Dose Increment – You can set the insulin dose increment
to either 1.0 or 0.5 units for use with insulin notes
Touch OK when you are done.
Living With Your FreeStyle Libre System
Your FreeStyle Libre Flash Glucose Monitoring System can be used during
a wide variety of activities.
Activity What You Need To Know
Bathing,
Showering, and
Swimming
The Reader is not water-resistant and should
NEVER be submerged in water or other liquid.
Your Sensor is water-resistant and can be worn
while bathing, showering, or swimming.
Note: Do NOT take your Sensor deeper than
3 feet (1 meter) or immerse it longer than 30
minutes in water.
Sleeping Your Sensor should not interfere with your sleep. It
is recommended that you scan your Sensor before
going to sleep and when you wake up because
your Sensor holds only 8 hours of data at a time.
For example, if you sleep for 9 hours without
scanning your Sensor, 1 hour of data will not be
collected and a gap will appear on your glucose
graph.
If you have reminders set to go o while you are
sleeping, place the Reader nearby.
79 80
ART38553-101_rev-A_manual.indd 79-80 2/1/18 2:13 PM
Page 45

Activity What You Need To Know
Traveling by Air You can safely use your System at all times while
on an aircraft.
• The Reader is classed as a Medical-Portable
Electronic Device (M-PED) that meets all required
M-PED emission standards for safe use onboard
an aircraft: RTCA/DO160, Section 21, Category M.
Please note though that you must still comply
with any requests from the ight crew to not
scan your Sensor due to the wireless connection
between the Reader and the Sensor. You will still
be able to do a blood glucose test by inserting a
strip into the Reader as this does not turn on the
wireless connection.
• Some airport full-body scanners include x-ray or
millimeter radio-wave, which you cannot expose
your System to. The eect of these scanners
has not been evaluated and the exposure may
damage the System or cause inaccurate results.
To avoid removing your System, you may request
another type of screening. If you do choose to go
through a full-body scanner, you must remove
your Sensor.
Activity What You Need To Know
Traveling by Air
(cont .)
• The System can be exposed to common
electrostatic (ESD) and electromagnetic
interference (EMI), including airport metal
detectors. You can keep your Reader on while
going through these.
Note: If you are changing time zones, you can
change the time and date settings on the Reader
by touching the Settings symbol
from the
Home Screen, then Time & Date. Changing the
time and date aects the graphs and statistics.
The
symb ol may appear on your glucose
graph indicating the Reader time was change d.
Gaps in the graph may result or glucose readings
may be hidden.
81 82
ART38553-101_rev-A_manual.indd 81-82 2/1/18 2:13 PM
Page 46

Maintenance and Disposal
Cleaning and Disinfecting the Reader
Cleaning and disinfecting your Reader is important to prevent the spread
of infectious diseases. The Reader has a mean use life of 3 years and has
been validated for 156 cleaning and disinfection cycles (the equivalent of
1 cycle per week for 3 years).
You should clean and disinfect the Reader once a week. The Reader should
also be cleaned and disinfected prior to being handled by any person
providing testing assistance to the user.
Cleaning is the physical removal of organic soil from the Reader surfaces.
Keeping the Reader clean helps ensure that it is working properly and
that no dirt gets in the device. Cleaning allows for successful, subsequent
disinfection.
Disinfection is a process that destroys pathogens, such as viruses and other
microorganisms, on the Reader surfaces. Disinfecting the Reader helps
ensure that no infection is passed on when you or others come in contact
with the Reader.
This device is not intended for use in healthcare or assisted-use settings
such as hospitals, physician oces, or long-term care facilities because
it has not been cleared by FDA for use in these settings, including for
routine assisted testing or as part of glycemic control procedures.
Use of this device on multiple patients may lead to transmission of Human
Immunodeciency Virus (HIV), Hepatitis C Virus (HCV), Hepatitis B Virus
(HBV), or other bloodborne pathogens.
83 84
ART38553-101_rev-A_manual.indd 83-84 2/1/18 2:13 PM
Page 47

To clean and disinfect your Reader, you will need Clorox Healthcare Bleach
Germicidal Wipes, EPA Reg. #67619-12.
These disinfectant wipes contain a 0.55% Sodium Hypochlorite (NaOCl)
solution and have been shown to be safe for use with the Reader. They
may be purchased at major online retailers, such as Walmart.com,
Amazon.com, and OceDepot.com.
Note: Additional information about the risks for transmitting bloodborne
pathogens to persons undergoing ngerstick procedures for blood
sampling can be found. See References section for more information.
Step Action
1
Turn o the Reader before you clean and disinfect it.
2
Clean the outside surfaces of the Reader with a bleach wipe
until visibly clean. Make sure liquid does not get into the test
strip and USB ports.
Step Action
3
For disinfection, use a second bleach wipe to wipe all outside
surfaces of the Reader until they are wet. Make sure liquid
does not get into the test strip and USB ports. Allow the
Reader surfaces to remain wet for 60 seconds.
4
Dry with clean paper towel to remove any residual moisture.
5
When nished, thoroughly wash your hands with soap and
water.
85 86
ART38553-101_rev-A_manual.indd 85-86 2/1/18 2:13 PM
Page 48

