Page 1

Use and Care Guide
F&P SleepStyle Auto
F&P SleepStyle CPAP
Page 2

Page 3

1
BEFORE YOU START
Caution: USA Federal Law restricts this device to sale by or on the order of a physician.
Before the device is used for the first time, it must be set up by a healthcare professional.
If your device or any accessories are not operating correctly, please contact your healthcare provider.
Clinicians: please contact your Fisher & Paykel Healthcare representative for a copy of the F&P SleepStyle
Clinician Guide.
TABLE OF CONTENTS
1. Overview ..................................................................................................................................................3
1.1 Intended use ........................................................................................................................................................................... 3
1.2 Contraindications .................................................................................................................................................................3
1.3 Warnings ..................................................................................................................................................................................3
1.3.1 To avoid death or serious injury ........................................................................................................................ 3
1.3.2 To avoid electric shock .......................................................................................................................................... 3
1.3.3 To avoid burns ..........................................................................................................................................................3
1.3.4 To avoid the risk of fire .......................................................................................................................................... 3
1.3.5 To avoid carbon dioxide re-breathing or asphyxiation ............................................................................4
1.3.6 To avoid choking, or inhalation of a foreign object ...................................................................................4
1.3.7 To avoid injury...........................................................................................................................................................4
1.3.8 To avoid incorrect therapy ................................................................................................................................... 4
1.3.9 General ........................................................................................................................................................................4
1.4 Cautions .................................................................................................................................................................................... 5
1.4.1 To prevent water damage to the device ........................................................................................................ 5
1.4.2 General ........................................................................................................................................................................5
1.5 Precautions .............................................................................................................................................................................. 5
1.6 Adverse eects ...................................................................................................................................................................... 5
2. Getting started ....................................................................................................................................... 6
2.1 Device and accessories .......................................................................................................................................................6
2.2 Setting up your device ....................................................................................................................................................... 7
3. Using your device ..................................................................................................................................10
3.1 Screen icons .......................................................................................................................................................................... 10
3.2 Device controls..................................................................................................................................................................... 10
3.3 Starting therapy.....................................................................................................................................................................11
3.4 Stopping therapy ..................................................................................................................................................................11
3.5 Stand-by mode ......................................................................................................................................................................11
3.6 Comfort settings ...................................................................................................................................................................11
3.6.1 Ramp ............................................................................................................................................................................11
3.6.2 Humidity ....................................................................................................................................................................12
3.6.3 Expiratory relief .......................................................................................................................................................12
3.6.4 SensAwake™ .............................................................................................................................................................12
Page 4

2
4. Viewing your therapy data .................................................................................................................... 13
4.1 View your therapy data on your device ......................................................................................................................13
4.1.1 Therapy data ............................................................................................................................................................13
4.2 View your therapy data on the SleepStyle App or website ............................................................................... 14
5. Uploading your therapy data ................................................................................................................ 15
5.1 Modem .....................................................................................................................................................................................15
5.2 F&P InfoUSB™ ........................................................................................................................................................................15
6. Caring for your device .......................................................................................................................... 17
6.1 Disassembly for cleaning ..................................................................................................................................................17
6.2 Cleaning your device and accessories at home .......................................................................................................18
6.2.1 Wash after each use ..............................................................................................................................................18
6.2.2 After 7 days’ use .....................................................................................................................................................19
6.3 Reassembly of the device .................................................................................................................................................19
6.4 Replacement parts ............................................................................................................................................................ 20
6.4.1 Air filter .......................................................................................................................................................................21
7. Traveling with your device ................................................................................................................... 22
7.1 Things to remember before you travel .......................................................................................................................22
8. Specifications ....................................................................................................................................... 23
8.1 SleepStyle device models and features matrix .......................................................................................................23
8.2 Symbol definitions ..............................................................................................................................................................24
8.3 Product specifications.......................................................................................................................................................24
8.4 Classifications ...................................................................................................................................................................... 26
8.5 Operating conditions ......................................................................................................................................................... 27
8.6 Storage and transport conditions ................................................................................................................................27
8.7 Disposal instructions ..........................................................................................................................................................27
8.8 Servicing .................................................................................................................................................................................27
8.9 Warranty statement ........................................................................................................................................................... 27
9. Troubleshooting ................................................................................................................................... 28
9.1 Device troubleshooting ....................................................................................................................................................28
9.2 Screen icons troubleshooting ....................................................................................................................................... 30
9.3 Error messages on SleepStyle screen ........................................................................................................................32
9.4 SleepStyle App troubleshooting...................................................................................................................................32
Page 5

3
1. OVERVIEW
WELCOME
Thank you for choosing your F&P SleepStyle device.
The F&P SleepStyle Auto is an auto-adjusting
positive airway pressure device.
The F&P SleepStyle CPAP is a continuous positive
airway pressure device (CPAP).
This guide refers to the F&P SleepStyle Auto
and F&P SleepStyle CPAP as the “device”. The
device treats Obstructive Sleep Apnea (OSA) by
delivering a flow of positive airway pressure at a
level prescribed by the physician, to splint open the
airway and prevent airway collapse.
Please read this guide carefully before you use your
device. Keep this guide in a safe place so you can
refer to it later if you need to.
1.1 INTENDED USE
The device is for use on adult patients for the
treatment of Obstructive Sleep Apnea (OSA).
The device is for use in the home or sleep laboratory.
1.2 CONTRAINDICATIONS
To avoid the risk of fire:
Do not use this device with
supplemental oxygen.
Warnings
Do not use this device if you have the
following pre-existing conditions as they may
contraindicate the use of positive airway pressure:
• Pneumothorax
• Bullous lung disease
• Pneumocephalus
• Cerebrospinal fluid leak
• Recent cranial surgery or head trauma
• Abnormalities of the cribriform plate
• Pathologically low blood pressure
• Bypassed upper airways.
If you are unsure about what pre-existing conditions
you have, check with your physician or healthcare
provider.
1.3 WARNINGS
1.3.1 To avoid death or serious
injury:
• The device must only be used on adult patients.
• The device must only be used for the treatment
of OSA.
• The device must only be used on prescription of
a physician.
• The device must not be used for life-support
applications.
1.3.2 To avoid electric shock:
• Do not use if the device, power cord or
accessories are damaged, deformed, or cracked.
• Do not pull on the power cord as it may become
damaged.
• Do not use bleach, alcohol, or cleaners with
citrus or other natural oils. These substances may
degrade the device and accessories.
• Do not immerse the device in water or any other
liquid.
• Do not modify the device or accessories.
• Do not take apart the device. Taking the device
apart, for example by unscrewing the underside
of the device, will damage pressure seals and
electrical components.
1.3.3 To avoid burns:
• Do not lie on, and avoid prolonged skin contact
with, the ThermoSmart™ breathing tube.
• Do not fill the water chamber with hot water as
this may lead to airway burns.
1.3.4 To avoid the risk of fire:
• Do not cover the ThermoSmart breathing tube
as this may overheat the tube.
• Do not connect electrical accessories not
approved for use with the device.
• Do not use this device with supplemental
oxygen.
• Sources of oxygen must be located more than 1
m (40 in.) from the device.
Page 6