IMPORTANT: If you require assistance or if you notice any signs
of deterioration on the Reader (such as clouding or crazing on the
display of the Reader, corroding or eroding of the plastic housing, or
cracking of plastic housing or display) or if the Reader does not turn
on, discontinue use of the Reader and contact Customer Service at
1-855-632-8658.
Maintenance
The FreeStyle Libre Flash Glucose Monitoring System has no serviceable
parts.
Disposal
This product should be disposed of in accordance with all applicable local
regulations related to the disposal of electronic equipment, batteries,
sharps, and materials potentially exposed to body uids.
Contact Customer Service for further information on the appropriate
disposal of system components.
CAUTIO N: Do NOT place the Reader in water or other liquids.
Avoid getting dust, dirt, blood, control solution, water, bleach,
or any other substance in the test strip or USB ports as this may
cause the Reader to not function properly.
Troubleshooting
This section lists problems or observations that you may have, the
possible cause(s), and recommended actions. If the Reader experiences an
error, a message will appear on the screen with directions to resolve the
error.
Reader Does Not Power On
Problem What It May Mean What To Do
Reader does not
power on after
you press the
Home Button or
insert a test strip.
Reader battery is
too low.
Charge the Reader.
Reader is outside
of its operating
temperature range.
Move the Reader to a
temperature between
50 °F and 113 °F and then
try to power it on.
If the Reader still does not power on after trying these steps, contact
Customer Service.
87 88
ART38553-101_rev-A_manual.indd 87-88 2/1/18 2:13 PM
Page 49

Problems at the Sensor Application Site
Problem What It May Mean What To Do
The Sensor is
not sticking
to your skin.
The site is not free
of dirt, oil, hair, or
sweat.
1. Remove the Sensor.
2. Consider shaving and/or
cleaning the site with soap
and water.
3. Follow the instructions in
Applying and Starting Your
Sensor sections.
Skin irritation
at the Sensor
application
site.
Seams or other
constrictive clothing
or accessories
causing friction at
the site.
Ensure that nothing rubs on the
site.
You may be sensitive
to the adhesive
material.
If the irritation is where the
adhesive touches skin, contact
your health care professional to
identify the best solution.
Problems Starting Your Sensor or Receiving Sensor
Readings
Display What It May Mean What To Do
New Sensor
Starting Up
Sensor is not ready
to read glucose.
Wait until the 12 hour Sensor
start-up period has completed.
Scan
Timeout
The Reader is not
held close enough
to the Sensor.
Hold the Reader within
1.5 inches (4 cm) of the Sensor.
Bring the screen of the Reader
close to the Sensor.
Sensor
Ended
The Sensor life has
ended.
Apply and start a new Senso r.
89 90
ART38553-101_rev-A_manual.indd 89-90 2/1/18 2:13 PM
Page 50

Display What It May Mean What To Do
New Sensor
Found
You scanned a new
Sensor before your
previous Sensor
ended.
Your Reader can only be used
with one Sensor at a time. If
you start a new Sensor, you will
no longer be able to scan your
old Sensor. If you would like to
begin using the new Sensor,
select “Yes”.
Scan Error The Reader
was unable to
communicate with
the Sensor.
Try scanning again.
Note: You may need to move
away from potential sources of
electromagnetic interference.
Sensor Error The System is
unable to provide a
glucose reading.
Scan again in 10 minutes.
Display What It May Mean What To Do
Glucose
Reading
Unavailable
Your Sensor is too
hot or too cold.
Move to a location where the
temperature is appropriate and
scan again in a few minutes.
Sensor
Already in
Use
The Sensor was
started by another
Reader.
A Sensor can only be scanned
by the Reader that started it.
Scan the Sensor again with the
Reader that started it. Or, apply
and start a new Sensor.
Check Sensor The Sensor tip may
not be under your
skin.
Try to start your Sensor again. If
Reader displays “Check Sensor”
again, your Sensor was not
applied properly. Apply and
start a new Sensor.
Replace
Sensor
The System has
detected a problem
with your Sensor.
Apply and start a new Senso r.
91 92
ART38553-101_rev-A_manual.indd 91-92 2/1/18 2:13 PM
Page 51

Blood Glucose Error Messages
Error
Message
What It May Mean What To Do
E-1
The temperature is
too hot or too cold
for the Reader to
work correctly.
1. Move the Reader and test
strips to a location where the
temperature is within the test
strip operating range. (See
test strip instructions for use
for the appropriate range).
2. Wait for the Reader and test
strips to adjust to the new
temperature.
3. Repeat the test using a new
test strip.
4. If the error reappears, contact
Customer Service.
E-2
Reader error. 1. Turn o the Reader.
2. Repeat the test using a new
test strip.
3. If the error reappears, contact
Customer Service.
Error
Message
What It May Mean What To Do
E-3
Blood drop is too
small.
or
Incorrect test
procedure.
or
There may be a
problem with the test
strip.
1. Review the testing
instructions.
2. Repeat the test using a new
test strip.
3. If the error reappears, contact
Customer Service.
E-4
The blood glucose
level may be too high
to be read by the
system.
or
There may be a
problem with the test
strip.
1. Repeat the test using a new
test strip.
2. If the error reappears, contact
your health care professional
immediately.
93 94
ART38553-101_rev-A_manual.indd 93-94 2/1/18 2:13 PM
Page 52

Error
Message
What It May Mean What To Do
E-5
Blood was applied
to the test strip too
soon.
1. Review the testing
instructions.
2. Repeat the test using a new
test strip.
3. If the error reappears, contact
Customer Service.
E-6
The test strip may not
be compatible with
the Reader.
1. Check that you are using
the correct test strip for
the Reader. (See test strip
instructions for use to verif y
your strip is compatible with
the Reader).
2. Repeat the test using a test
strip for use with your Reader.
3. If the error reappears, contact
Customer Service.
Error
Message
What It May Mean What To Do
E-7
Test strip may be
damaged, used, or
the Reader does not
recognize it.
1. Check that you are using
the correct test strip for
the Reader. (See test strip
instructions for use to verif y
your strip is compatible with
the Reader).
2. Repeat the test using a test
strip for use with your Reader.
3. If the error reappears, contact
Customer Service.
E-9
Reader error. 1. Turn o the Rea der.
2. Repeat the test using a new
test strip.
3. If the error reappears, contact
Customer Service.
95 96
ART38553-101_rev-A_manual.indd 95-96 2/1/18 2:13 PM
Page 53