4
1.3.5 To avoid carbon dioxide rebreathing or asphyxiation:
• Do not use masks that do not contain a
vent suitable for CPAP therapy, or are not
recommended by Fisher & Paykel Healthcare or
your healthcare provider.
• Remove the mask immediately if the device is
powered o (including in the event of a power
failure or device malfunction). The flow through
the mask may be insucient to clear all exhaled
gas.
1.3.6 To avoid choking, or inhalation
of a foreign object:
• Ensure the breathing tube and power cord,
including any extension cords, are correctly
positioned so they will not become entangled
with the body or furniture during sleep.
• Do not use the device without the recommended
air filter fitted. This will reduce dust or particles
entering the device and breathing tube.
• Do not place the device above head height to
prevent water from entering the breathing tube.
• Do not use the device with water in the water
chamber if the device is being used in a moving
vehicle or ship.
1.3.7 To avoid injury:
• Do not place the device above head height as
the device may fall.
• Do not use breathing tubes, parts, and
accessories that are not distributed for use with
this device or recommended by Fisher & Paykel
Healthcare.
• Do not use the breathing tubes or accessories
with any other device.
1.3.8 To avoid incorrect therapy:
• Do not cover the device or place it where the
air inlet could be obstructed (such as next to
curtains).
• Do not use the device adjacent to electrical
equipment.
• Do not adjust the pressure. Pressure adjustments
should only be made by a qualified healthcare
provider.
• Refer to the mask’s Use and Care Guide prior to
use to ensure correct fit of the mask. Incorrect fit
of the mask may aect consistent operation of
this device.
• Only clean the device and accessories according
to the cleaning instructions set out in section
6 – Caring for Your Device.
• Do not clean or disinfect the ThermoSmart
breathing tube with hot water. This may cause
deformation of the tube and reduce therapeutic
pressure.
• Use the elbow when rotating the ThermoSmart
breathing tube. Incorrect handling may damage
the tube.
• Do not remove the InfoUSB, or power o the
device, before you see this screen when updating
your prescription using InfoUSB:
Press any button to acknowledge and clear this
message.
1.3.9 General:
• Only use the device within the operating
conditions specified, otherwise the performance
of the device could be compromised. See section
8.5 – Operating Conditions.
• Do not place any part of the device or
accessories within 30 cm (12 in.) of any portable
mobile radio frequency communication
equipment. The device complies with the
electromagnetic compatibility requirements of
IEC 60601-1-2 and the device may aect or be
aected due to the eects of electromagnetic
interference, in certain circumstances. If
interference should occur, try moving your
device or the equipment causing interference.
Alternatively, consult your healthcare provider.
• Do not use accessories or power cables which
are not provided, or recommended, by Fisher &
Paykel Healthcare. This could result in increased
electromagnetic emissions or decreased
electromagnetic immunity.
• California residents please be advised of the
following, pursuant to Proposition 65: This
product contains chemicals known to the State
of California to cause cancer, birth defects and
other reproductive harm. For more information,
please visit: www.fphcare.com/prop65.
• This device is not repairable and does not
contain any repairable parts. Please refer queries
relating to the device or accessories to your
healthcare provider.
Page 7

5
1.4 CAUTIONS
1.4.1 To prevent water damage to
the device:
• Do not use if the water chamber is damaged.
• Do not fill the chamber housing with water. Only
place water in the water chamber.
• Do not fill the water chamber above the
maximum water-level line.
• Replace water before each use.
• Do not use the device without the chamber seal
fitted to the water chamber.
• Do not fill the water chamber while it is in the
device.
• Empty the water chamber before transporting or
packing the device.
• Do not use the device with an empty water
chamber unless the humidity level is set to 0.
• Do not add aromatic-based or scented oils to the
water chamber as these oils can cause damage
to the device.
1.4.2 General:
• Changes or modifications not expressly
approved by Fisher & Paykel Healthcare voids
the user’s authority to operate the device.
• Position the device so the power cord connection
to the power supply is easily accessible and able
to be disconnected.
• Do not use USB drives with the device which are
not provided by Fisher & Paykel Healthcare. Use
of USB drives other than the InfoUSB may cause
data corruption. Do not attempt to change the
directories or view the data without software
distributed or designed for use with the device.
• Replace the device and accessories if there is
any sign of cracking, deformation, discoloration
or leaking. It is recommended that you inspect
the device, breathing tube, water chamber,
chamber seal, outlet seal, air filter and elbow, on
a regular basis after cleaning. See section 6.4 -
Replacement Parts.
• Use distilled water to reduce residue build-up
on the chamber base. This will extend the life of
your water chamber.
1.5 PRECAUTIONS
• The safety and eectiveness of the CPAP
device has not been established in patients
with respiratory failure or chronic obstructive
pulmonary disease (COPD).
• The safety and eectiveness of the autoadjusting positive airway pressure device has
not been established in patients with congestive
heart failure, obesity hypoventilation syndrome,
or central sleep apnea.
1.6 ADVERSE EFFECTS
• Nosebleeds, perforated ear drum, dryness of
the nasopharynx, sinus infection, and middle
ear infection may occur from the use of positive
airway pressure therapy.
Page 8
6
2. GETTING STARTED
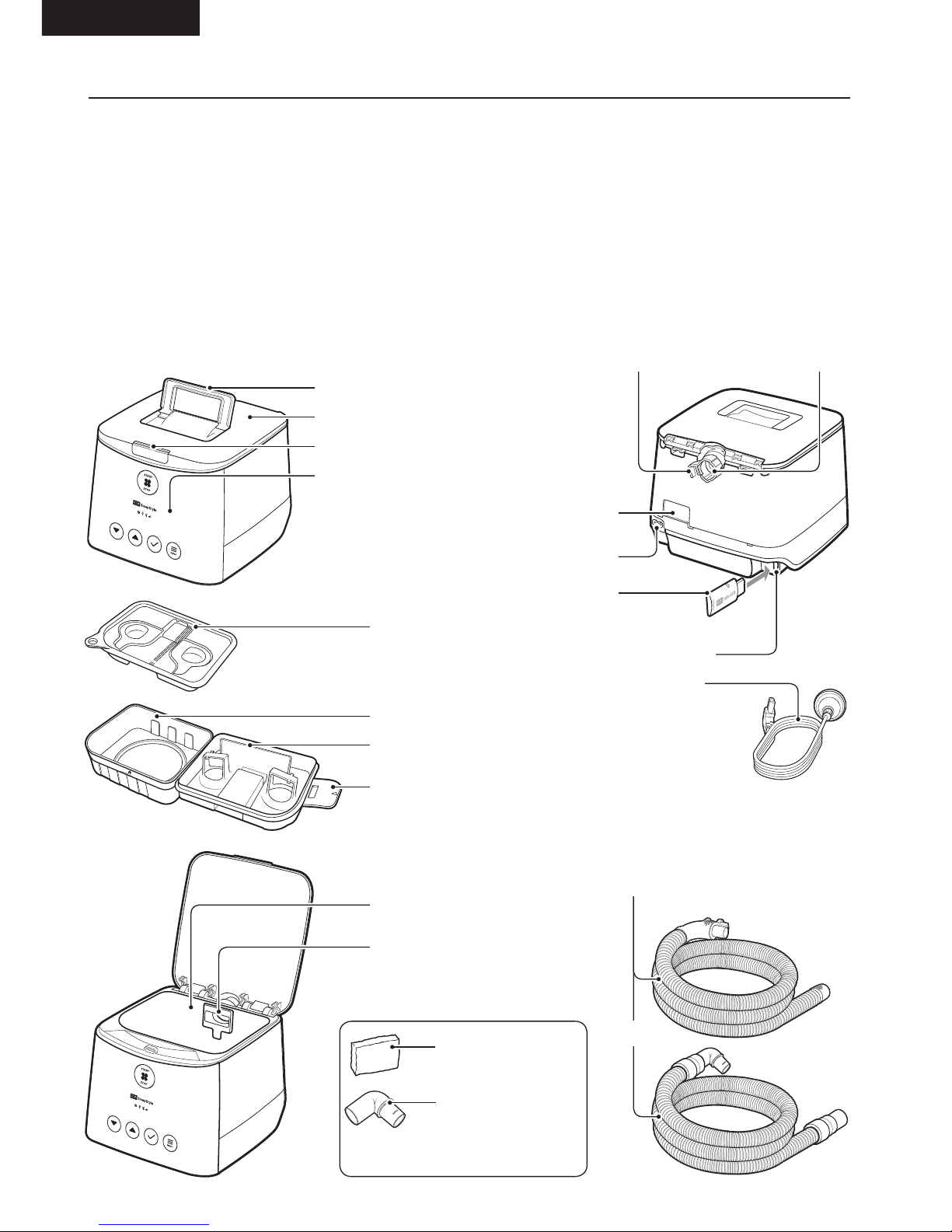
2.1 DEVICE AND ACCESSORIES
1 x Carry-bag
1 x SleepStyle device
1 x Breathing tube
1 x Power cord
1 x F&P SleepStyle Use and Care Guide
1 x F&P SleepStyle Quick Reference Guide
1 x Water chamber
1 x Chamber seal
1 x Outlet seal
1 x F&P InfoUSB (already in InfoUSB port)
1 x Air filter (already in the device)
1 x Spare air filter
1 x Spare elbow (for use with a standard breathing
tube)
Device lid
Lid latch
Display screen
Air filter
Power inlet
F&P InfoUSB
Water chamber
Chamber lid
Chamber seal
Outlet seal
Chamber housing
Handle
Chamber tab
Spare elbow
These are in a bag together
Spare air filter
InfoUSB port
Power cord
Air outlet
or
ThermoSmart breathing tube
or standard breathing tube with
elbow
ThermoSmart connection
Page 9
7
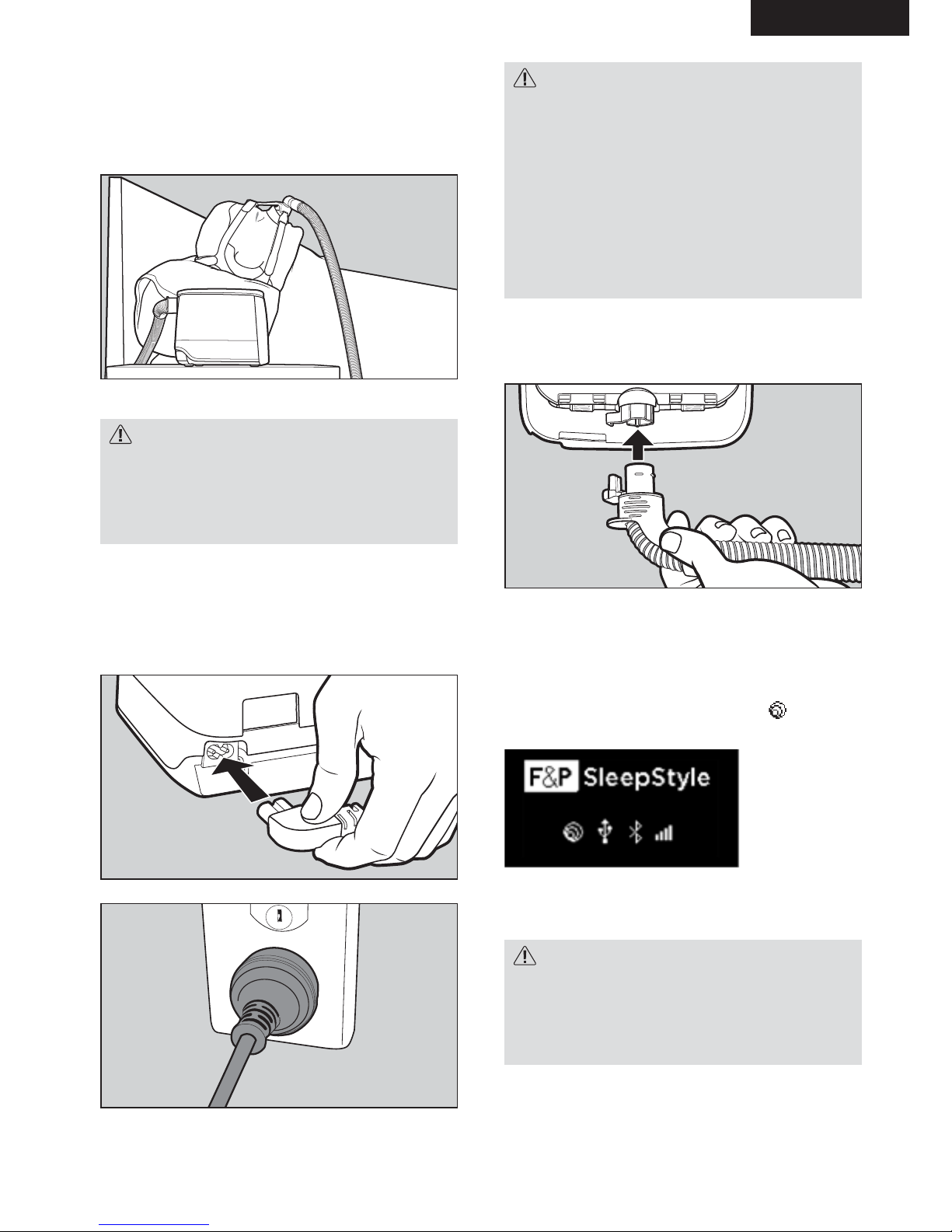
2.2 SETTING UP YOUR DEVICE
1. Place the device below head height on
a stable and level surface, like a bedside
table.
Warnings
To avoid injury, choking, or inhalation of a
foreign object:
Do not place the device above head height to
prevent water from entering the breathing tube.
2. Connect the power cord and the
breathing tube.
Connect the power cord into the power inlet of the
device. Connect the other end of the power cord
into the power supply.
Warnings
To avoid electric shock:
Do not use if the device, power cord, or
accessories are damaged, deformed or cracked.
To avoid choking, or inhalation of a foreign
object:
Ensure the breathing tube and power cord,
including any extension cords, are correctly
positioned so they will not become entangled
with the body or furniture during sleep.
ThermoSmart breathing tube
Connect the ThermoSmart breathing tube into the
air outlet.
Note: Make sure the connectors on the
ThermoSmart breathing tube click into position
with the ThermoSmart connection.
If you have connected the ThermoSmart breathing
tube correctly, the ThermoSmart icon
will
appear on your home screen:
Note: Screen may dier, depending on the model
of the device.
Warnings
To avoid incorrect therapy:
Use the elbow when rotating the ThermoSmart
breathing tube. Incorrect handling may damage
the tube.
Page 10
8
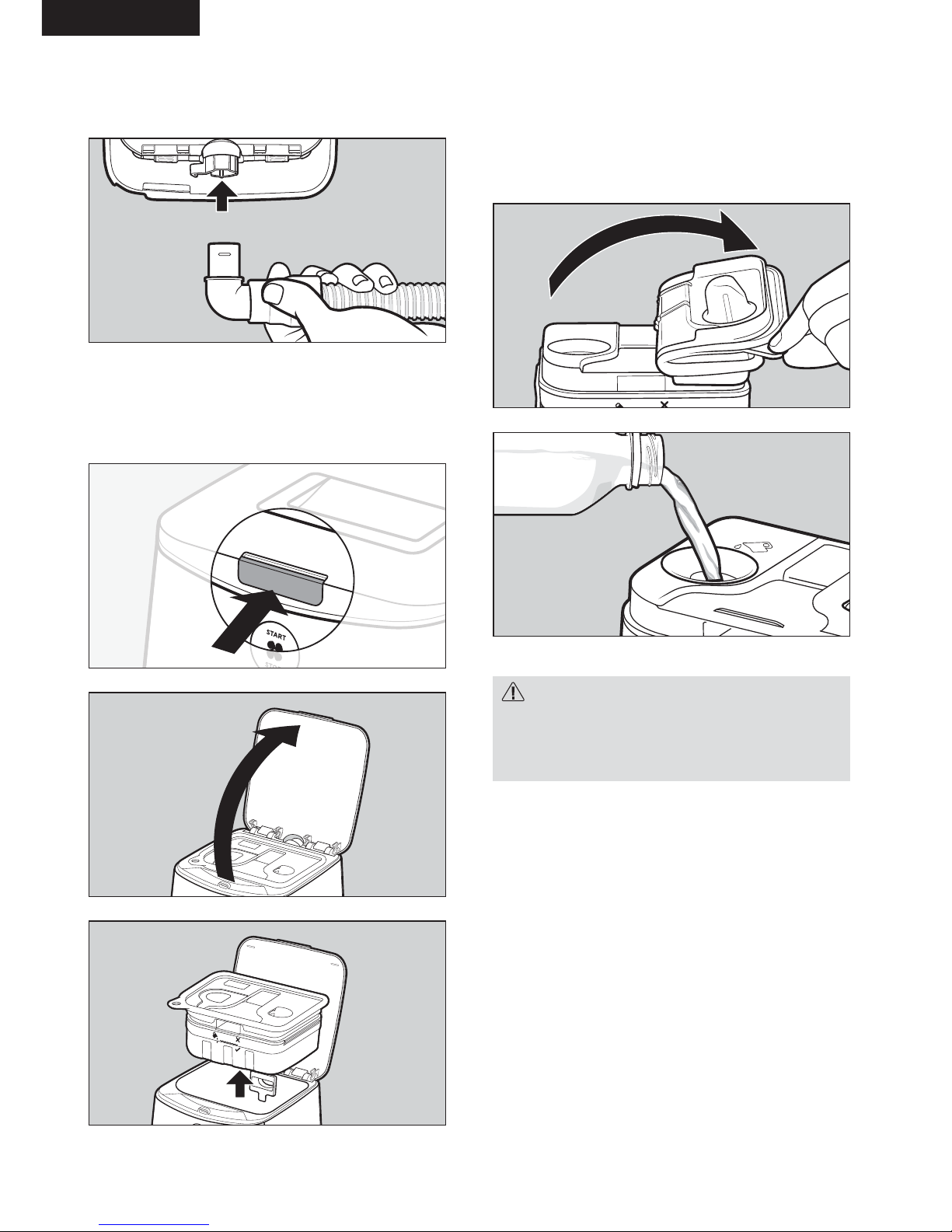
Standard breathing tube
Connect the standard breathing tube with the
elbow into the air outlet.
3. Remove the water chamber from the
device.
Press the lid latch and open the device lid. Take the
water chamber out of the device.
4. Fill the water chamber with water
(i) Peel back the chamber seal on the left-hand
side of the water chamber.
(ii) Fill the water chamber with water up to the
maximum water-level line, as indicated on the
side and inside of the water chamber.
Warnings
To avoid burns:
Do not fill the water chamber with hot water as
this may lead to airway burns.
Page 11
9
Cautions
To prevent water damage to the device:
• Do not use if the water chamber is damaged.
• Do not fill the chamber housing with water.
Only place water in the water chamber.
• Do not fill the water chamber above the
maximum water-level line.
• Replace water before each use.
• Do not fill the water chamber while it is in the
device.
• Do not use the device with an empty water
chamber unless the humidity level is set to 0.
• Do not add aromatic-based or scented oils
to the water chamber as these oils can cause
damage to the device.
General:
Use distilled water to reduce residue build-up
on the chamber base. This will extend the life of
your water chamber.
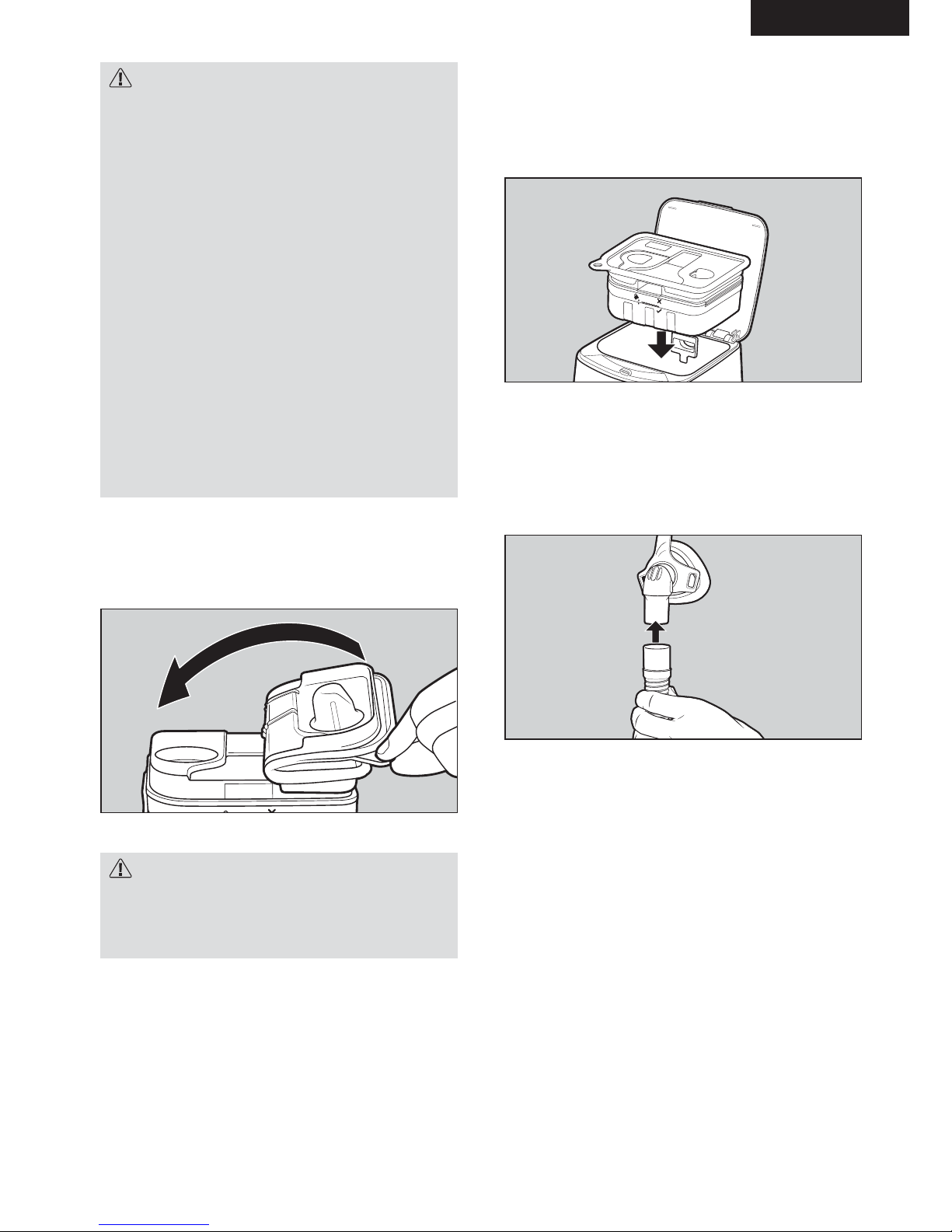
5. Secure the chamber seal.
Unfold the chamber seal back onto the water
chamber. Push down in the finger holds to secure
it in place.
Cautions
To prevent water damage to the device:
Do not use the device without the chamber seal
fitted to the water chamber.
6. Put the water chamber back into the
device.
Place the water chamber back into the device.
Push the device lid down until the lid latch clicks
into position.
7. Connect the mask to the breathing
tube.
Holding the mask and the other end of the
breathing tube, connect the mask swivel firmly into
the breathing tube.
Page 12