Problems Checking Your Blood Glucose
Problem What It May Mean What To Do
The Reader
does not
start a
test after
inserting a
test strip.
Test strip is not
inserted correctly
or not inserted fully
into the strip port.
1. With the 3 black lines facing
up, insert the test strip into
the strip port until it stops.
2. If the Reader still does not
start a test, contact Customer
Service.
Reader battery is too
low.
Charge the Reader.
The test strip is
damaged, used, or
unrecognizable by
the Reader.
Insert a new FreeStyle Precision
Neo test strip.
Reader is outside
of its operating
temperature range.
Move the Reader to a
temperature between 50 °F and
113 °F and then try to power it
on.
Reader is in a power
saving mode.
Press the Home Button then
insert a test strip.
Problem What It May Mean What To Do
The test
does not
start after
applying
the blood
sample.
Blood sample is too
small.
1. See test strip instructions
for use for re-application
instructions.
2. Repeat the tes t using a new
test strip.
3. If the test sti ll does not start,
contact Customer Service.
Sample applied after
the Reader turned
off.
1. Review the testing
instructions.
2. Repeat the tes t using a new
test strip.
3. If the test sti ll does not start,
contact Customer Service.
Problem with
Reader or test strip.
1. Repeat the test using a new
test strip.
2. If the test still d oes not start,
contact Customer Service.
97 98
ART38553-101_rev-A_manual.indd 97-98 2/1/18 2:13 PM
Page 54

Perform a Reader Test
If you think the Reader is not working prop erly, you
can check the Reader by per forming a Reader Test.
Touch the Settings symbol
from the Ho me
Screen, select System Status and then selec t
Reader Test.
Note: The Reader Test will perform internal
diagnostics and will allow you to check that the
display, sounds, and touchscreen are working
properly.
Customer Service
Customer Service is available to answer any questions you may have
about your FreeStyle Libre Flash Glucose Monitoring System. Please go to
the back cover of this manual for your Customer Service phone number.
System Info
Reader Test
Event Log
System Status
OK
System Specifications
See test strip and control solution instructions for use for additional
specications.
Sensor Specifications
Sensor glucose assay
method
Amperometric electrochemical sensor
Sensor glucose reading
range
40 to 500 mg/dL
Sensor size 5 mm height and 35 mm diameter
Sensor weight 5 grams
Sensor power source One silver oxide battery
99 100
ART38553-101_rev-A_manual.indd 99-100 2/1/18 2:13 PM
Page 55

Sensor data Up to 10 days
Sensor memory
8 hours (glucose readings stored every
15 minutes)
Operating temperature 50 °F to 113 °F
Sensor Applicator and
Sensor Pack storage
temperature
39 °F to 77 °F
Operating and storage
relative humidity
10-90%, non-condensing
Sensor water resistance
IP27: Can withstand immersion into
3 ft (one meter) of water for up to 30
minutes. Protected against insertion of
objects > 12mm diameter.
Operating and storage
altitude
-1,250 ft (-381 meters) to 10,000 ft
(3,048 meters)
Reader Specifications
Blood glucose assay
range
20 to 500 mg/dL
Reader size 95 mm x 60 mm x 16 mm
Reader weight 65 grams
Reader power source One lithium-ion rechargeable battery
Reader battery life 7 days of typical use
Reader memory 90 days of typical use
Reader operating
temperature
50 °F to 113 °F
Reader storage
temperature
-4 °F to 140 °F
Operating and storage
relative humidity
10-90%, non-condensing
101 102
ART38553-101_rev-A_manual.indd 101-102 2/1/18 2:13 PM
Page 56

Reader moisture
protection
Keep dry
Operating and storage
altitude
-1,250 ft (-381 meters) to 10,000 ft
(3,048 meters)
Reader display timeout
60 seconds (120 seconds when test
strip is inserted)
Radio Frequency
Near Field Communication* (13.56 MHz
RFID); ASK Modulation; 124 dBuV/m;
1.5 inch communication range
Data port Micro USB
Minimum Computer
Requirements
System must only be used with
EN60950-1 rated computers
Mean use life 3 years of typical use
Reader cleaning and
disinfection
The Reader has a mean use life of
3 years, which is 156 cleaning and
disinfection cycles (1 cycle per week
for 3 years).
Power Adapter
Abbott Diabetes Care PRT25611
Operating temperature: 50 °F to 104 °F
USB Cable
Abbott Diabetes Care PRT21373
Length: 37 inches (94 cm)
* Security measures: The communication between Reader and Sensor is a short range near
eld communication method making it dicult to interfere with or intercept data that is
being transferred. The Sensor and Reader are protected by proprietary data format, memory
mapping, and cyclic redundancy check (CRC) generation and verication of data.
Quality of Service (QoS): QoS for the FreeStyle Libre Reader and Sensor wireless
communications using the near eld communications is assured within the eective range
of 4 cm between the Sensor and Reader that is specied to occur within 15 seconds.
103 104
ART38553-101_rev-A_manual.indd 103-104 2/1/18 2:13 PM
Page 57

Labeling Symbols
Consult instructions for
use
Use-by date
Temperature limit Catalog number
Manufacturer Serial number
Batch code
Keep dry
Type BF applied part Non-ionizing radiation
CODE
Sensor code Caution
Do not re-use
Sterilized using
irradiation
MR unsafe Humidity limitation
FCC Declaration of
Conformity mark
Do not use if package
is damaged
Not made with natural rubber latex
CAUTION: Federal law restricts this device to sale by or on
the order of a physician.
This product contains electronic equipment, batteries, sharps
and materials that may contact bodily uids during use.
Dispose of product in accordance with all applicable local
regulations.
105 106
ART38553-101_rev-A_manual.indd 105-106 2/1/18 2:13 PM
Page 58

Performance Characteristics
Clinical Study Overview
Performance of the FreeStyle Libre Flash Glucose Monitoring System (the System) was evaluated in a
clinical study. The study was conducted at 4 centers with a total of 48 subjects with diabetes (95.8%
Type 1, 4.2% Type 2). All subjects were aged eighteen and older. Subjects in the study required
insulin to manage their diabetes. Each subject wore up to two FreeStyle Libre sensors on the back
of the upper arm. During the study, subjects tested their blood glucose using ngerstick capillary
samples at least eight times during each day of the study. Subjects used the blood glucose meter
built into the FreeStyle Libre reader. Additionally, subjects had their venous blood glucose analyzed
up to 128 times over four separate visits to the clinical center. Venous blood was analyzed using
the Yellow Springs Instrument Life Sciences 2300 STAT Plus™ Glucose & Lactate Analyzer (YSI). YSI
is a laboratory glucose and lactate analyzer of whole blood and plasma and is a widely recognized
standard in laboratory analysis of blood glucose. Glucose readings obtained from the System were
compared to glucose readings obtained from the YSI to evaluate the performance of the System.
Three lots of sensors were evaluated in the study.
Agreement with YSI Levels
Agreement between FreeStyle Libre Glucose Measurement (CGM) and venous blood was characterized
by using paired CGM and Yellow Springs Instrument measurements (YSI). The accuracy of CGM versus
YSI reference was assessed by calculating the percentage of System readings that were within 15%,
20%, 30% and 40% for reference values 80 mg/dL and above, and 15 mg/dL, 20 mg/dL, 30 mg/dL and
40 mg/dL for values below 80 mg/dL when glucose levels are assigned using the YSI values. Overall
91.1% of results were within ±20 mg/dL / 20% of YSI reference.
Agreement with CGM Glucose Levels
Agreement between CGM and venous blood was characterized by using paired CGM and Yellow
Springs Instrument measurements (YSI). The accuracy of CGM versus YSI reference was assessed
by calculating the percentage of System readings that were within 15%, 20%, 30% and 40% for
reference values 80 mg/dL and above, and 15 mg/dL, 20 mg/dL, 30 mg/dL and 40 mg/dL for values
below 80 mg/dL. The results are presented in Table 1 for YSI reference. Overall 91.0% of results
were within ±20 mg/dL / 20% of YSI reference.
Table 1: Number and Percent of Results within YSI Reference
CGM Glucose
Level
(mg/dL)
Number of
CGM-Reference
Pairs
Within
±15% /
±15mg/dL
Within
±20% /
±20mg/dL
Within
±30% /
±30mg/dL
Within
±40% /
±40mg/dL
Outside
±40% /
±40mg/dL
Overall 5772 82.1 91.0 97.8 99.3 0.7
40-50 38 44.7 57.9 81.6 94.7 5.3
51-80 461 72.2 81.1 92.0 97.6 2.4
81-180 3236 82.9 91.2 97.9 99.3 0.7
181-300 1799 84.9 93.6 99.2 99.7 0.3
301-400 226 77.0 95.1 99.6 99.6 0.4
401-500 12 58.3 75.0 100.0 100.0 0.0
107 108
ART38553-101_rev-A_manual.indd 107-108 2/1/18 2:13 PM
Page 59

Agreement on Day 1 against YSI Reference
The accuracy of CGM versus YSI reference on the rst day of sensor wear was assessed by calculating
the percentage of System readings that were within 15%, 20%, 30% and 40% for reference values
80 mg/dL and above, and within 15 mg/dL, 20 mg/dL, 30 mg/dL and 40 mg/dL for values below
80 mg/dL by hourly intervals. The results are presented in Table 2.
Table 2: Number and Percent of Results within YSI Reference
Time Interval
(hours)
Number of CGMReference Pairs
Within
±15% /
±15mg/dL
Within
±20% /
±20mg/dL
Within
±30% /
±30mg/dL
Within
±40% /
±40mg/dL
Outside
±40% /
±40mg/dL
(0-2) 81 69.1 87.7 100.0 100.0 0.0
(2-4) 318 73.9 84.6 97.2 99.7 0.3
(4-6) 374 76.7 88.0 97.3 99.7 0.3
(6-8) 369 79.9 90.8 99.2 100.0 0.0
Overall Accuracy against YSI reference
Accuracy was measured by comparing the absolute relative dierence between the System and
reference YSI glucose values. The absolute relative dierence measures the level of disagreement
between the System and the reference value, but does not tell you whether the System glucose
value was, on average, higher or lower than the reference glucose value. The Mean Absolute Relative
Dierence gives an indication of the average percent disagreement between the CGM
and the reference. Table 3 shows the overall absolute dierence measure. Overall the Mean Absolute
Relative Dierence was 9.7% for the comparison with YSI reference. The Median Absolute Relative
Dierence shows that half of the time the System was within 7.7% of the YSI reference.
Table 3: Dierence Measures with YSI Reference
Number of
CGM-Reference Pairs
Median Absolute Relative
Dierence
Mean Absolute Relative
Dierence
5772 7.7% 9.7%
Agreement with BG Levels
Agreement between the System and capillary blood glucose values (BG) as measured by the Reader’s
built-in meter was characterized by using paired System CGM and BG value. The accuracy of CGM
versus BG value was assessed by calculating the percentage of System readings that were within 15%,
20%, 30% and 40% for BG values 80 mg/dL and above, and within 15 mg/dL, 20 mg/dL, 30 mg/dL
and 40 mg/dL for values below 80 mg/dL. The results are presented in Table 4 for BG values. Overall
84.3% of results were within ±20 mg/dL / 20% of BG values.
109 110
ART38553-101_rev-A_manual.indd 109-110 2/1/18 2:13 PM
Page 60