10
3. USING YOUR DEVICE
3.1 SCREEN ICONS
When your device is plugged in and switched on,
you will see the home screen appear with up to
four icons. These icons indicate the status of a
setting or accessory, as follows:
ThermoSmart Icon*
Indicates that the ThermoSmart breathing
tube is connected and working correctly
InfoUSB Icon
Indicates that the F&P InfoUSB is connected
and working correctly
Bluetooth® Icon
Indicates that Bluetooth technology is turned
“On” on your device and is working correctly
Modem Icon*
Indicates that modem is turned “On” on your
device and is working correctly
Note: If there is a line through one of these icons, or if there is a gap where an icon usually appears, refer to
section 9.2 - Screen Icons Troubleshooting for more information.
* Not available in all models.
3.2 DEVICE CONTROLS
Down
• Press to decrease a comfort
setting.
• Press to move between
options in a setting.
• Press to show the
“Humidity” setting at any
time
Up
• Press to increase a comfort
setting.
• Press to move between
options in a setting.
• Press to show the
“Humidity” setting at any
time
OK
• Press to make a selection.
• Press to accept an
instruction on the display
screen.
Start/Stop
• Press to start and stop
therapy.
• Press and hold for 3
seconds to start Ramp.
Menu
• Press to enter the Menu at
any time.
• Press to scroll between
settings or data screens.
Page 13

11
3.3 STARTING THERAPY
1. Fit your mask.
Note: Refer to your mask’s Use and Care Guide for
more information on how to fit and remove your
mask.
2. Press Start/Stop
to begin therapy.
The screen below will appear:
3.4 STOPPING THERAPY
1. Remove your mask.
2. Press Start/Stop
to stop therapy.
Note: To reduce condensation, please keep the
device plugged in and switched on at the power
supply after stopping therapy.
The screen below will appear:
Your device will then scroll through your therapy
data screens:
Therapy Hours: The number of hours that you
used your device last night.
Mask Leak: If your mask leak was “Normal” or
“High” last night.
AHI (Apnea Hypopnea Index): The average
number of airway breathing events (apneas and/or
hypopneas) you had per hour last night.
For more information on viewing your therapy
data, see section 4.1 – View Your Therapy Data on
Your Device.
Note: The AHI screen may be restricted by your
healthcare provider.
3.5 STAND-BY MODE
The device will enter stand-by mode after 30
seconds if no button has been pressed on the
device.
The display screen light will dim but will still be
visible to show that your device is still powered on:
Press Down , Up , OK , or Menu to
wake up the device.
3.6 COMFORT SETTINGS
3.6.1 Ramp
Ramp works by gradually increasing to your
prescribed pressure over a 20-minute period.
To start Ramp:
Press and hold Start/Stop
for 3 seconds until
the Ramp symbol appears on the display
screen:
Page 14

12
If you need to restart Ramp, press and hold Start/
Stop for 3 seconds.
Note: If SensAwake is “On” (see section 3.6.4 SensAwake) then you don’t need to start Ramp.
The device will automatically reach your prescribed
pressure when you fall asleep. However, if you feel
the SensAwake pressure is too high, you can use
Ramp.
3.6.2 Humidity
Humidification is the process by which moisture is
added to the air you breathe.
You can set the humidity level from 0 (all droplets
are transparent) to 7 (all droplets are shaded).
To use humidity, you will need to fill your water
chamber with water and ensure the humidity level
is at least 1 (one droplet shaded).
To adjust humidity at any time:
1. Press Down
, Up or Menu .
2. Press Down
or Up to change the level
of humidity.
The device will save your changes and time out
after a period of no interaction. Alternatively,
you can exit this setting by pressing Menu
until you reach the previous screen.
Note: The default humidity level is 5. If using
without a water chamber, or where low power
consumption is required, set the humidity level to
0.
3.6.3 Expiratory relief
Expiratory relief reduces the pressure when
you breathe out, and returns to your prescribed
pressure when you breathe in.
How to set the expiratory relief level:
1. Press Menu to scroll to the “Expiratory
relief”’ setting.
2. Press Down
or Up to change the level
of expiratory relief:
• O (no circles shaded)
• Low (1 circle shaded)
• Medium (2 circles shaded)
• High (3 circles shaded).
Note: Expiratory relief may be restricted by your
healthcare provider.
3.6.4 SensAwake™
We all experience subconscious waking during the
night. When this happens, SensAwake will provide
pressure relief to help ease your return to sleep.
How to turn SensAwake on or o:
1. Press Menu to scroll to the “SensAwake”
setting.
2. Press Down
or Up to move between
“ON” and “OFF”.
Note: SensAwake may be restricted by your
healthcare provider.
Page 15

13
4. VIEWING YOUR THERAPY DATA
4.1 VIEW YOUR THERAPY
DATA ON YOUR DEVICE
Your device records your therapy data for the last
night, last 7 days, and last 30 days, which you can
view at any time.
How to view your therapy data on your
device:
1. Press Menu to scroll to “My Data”.
2. Press Down
or Up to move between
the following options:
• “D” – Day (last night’s therapy data)
• “W” – Week (average over the last 7 days
of therapy data)
• “M” – Month (average over the last 30 days
of therapy data).
3. Press OK
to view the range of therapy data
you would like see.
The device will automatically scroll through the
following therapy data:
Note: If you would like to bypass this automated
scrolling, press Menu
to scroll through the data
screens manually.
4.1.1 Therapy Data:
Therapy hours
Day View: Displays the number of hours that you
used your device last night.
Week View: Displays the average number of hours
that you used your device over the last 7 days.
Month View: Displays the average number of hours
that you used your device over the last 30 days.
Mask leak
Day View: Indicates whether the leak from your
mask last night was “Normal” or “High”.
Week View: Indicates whether, on average, the
leak from your mask was “Normal” or “High” over
the last 7 days.
Month View: Indicates whether, on average, the
leak from your mask was “Normal” or “High” over
the last 30 days.
AHI
Day View: Displays the average number of airway
breathing events you had per hour last night.
Week View: Displays the average number of
airway breathing events you had over the last 7
days.
Month View: Displays the average number of
airway breathing events you had over the last 30
days.
Note: The AHI screen may be restricted by your
healthcare provider.
Page 16