Table 4: Number and Percent of Results within BG Values*
CGM Glucose
Level
(mg/dL)
Number of
CGM-Reference
Pairs
Within
±15% /
±15mg/dL
Within
±20% /
±20mg/dL
Within
±30% /
±30mg/dL
Within
±40% /
±40mg/dL
Outside
±40% /
±40mg/dL
Overall 3680 72.8 84.3 95.0 98.0 2.0
40-50 23 47.8 73.9 87.0 95.7 4.3
51-80 288 65.6 77.1 89.6 97.6 2.4
81-180 1722 71.7 82.9 94.7 97.4 2.6
181-300 1193 75.0 87.3 96.2 98.4 1.6
301-400 362 76.8 87.8 97.2 99.2 0.8
401-500 92 78.3 85.9 95.7 98.9 1.1
* Comparison to BG was performed using the FreeStyle Libre Reader’s built-in blood glucose meter. Dierent
performance may be expected when compared to other models of blood glucose meters.
Overall Accuracy against BG values
Accuracy was measured by comparing the absolute relative dierence between the System and BG
values. The absolute relative dierence measures the level of disagreement between the System
and the BG value, but does not tell you whether the System glucose value was, on average, higher
or lower than the BG glucose value. The Mean Absolute Relative Dierence gives an indication of
the average percent disagreement between the CGM and the BG value. Table 5 shows the overall
absolute dierence measure. Overall the Mean Absolute Relative Dierence was 12.1% for the
comparison with BG value. The Median Absolute Relative Dierence shows that half of the time the
System was within 9.4% of the BG value.
Table 5: Dierence Measures with BG Value *
Number of
CGM-Reference Pairs
Median Absolute Relative
Dierence
Mean Absolute Relative
Dierence
3680 9.4% 12.1%
* Comparison to BG was performed using the FreeStyle Libre Reader’s built-in blood glucose meter. Dierent
performance may be expected when compared to other models of blood glucose meters.
111 112
ART38553-101_rev-A_manual.indd 111-112 2/1/18 2:13 PM
Page 61

Concurrence of System and Reference (CGM vs. YSI)
The percentage of concurring glucose values (CGM vs. YSI) in each glucose reference range is
presented for each CGM range in Table 6. For example, in the clinical study, when the System glucose
results were within the 81 to 120 mg/dL range, actual blood glucose values were less than 40 mg/dL
0% of the time, between 40 and 60 mg/dL 0.2% of the time, between 61 and 80 mg/dL 5.6% of the
time, between 81 and 120 mg/dL 75.9% of the time, between 121 and 160 mg/dL 17.6% of the time,
between 161 and 200 mg/dL 0.6% of the time, between 201 and 250 mg/dL 0.1% of the time and
above 250 mg/dL 0% of the time.
Table 6: Concurrence Analysis by Glucose Level
CGM
(mg/dL)
YSI Glucose Level (mg/dL)
N
<40* 40-60 61-80
81120
121160
161200
201250
251300
301350
351400
401500
>500*
<40 0.0 19.0 61.9 19.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 21
40-60 0.7 25.2 58.7 15.4 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 143
61-80 0.0 7.3 45.8 46.3 0.6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 356
81-120 0.0 0.2 5.6 75.9 17.6 0.6 0.1 0.0 0.0 0.0 0.0 0.0 1222
121-160 0.0 0.0 0.1 13.2 72.0 14.3 0.4 0.0 0.0 0.0 0.0 0.0 1435
161-200 0.0 0.0 0.0 0.3 21.9 67.5 10.2 0.1 0.0 0.0 0.0 0.0 1087
201-250 0.0 0.0 0.0 0.0 0.9 28.8 64.5 5.7 0.0 0.0 0.0 0.0 905
251-300 0.0 0.0 0.0 0.0 0.0 0.3 41.2 53.4 5.2 0.0 0.0 0.0 386
301-350 0.0 0.0 0.0 0.0 0.0 0.0 1.2 55.3 40.6 2.9
0.0
0.0 170
351-400 0.0 0.0 0.0 0.0 0.0 0.0 0.0 3.6 66.1 30.4
0.0
0.0 56
401-500 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 25.0 33.3 41.7
0.0
12
>500 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0
* Levels out of system dynamic range.
113 114
ART38553-101_rev-A_manual.indd 113-114 2/1/18 2:13 PM
Page 62