14
4.2 VIEW YOUR THERAPY
DATA ON THE SLEEPSTYLE
APP OR WEBSITE
Your SleepStyle device allows you to view your
therapy data on the SleepStyle App or website.
The SleepStyle App uses Bluetooth wireless
technology to communicate with your device.
You can download the SleepStyle App, available
on the Apple App Store or on Google Play™ for
Android™.
You can install the SleepStyle App on iPhone 6s
Plus, iPhone 6s, iPhone 6 Plus, iPhone 6, iPhone 5s,
iPhone 5c, iPhone 5, or any leading smartphone
with Android.
To pair your SleepStyle device to your
mobile device, follow these steps:
1. Turn on your SleepStyle device. The device will
remain discoverable for a period of 15 minutes.
Make sure your mobile device is within range.
Note: Changing the SleepStyle device’s
Bluetooth setting to “On” will also make it
discoverable for 15 minutes. See below for
instructions on how to change your Bluetooth
setting.
2. Open your mobile device’s Settings menu and
turn on Bluetooth.
Note: You might need to refer to your mobile
phone’s user manual for specific instructions on
how to turn on Bluetooth.
3. Open the SleepStyle App and follow the
instructions on how to register an account.
4. Follow the instructions in the app on how to
pair your mobile device with your SleepStyle
device
Your devices should now be paired. The SleepStyle
App will stay up to date with daily therapy data
from your SleepStyle device as long as Bluetooth is
turned on for both devices.
You only need to do the pairing once. After you
have paired your SleepStyle device to your mobile
device, it will stay paired and will re-connect
automatically until you choose to unpair them.
How to change your Bluetooth setting:
If Bluetooth is “O” on your device, there will be a
line through the Bluetooth
on your home screen:
To change your Bluetooth setting, follow these
steps:
1. From the home screen, hold Menu
for 5
seconds.
2. Press Menu
to scroll to the ‘Bluetooth’
setting.
3. Press Down or Up to change the
setting. Your selection will flash to confirm your
selection.
Page 17

15
5. UPLOADING YOUR THERAPY DATA
5.1 MODEM
If your SleepStyle device has a cellular modem,
therapy data will automatically upload to your
healthcare provider. This will occur as long as your
SleepStyle device is turned on at the power supply.
Only your healthcare provider has access to this
data.
Note: The cellular modem is not available in all
models. To identify whether your SleepStyle device
has a cellular modem, look for the modem icon
on the front of your device. If your device has a
cellular modem, and modem is turned on, ensure
that the device is placed at least 20 cm (8 in.) away
from your body while in use.
How to change the modem setting:
Your modem should remain “On” so that your
therapy data will upload to your healthcare
provider.
If you need to change your modem setting, follow
these steps:
1. From the home screen, hold Menu
for 5
seconds.
2. Press Menu
to scroll through the screens
until you reach the “Cellular Modem” setting.
3. Press Down or Up to change the
setting. Your selection will flash to confirm your
selection.
If you have turned the modem “O”, it will turn
back on automatically after 3 days.
5.2 F&P InfoUSB™
The InfoUSB automatically stores your therapy
data. To ensure your therapy data is recorded to
the InfoUSB, you will need to make sure that the
InfoUSB is in the InfoUSB port.
If the InfoUSB is connected correctly, the InfoUSB
icon
will appear on the device home screen:
You can remove the InfoUSB from the InfoUSB
port if requested by your healthcare provider. You
can then upload your therapy data, or take your
InfoUSB with you when you visit them next, or post
the InfoUSB to them.
If your healthcare provider updates your
prescription or device settings on the InfoUSB,
these changes will automatically transfer to your
device when the InfoUSB is next inserted into the
InfoUSB port.
Note: The InfoUSB icon
will not appear on the
display screen while it is in stand-by mode. To
check that the InfoUSB is connected correctly,
press any button to wake up the device. You should
see the InfoUSB icon
on screen.
Cautions
General:
Only use the InfoUSB with the device. Use of any
other USB drives may cause data corruption.
Do not attempt to change the directories or
view the data without software distributed or
designed for use with the device.
Page 18

16
InfoUSB application
The InfoUSB application allows you to upload your
therapy data to your healthcare provider in 5 easy
steps.
1. Insert the InfoUSB into a computer’s
USB port
When requested by your healthcare provider,
remove the InfoUSB from your device and insert
it into the USB port of a computer. A small light
illuminates when connected to your computer.
If the light does not illuminate, please turn the
InfoUSB around or make sure that it is inserted
fully into the USB port.
Note: To avoid getting computer viruses on the
InfoUSB, keep your computer’s anti-virus software
up-to-date and do not use the InfoUSB to transfer
and store files from your computer.
2. Install InfoUSB application
From the Mac App Store
Launch the Mac App Store and search for the
InfoUSB app. Install this free application. Upon
successful installation, open Launchpad and then
open the InfoUSB app.
Note: A Mac running OS X 10.8 or later with a USB
port and an internet connection are required.
From the Windows® Store
Launch the Windows Store and search for the
InfoUSB app. Install this free application. Upon
successful installation, open the app.
A PC or tablet running Windows 8 or later with a
USB port and an Internet connection are required.
From the InfoUSB
If you cannot access the Windows Store, click
on the Start button and open “My Computer”.
Navigate to the drive called “FPHCARE”. Open
this folder and double-click on the Setup.exe file.
Follow the on-screen instructions.
Note: A PC running a Windows operating system
with a USB port and an internet connection are
required.
3. Data transfer
Upon detection of an InfoUSB in your computer,
the message below will appear:
Enter your Date of Birth and select the Upload
button. Ensure that your computer is connected
to the internet for successful data transfer to your
healthcare provider.
4. Confirmation
After the data has been sent successfully, the
confirmation message below will appear. If your
prescription is updated, you will also see the
message “Your healthcare provider has updated
your prescription”.
5. Future data transfer
Remove the InfoUSB from your computer and
place it back into the InfoUSB port of your device.
You can now use your device.
The next time you need to upload your therapy
data to your healthcare provider, simply insert the
InfoUSB into your computer. The message in Step
3 above will automatically appear.
Page 19

17
6. CARING FOR YOUR DEVICE
6.1 DISASSEMBLY FOR
CLEANING
Breathing tube
ThermoSmart breathing tube
1. Hold the plastic connector and gently pull it
away from the device.
2. Hold both the mask end of the tube and the
mask swivel and gently pull them apart.
Standard breathing tube with elbow
1. Hold the elbow and gently pull it away from the
device.
2. Hold both the mask end of the tube and the
mask swivel and gently pull them apart.
Water chamber and chamber seal
1. Press the lid latch and open the device lid.
2. Take the water chamber out of the device.
3. Remove the chamber seal from the top of the
water chamber and put aside.
4. Lift the tab on the side of the water chamber
and lift the chamber lid to open.
Page 20

18
Outlet seal
1. Grip the outlet seal tab.
2. Gently pull the outlet seal out of the device.
6.2 CLEANING YOUR DEVICE
AND ACCESSORIES AT HOME
Cleaning your device and accessories can help
extend their life and ensure that you continue to
receive eective therapy. Below is information on
when and how to clean the device and accessories.
Refer to your mask’s Use and Care Guide on how
to clean your mask.
Warnings
To avoid electric shock:
Do not use bleach, alcohol, or cleaners with
citrus or other natural oils. These substances
may degrade the device and accessories.
To avoid incorrect therapy:
Only clean the device and accessories according
to the cleaning instructions below.
Cautions
General:
Replace the device and accessories if there is
any sign of cracking, deformation, discoloration
or leaking. It is recommended that you inspect
the device, breathing tube, water chamber,
chamber seal, outlet seal, air filter and elbow, on
a regular basis after cleaning. See section 6.4 -
Replacement Parts.
6.2.1 Wash after each use
The following accessories should be cleaned after
each use:
• Breathing tube
• Water chamber
• Chamber seal.
Breathing tube
Note: The elbow on the standard breathing tube
can remain attached when washing after each use.
1. Hand-wash the breathing tube in a tub of
warm, soapy water with a mild dishwashing
detergent. Ensure that all visible soil is
removed.
2. Rinse the breathing tube thoroughly in a tub
of clean water for 30 seconds. Ensure that all
soap residue has been removed.
3. Repeat the rinsing process again, using clean
wate r.
4. Hang the breathing tube, with both ends
pointing to the floor, to dry away from direct
sunlight or heat e.g. heated towel rails.
Note: If dirt remains inside the breathing tube after
rinsing, use a soft, non-metallic brush to remove it.
Rinse the tube again. If the dirt cannot be removed,
the breathing tube should be replaced.
Warnings
To avoid incorrect therapy:
Do not clean or disinfect the ThermoSmart
breathing tube with hot water. This may cause
deformation of the tube and reduce therapeutic
pressure.
Water chamber and chamber seal
1. Hand-wash the water chamber and chamber
seal in a tub of warm, soapy water with a mild
dishwashing detergent. Ensure that all visible
soil is removed.
2. Rinse the water chamber and chamber seal
thoroughly in a tub of clean water for 30
seconds. Ensure that all soap residue has been
removed.
3. Repeat the rinsing process again, using clean
wate r.
4. Leave the parts to dry out of direct sunlight or
heat before reassembling.
Note: The use of distilled water is recommended
during therapy to reduce mineral deposits and
stains. Should mineral deposits occur, you can
reduce these by soaking the water chamber for
10 minutes in a solution of 1 part white vinegar
to 2 parts water. Empty the solution and rinse
thoroughly with clean water. Repeat the rinsing
process again, using clean water. Leave to dry out
of direct sunlight or heat before reassembling.
Page 21

19
6.2.2 After 7 days’ use
The device and accessories below should be
washed after 7 days’ use:
• Outlet seal
• Elbow
• Device.
Note: Once every 7 days, you can clean the water
chamber, chamber seal, and outlet seal in a
domestic dishwasher. Place the water chamber
on the top shelf of the dishwasher and ensure the
chamber seal and outlet seal are placed in a secure
location.
Outlet seal and elbow
1. Disconnect the elbow from the standard
breathing tube.
2. Hand-wash the outlet seal and elbow in a tub
of warm, soapy water with a mild dishwashing
detergent. Ensure that all visible soil is
removed.
3. Rinse the outlet seal and elbow thoroughly in a
tub of clean water for 30 seconds. Ensure that
all soap residue has been removed.
4. Repeat the rinsing process again, using clean
wate r.
5. Leave out of direct sunlight or heat before
reassembling.
6. Reconnect the elbow to the standard breathing
tube.
Device
1. Turn the device o at the power supply, then
remove the power cord from the rear of the
device.
2. Wipe the exterior and chamber housing of
the device with a clean, damp (not dripping
wet) cloth and warm, soapy water using a mild
dishwashing detergent.
3. Leave to dry out of direct sunlight or heat.
Warnings
To avoid electric shock:
• Do not pull on the power cord as it may
become damaged.
• Do not immerse the device in water or any
other liquid.
6.3 REASSEMBLY OF THE
DEVICE
Once the parts you have cleaned are dry, you can
reassemble the parts.
Breathing tube
ThermoSmart breathing tube
1. Hold the end of the breathing tube with the
electrical connectors and push it into the air
outlet of the device.
Note: Make sure the connectors on the
ThermoSmart breathing tube click into position
with the ThermoSmart connection.
2. Holding the mask and the other end of the
breathing tube, push the mask swivel firmly
into the breathing tube.
Standard breathing tube with elbow
1. Hold the elbow end of the breathing tube and
push it into the air outlet of the device.
2. Hold both the mask end of the breathing tube
and the mask swivel, and push them together.
Outlet seal
Hold the tab on the outlet seal and push it into the
chamber housing inlet. Ensure the tab is sitting flat
against the wall of the chamber housing.
Page 22

20
Water chamber and chamber seal
1. Close the chamber lid. Press the chamber tab down until it clicks into place.
2. Fill the water chamber with water through either of the filling holes in the top.
3. Secure the chamber seal back onto the water chamber by pressing down in the finger holds. Ensure it is
sitting flat and seals the holes on the chamber lid.
4. Place the water chamber back into the device.
6.4 REPLACEMENT PARTS
Below is a list of replacement parts that are available. Contact your healthcare provider to order these.
Product code Description
900SPS100 Water chamber
900SPS101 Chamber seal
900SPS111 Air filter (single)
900SPS110 Air filters (2-pack)
900SPS120 ThermoSmart breathing tube*
900SPS121 Standard breathing tube with elbow*
900SPS122 Elbow (for use with a standard breathing tube)
900SPS140 Device lid
900SPS141 Outlet seal
900SPS142 Carry-bag
900SPS160 North American power cord
900SPS161 Australasian power cord
900SW101 F&P InfoUSB
* Applied Parts – to fit 22 mm (0.86 in.) Conical Connector.
Warnings
To avoid injury:
Do not use breathing tubes, parts, and accessories that are not distributed for use with this device or
recommended by Fisher & Paykel Healthcare.
General:
Do not use accessories or power cables which are not provided, or recommended, by Fisher & Paykel
Healthcare. This could result in increased electromagnetic emissions or decreased electromagnetic
immunity.
Page 23

21
6.4.1 Air filter
The air filter is located at the rear of the device. Replace the air filter at least once every 3 months, or more
frequently if it becomes blocked with dirt or dust. To replace the air filter, please follow the instructions below.
Warnings
To avoid choking, or inhalation of a foreign object:
Do not use the device without the recommended air filter fitted. This will reduce dust or particles entering
the device and breathing tube.
1. To remove, pinch the air filter with your fingers and pull it out of the device.
2. Hold onto the short side of the new air filter. Push into the device so there are no gaps.
Page 24

22
7. TRAVELING WITH YOUR DEVICE
7.1 THINGS TO REMEMBER BEFORE YOU TRAVEL
The device has a universal voltage feature that allows it to operate on any domestic AC mains voltage
(between 100 and 240V AC). With the use of the appropriate pin/plug adapter the device can operate in
most countries.
Below is a checklist of what to take with you when you travel:
Carry-bag Power cord
SleepStyle device Air filter
Water chamber (empty) Outlet seal
Chamber seal F&P SleepStyle Use and Care Guide
F&P InfoUSB Mask
ThermoSmart breathing tube or standard
breathing tube with elbow
You may also need:
Extension cord Plug adapter
Cautions
To prevent water damage to the device:
Empty the water chamber before transporting or packing.
Note: The device is not certified for use on an aircraft. Confirm with your airline whether you can take the
device with you as carry-on luggage.
Page 25