Concurrence Analysis by Glucose Trend Arrow
Trend Arrow accuracy of the System, as assessed by concurrence analysis, is presented in Table 7. For
example, in the clinical study, when the trend arrow indicated that glucose was changing slowly (-1 to
1 mg/dL/min (g) ), actual glucose levels in the body were falling quickly (i) 0.3% of the time, falling
(m) 3.7% of the time, changing slowly (g) 83.0% of the time, rising (k) 3.9% of the time, and rising
quickly (h) 0.5% of the time.
Table 7: Concurrence Analysis by Glucose Trend Arrow
CGM
(mg/dL/min)
YSI (mg/dL/min)
N
<-2 [-2, -1] [-1, 0] [0, 1] [1, 2] >2 NA*
<-2 (i)
26.3 45.5 10.3 1.3 0.0 0.0 16.7 156
-2 to -1 (m)
4.3 27.0 54.6 3.8 0.6 0.0 9.7 652
-1 to 1 (g)
0.3 3.7 49.4 33.6 3.9 0.5 8.6 4175
1 to 2 (k)
0.0 0.6 8.8 38.6 33.3 9.2 9.4 477
>2 (h)
0.0 0.0 2.8 14.6 34.9 40.6 7.1 212
NA† 2.5 9.1 33.1 20.7 14.0 9.1 11.6 121
* Glucose rate of change not available due to the time dierence between glucose readings exceeding 30 minutes.
† Glucose Trend Arrow not available.
Agreement with ‘LO’ and ‘HI’ CGM Reading against YSI Reference
The System reports glucose concentrations between 40 and 500 mg/dL. When the System
determines that glucose level is below 40 mg/dL, it will report as ‘LO’. When the System determines
that glucose level is above 500 mg/dL, it will report as ‘HI’. No measurements were obtained above
500 mg/dL in the clinical study. Table 8 displays the concurrence between the CGM and YSI reference
glucose when CGM reads ‘LO’. For example, in the clinical study, when CGM reading was ‘LO’ YSI
glucose values were less than 40 mg/dL 0.0% of the time, above 40 mg/dL 100.0% of the time,
above 50 mg/dL 95.2% of the time, above 60 mg/dL 80.9% of the time, above 70 mg/dL 42.8% of
the time, and above 80 mg/dL 19.0% of the time.
Table 8: Concurrence Analysis with ‘LO’ CGM Reading
YSI (mg/dL)
Total
<40 >40 >50 >60 >70 >80
% of CGM points in
YSI range
0.0 100.0 95.2 80.9 42.8 19.0
Number of CGM
points in YSI range
0 21 20 17 9 4 21
115 116
ART38553-101_rev-A_manual.indd 115-116 2/1/18 2:13 PM
Page 63

Accuracy by Day of Wear
After the 12 hour start-up period, the sensor can be worn for up to 10 days. To show sensor
performance over time, the absolute relative dierence between the System and reference YSI
glucose values over the wear is presented in Table 9.
Table 9: Dierence Measures by Day (YSI Reference)
Day
Number of CGMReference Pairs
Median Absolute
Relative Dierence (%)
Mean Absolute
Relative Dierence (%)
1
1497 8.7 10.7
4
1470 7.4 9.6
7
1394 7.4 9.1
10
1411 7.5 9.3
The accuracy of CGM versus YSI reference and BG reference was assessed by calculating the
percentage of System readings that were within 15%, 20%, 30% and 40% for reference values
80 mg/dL and above, and 15 mg/dL, 20 mg/dL, 30 mg/dL and 40 mg/dL for values below
80 mg/dL. The results for CGM vs. YSI reference are presented in Table 10.
Table 10: Number and Percent of Results within YSI Reference
Day Number of CGM-
Reference Pairs
Within
±15% /
±15mg/dL
Within
±20% /
±20mg/dL
Within
±30% /
±30mg/dL
Within
±40% /
±40mg/dL
Outside
±40% /
±40mg/dL
1
1497 76.2 87.4 97.9 99.5 0.5
4
1470 82.3 91.4 97.6 99.3 0.7
7
1394 85.0 93.5 98.6 99.3 0.7
10
1411 85.3 92.3 97.9 99.4 0.6
117 118
ART38553-101_rev-A_manual.indd 117-118 2/1/18 2:13 PM
Page 64

System Glucose Availability
The System is designed to produce a glucose reading after each user initiated scan that is performed
after the start-up time, through the wear period. Table 11 shows the number of available glucose
readings reported by all sensors and the expected number based on the total number of scan
attempts. Results are shown for sensors which produced at least one CGM reading during the
clinical study over the total wear period. The percentage of available CGM readings is presented in
comparison to the number of expected CGM readings. Overall, 99.5% of CGM readings (9,228 CGM
readings out of an expected 9,272) were available.
Table 11: CGM Availability
No. CGM No. Scan %
9228 9272 99.5
Detection of Hypoglycemic and Hyperglycemic Events
Table 12 shows the accuracy of the System’s Glucose Messages in informing the user of low or
high glucose events within 15 minutes before or after the true low or high blood glucose value.
Percentages are displayed for three dierent parameters:
• Detection Rate – amount of time the System displays a Glucose Message correctly.
• Missed Detection Rate – amount of time the System did not display a Glucose Message when it
should have.
• False Notication Rate – amount of time the System displays a Glucose Message when it shouldn’t
have.
For example, in the clinical study, the System was able to detect 85.4% of ac tual low glucose
events (detection rate), but 39.9% of the time a Low Glucose message was displayed in error (false
notication rate) and 14.6% of the time a Low Glucose message was not displayed when it should
have been (missed detection rate).
119 120
ART38553-101_rev-A_manual.indd 119-120 2/1/18 2:13 PM
Page 65

Table 12: Detection of Hypoglycemic and Hyperglycemic Events
Type of Notication Notication Status
15 Minute
Interval
Notication of Hypoglycemic Events
(Low Glucose message)
Detection Rate (%) 85.4
Missed Detection Rate (%) 14.6
False Notication Rate (%) 39.9
Notication of Hyperglycemic Events
(High Glucose message)
Detection Rate (%) 95.1
Missed Detection Rate (%) 4.9
False Notication Rate (%) 22.1
Impending Notication of Hypoglycemic
Events (Glucose Going Low message)
Detection Rate (%) 95.0
Missed Detection Rate (%) 5.0
False Notication Rate (%) 46.8
Impending Notication of Hyperglycemic
Events (Glucose Going High message)
Detection Rate (%) 97.2
Missed Detection Rate (%) 2.8
False Notication Rate (%) 28.4
Precision
Precision of the System was evaluated by comparing the results from two separate sensors worn on
the same subject at the same time. Table 13 provides data from two separate sensors worn on 47
subjects at the same time, providing 7,319 real-time pairs of CGM measurements, with a mean CV
of 6.0%.
Table 13: Overall between Sensor Precision
Mean Glucose
(mg/dL)
Median CV Mean CV
Number of
Subjects
Number of
Paired Readings
175.3
4.6 6.0 47 7319
Sensor Wear Duration
After the 12 hour start-up period, the Sensor can be worn for up to 10 days. To estimate how long a
Sensor will work over the wear duration, 97 Sensors were evaluated in the clinical study to determine
how many days of readings each Sensor provided. Of these 97 sensors, 75 (77.3%) lasted until the
nal day of use. 84 sensors (86.6%) lasted more than 5 days. There were 22 (22.7%) sensors that
failed early, of which 11 (11.3%) failed on or before the fth day of wear.
Adverse Events
No device related serious adverse events occurred during the study. Mild skin irritations, such as
erythema, edema, rash, bleeding, itching, induration, and infection were reported around the
insertion site and adhesive area by a moderate frequency of subjects (5 out of 48 or 10.4%). Pain
was mostly reported as none with only one instance of mild pain.
121 122
ART38553-101_rev-A_manual.indd 121-122 2/1/18 2:13 PM
Page 66

Electromagnetic Compatibility (EMC)
• The System needs special precautions regarding EMC and needs to be installed and put into service
according to the EMC information provided in this manual.
• Portable and mobile RF communications equipment can aect the System.
• The use of accessories, transducers and cables other than those specied by Abbott Diabetes Care
may result in increased EMISSIONS or decreased IMMUNITY of the System.
• The System should not be used adjacent to or stacked with other equipment and that if adjacent
or stacked use is necessary, the System should be observed to verify normal operation in the
conguration in which it will be used.
• This device complies with part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) This device may not cause harmful interference, and (2) this device must accept any
interference received, including interference that may cause undesired operation.
• Changes or modications not approved by Abbott could void the user’s authority to operate the
equipment.
Guidance and manufacturer’s declaration –
electromagnetic emissions
The System is intended for use in the electromagnetic environment specied below. The customer or
the user of the System should assure that it is used in such an environment.
Emissions test Compliance
Electromagnetic
environment – guidance
RF emissions
CISPR 11
Group 1 The System uses RF energy
only for its internal function.
Therefore, its RF emissions
are very low and are not likely
to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11
Class B The System is suitable for use
in all establishments, including
domestic establishments and
those directly connected to
the public low voltage power
supply network that supplies
buildings used for domestic
purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations /
flicker emissions
IEC 61000-3-3
Complies
123 124
ART38553-101_rev-A_manual.indd 123-124 2/1/18 2:13 PM
Page 67

Guidance and manufacturer’s declaration –
electromagnetic immunity
The System is intended for use in the electromagnetic environment specied below. The customer or
the user of the System should assure that it is used in such an environment.
IMMUNITY
test
IEC 60601
test level
Compliance
Level
Electromagnetic
environment – guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 8 kV contact
± 15 kV air
± 8 kV contact
± 15 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered
with synthetic material, the relative
humidity should be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
± 2 kV for
power supply
lines
± 1 kV for input/
output lines
± 2 kV for
power supply
lines
± 1 kV for input/
output lines
Mains power quality should be that
of a typical domestic, commercial,
or hospital environment.
IMMUNITY
test
IEC 60601
test level
Compliance
Level
Electromagnetic
environment – guidance
Surge
IEC 61000-4-5
±1 kV
differential
mode
±2 kV common
mode
±1 kV
differential
mode
±2 kV common
mode
Mains power quality should be that
of a typical domestic, commercial,
or hospital environment.
Voltage
dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % U
T
(>95 % dip in
U
T
) for 0.5 cycle
40 % U
T
(60 % dip in U
T
)
for 5 cycles
70 % U
T
(30 % dip in U
T
)
for 25 cycles
<5 % U
T
(>95 % dip
in U
T
) for 5
seconds
<5 % U
T
(>95 % dip in
U
T
) for 0.5 cycle
40 % U
T
(60 % dip in U
T
)
for 5 cycles
70 % U
T
(30 % dip in U
T
)
for 25 cycles
<5 % U
T
(>95 % dip
in U
T
) for 5
seconds
Mains power quality should be that
of a typical domestic, commercial,
or hospital environment. If the user
of the System requires continued
operation during power mains
interruptions, it is recommended
that the System be powered from
an uninterruptible power supply or
a battery.
125 126
ART38553-101_rev-A_manual.indd 125-126 2/1/18 2:13 PM
Page 68

IMMUNITY
test
IEC 60601
test level
Compliance
Level
Electromagnetic
environment – guidance
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
30 A/m 30 A/m Power frequency magnetic fields
should be at levels characteristic
of a typical location in a typical
domestic, commercial, or hospital
environment.
NOTE U
T
is the a.c. mains voltage prior to application of the test level.
IMMUNITY
test
IEC 60601
test level
Compliance
Level
Electromagnetic
environment – guidance
Conducted RF
IEC 61000-4-6
6 Vrms
150 kHz to
80 MHz
6 Vrms Portable and mobile RF
communications equipment should
be used no closer to any part of the
System, including cables, than the
recommended separation distance
calculated from the equation
applicable to the frequency of the
transmitter.
Recommended separation
distance
=
1.2
d
127 128
ART38553-101_rev-A_manual.indd 127-128 2/1/18 2:13 PM
Page 69

IMMUNITY
test
IEC 60601
test level
Compliance
Level
Electromagnetic
environment – guidance
Radiated RF
IEC 61000-4-3
10 V/m
80 MHz to
2.7 GHz
10 V/m Recommended separation
distance
80 MHz to 800 MHz
800 MHz to 2.5 GHz
P is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,
a
should
be less than the compliance level in each frequency range.
b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
=
1.2
d
=
2.3
d
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured
field strength in the location in which the System is used exceeds the applicable RF compliance
level above, the System should be observed to verify normal operation. If abnormal performance
is observed, additional measures may be necessary, such as re-orienting or relocating the System.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 10 V/m.
129 130
ART38553-101_rev-A_manual.indd 129-130 2/1/18 2:13 PM
Page 70

Recommended separation distances between portable
and mobile RF communications equipment and the
System
The System is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the System can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and the System as recommended below, according to the maximum output power of
the communications equipment.
Rated maximum
output power of
transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to
80 MHz
80 MHz to
800 MHz
800 MHz to
2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
=
1.2
d
=
1.2
d
=
2.3
d
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
131 132
ART38553-101_rev-A_manual.indd 131-132 2/1/18 2:13 PM
Page 71

Font License
©2013 Abbott
Licensed under the Apache License, Version 2.0 (the “License”); you may
not use this le except in compliance with the License. You may obtain a
copy of the License at: http://www.apache.org/licenses/LICENSE-2.0
Unless required by applicable law or agreed to in writing, software
distributed under the License is distributed on an “AS IS” BASIS,
WITHOUT WARRANTIES OR CONDITIONS OF ANY KIND, either express or
implied. See the License for the specic language governing permissions
and limitations under the License.
Limited Warranty
We hope that you are happy with your FreeStyle Libre system. Please refer to the
User’s Manual before using your Reader for the first time.
Abbott Diabetes Care (“Abbott”) warrants that the FreeStyle Libre reader (“Reader ”)
shall be free from defects in material and workmanship for a period of one (1) year
from the date of manufacture or one (1) year from the original date of purchase
with proof of purchase (whichever is later). This Limited Warranty is invalid if the
Reader is modified, altered, damaged, misused or used other than in accordance
with the User’s Manual, applicable labeling, and/or inserts. Abbott’s sole obligation
is to replace the Reader, free of charge, with the same or an alternative reader as
determined by Abbott in its sole discretion. Your replacement may be a different
model or type. Abbott may require, as a condition of obtaining limited warranty
service, that you return the Reader postage prepaid, with proof of purchase to an
address specified by Abbott. The Limited Warranty on the replacement Reader
will expire on the date of the original Limited Warranty expiration or 90 days
after the shipment of a replacement Reader, whichever period is longer. This
Limited Warranty covers only the Reader, does not cover the sensor or disposable
accessories, extends only to the original purchaser, and is not assignable or
transferable.
TO THE EXTENT POSSIBLE UNDER LAW, THE FOREGOING ARE ABBOTT’S ONLY
WARRANTIES FOR THE READER AND STATE YOUR EXCLUSIVE REMEDY. ABBOTT
MAKES NO OTHER WARRANTIES, EXPRESS OR IMPLIED, AND ABBOTT EXCLUDES
AND DISCLAIMS ANY OTHER WARRANTIES INCLUDING, BUT NOT LIMITED TO,
IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR
PURPOSE. ABBOTT DOES NOT WARRANT THAT OPERATION OF THE READER WILL
133 134
ART38553-101_rev-A_manual.indd 133-134 2/1/18 2:13 PM
Page 72

BE UNINTERRUPTED OR ERROR FREE AND ABBOTT WILL NOT BE LIABLE FOR
ANY LOST PROFITS, LOST SAVINGS OR OTHER SPECIAL, PUNITIVE, INCIDENTAL
OR CONSEQUENTIAL DAMAGES RESULTING DIRECTLY OR INDIRECTLY FROM
PURCHASE, OPERATION OR USE OF THE READER OR FAILURE OF THE READER
TO PERFORM IN ACCORDANCE WITH SPECIFICATIONS. NO WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, IF ANY IS IMPLIED
FROM THE SALE OF THE READER DESPITE ABBOTT’S SPECIFIC DISCLAIMER OF SUCH
WARRANTIES, SHALL EXTEND FOR A LONGER DURATION THAN ONE YEAR FROM
THE ORIGINAL DATE OF PURCHASE OF THE READER.
This Limited Warranty and any dispute or claim arising out of or in connection with
it shall be governed by and construed in accordance with the laws of Delaware.
Some states do not allow limitations on how long an implied warranty lasts or
the exclusion or limitation of incidental or consequential damages, so the above
limitations or exclusions may not apply to you.
Your Rights Under State Law: This Limited Warranty gives you specific legal rights,
and you may also have other rights that vary from state to state.
FreeStyle, Libre and related brand marks are trademarks of Abbott Diabetes Care
Inc. in various jurisdictions.
LIMITED WARRANTY SERVICES
For questions or warranty service, contact Customer Service at 1-855-632-8658.
ABBOTT MAY MODIFY OR DISCONTINUE THIS PROGRAM AT ANY TIME
WITHOUT NOTICE.
References:
1
“FDA Public Health Notification: Use of Fingerstick Devices on More than One
Person Poses Risk for Transmitting Bloodborne Pathogens: Initial Communication”
(2010) http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm224025.
htm
2
“CDC Clinical Reminder: Use of Fingerstick Devices on More than One Person Poses
Risk for Transmitting Bloodborne Pathogens” (2010)
http://www.cdc.gov/injectionsafety/Fingerstick-DevicesBGM.html
3
American Diabetes Association, Classification and Diagnosis of Diabetes, 2017.
Diabetes Care 40(Suppl. 1):S11–S2
135 136
ART38553-101_rev-A_manual.indd 135-136 2/1/18 2:13 PM
Page 73

ART38553-101_rev-A_manual.indd 137 2/1/18 2:13 PM
 Loading...
Loading...