23
8. SPECIFICATIONS
8.1 SLEEPSTYLE DEVICE MODELS AND FEATURES MATRIX
Performance features SleepStyle Auto SleepStyle CPAP
Fully integrated humidifier*
Auto-Adjusting Pressure
ThermoSmart Technology**
SensAwake
Expiratory relief
Central Sleep Apnea detection
Ramp
Auto-Altitude Adjustment
Leak Compensation
Ecacy reporting
Compliance reporting
F&P InfoUSB
Bluetooth wireless technology
Cellular modem*
* Not available in all models.
** In some countries, the ThermoSmart breathing tube needs to be purchased as an accessory to activate
ThermoSmart.
Device model SleepStyle Auto SleepStyle CPAP
Australasia SPSAAA/SPSABA SPSCAA/SPSCBA
North America SPSAAN SPSCAN
Page 26

24
8.2 SYMBOL DEFINITIONS
For safety reasons, refer to the
instructions for use
Catalogue number
Caution Serial number
Consult instructions for use Batch code
Do not use this device with
supplemental oxygen
Humidity range
Fill with water here Temperature range
Maximum water level (do not fill
above the water line)
Protected against ingress of small
objects and water drops
Manufacturer Do not use if package is damaged
Date of manufacture Regulatory Compliance Mark
Date of shelf life expiry Prescription only
Type BF applied part UL Classified mark symbol
Non-ionizing electromagnetic
radiation
Do not discard as regular waste
Class II equipment
8.3 PRODUCT SPECIFICATIONS
Dimensions 144 H x 177 W x 183 D mm (5.7 H x 7.0 W x 7.2 D in.)
Weight 1.7 kg (3.7 lb)
Packed Weight (max.): 2.7 kg (5.9 lb)
Performance Pressure Range:
4 to 20 cmH
2
O/hPa (in the unlikely event of fault conditions, pressure limited
to < 30 cmH2O)
Maximum flow rates
CPAP pressure setting (cmH
2
O) 4 8 12 16 20
Measured flow at patient connection port (L/min) 97 150 175 159 137
Dynamic pressure stability*
BPM Test pressure
4.0
cmH
2
O
8.0
cmH2O
12.0
cmH2O
16.0
cmH2O
20.0
cmH2O
Dynamic pressure stability
(cmH
2
O)
10
± 0.5 ± 0.815
20
BPM - Breaths Per Minute
Page 27

25
Static pressure stability*
Auto-adjusting and fixed pressure
Pressure change (cmH
2
O) at connection port at a
pressure setting of 10 cmH2O
± 0.5
*Pressure measurement including uncertainty: ± (0.04cmH
2
O + 0.026% of reading)
The pneumatic flow path:
7
8
11
12
1 4
10
2 3
5 6
9
1. Air inlet filter 7. Water chamber
2. Ambient temperature sensor 8. Heater plate
3. Flow sensor 9. Heater plate temperature sensor
4. Blower 10. Control system
5. Relative humidity sensor 11. Breathing tube
6 Pressure sensor 12. Mask
Humidity output
Tested at 23 °C (73.4 °F) ambient temperature AH (mgH
2
O/L BTPS)
With ThermoSmart breathing tube
Humidity level 7 > 23
Humidity level 5 > 18
With standard breathing tube
Humidity level 7 > 15
Humidity level 5 > 10
AH – Absolute Humidity
BTPS – Body Temperature Pressure Saturated
Page 28

26
Electrical ratings
Rated supply voltage Rated current input Rated supply frequency
100–115 V 1.2 A (2.5 A max.) 50–60 Hz
220–240 V 1.1 A (2.3 A max.) 50–60 Hz
Gas temperatures Maximum = 38 °C (100 °F)
Noise level Sound pressure level 28 ±1.5 dBA; average sound power level <35 dBA.
Water chamber volume 380 mL up to the maximum water-level line
Standards compliance IEC 60601-1 {Ed 3.1}:2012; IEC 60601-1-2:2014; IEC 60601-1-2:2007;
IEC 60601-1-11:2015; ISO 80601-2-70:2015; ISO 5356-1:2004; ISO 17510-1:2007;
ISO 8185:2007
Cellular modem: UMTS 3G: B1, B2, B5, B6, B8, B19; Maximum power +23 dBm
GSM 2G: 850MHz/900MHz/1800MHz/1900MHz; Maximum power +33 dBm
Bluetooth technology: 2402 – 2480 MHz; Maximum power +6 dBm, GFSK, π/4-DQPSK, 8DPSK
FCC compliance This device has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed
to provide reasonable protection against harmful interference in a residential
installation. This device generates, uses, and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may
cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this
device does cause harmful interference to radio or television reception, which
can be determined by turning the device o and on, the user is encouraged
to try to correct the interference by one or more of the following measures:
• Reposition or relocate the receiving antenna.
• Increase the separation between the device and receiver.
• Connect the device into an outlet on a circuit dierent from that to which
the receiver is connected.
• Consult your healthcare provider or your Fisher & Paykel Healthcare
representative for help.
Data recording The InfoUSB will store up to 5 years of summary ecacy data, 365 days of
detailed ecacy data, and 140 hours of high-resolution pressure, leak and
flow data. Without an InfoUSB, the device’s internal memory is capable of
storing up to 1 year of summary ecacy data, 30 days of detailed ecacy
data, and 20 hours of high-resolution pressure, leak, and flow data.
Service life Device 5 years
Breathing tubes 12 months
Water chamber 12 months
Air filter 3 months
General The patient is an intended operator.
8.4 CLASSIFICATIONS
Mode of operation Continuous operation
Electric shock protection Type BF
Ingress protection IP22
Page 29

27
8.5 OPERATING CONDITIONS
Ambient temperature 5 to 35 °C (41 to 95 °F)
Humidity 15 to 90% RH
Altitude 0 to 3,000 m (0 to 9,000 ft)
Cautions
General:
Only use the device within the operating conditions specified, otherwise the performance of the device
could be compromised.
Note: Above 1,500 m (4,500 ft) the maximum operating pressure will be reduced at high flow rates.
8.6 STORAGE AND TRANSPORT CONDITIONS
The device should always be stored and transported within the following temperatures and humidity ranges.
Temperature -10 °C to 60 °C (14 to 140 °F)
Humidity 15 to 90% RH
Note: The device is immediately suitable for use if transported and stored according to the specified storage
and transport conditions. Refer to section 8.6 – Storage and transport conditions.
8.7 DISPOSAL INSTRUCTIONS
Device disposal instructions
This device contains electronics and a lithium battery. Please do not discard as regular waste.
Dispose of electronics and lithium battery according to local guidelines.
Accessory and spare part disposal instructions
Dispose of mask, breathing tube, water chamber, and other spare parts according to local
guidelines. Place the mask, breathing tube, and water chamber in a waste bag at the end of use
and discard with normal waste.
8.8 SERVICING
Warnings
General:
This device is not repairable and does not contain any repairable parts. Please refer queries relating to the
device or accessories to your healthcare provider.
The device does not require preventative maintenance.
8.9 WARRANTY STATEMENT
Fisher & Paykel Healthcare warrants that the device (excluding consumable items forming part of the
CPAP delivery system), when used in accordance with the instructions for use, shall be free from defects
in workmanship and materials and will perform in accordance with Fisher & Paykel Healthcare’s ocial
published product specifications for a period of 2 years from the date of purchase by the end-user. This
warranty is subject to the limitations and exceptions set out in detail here: www.fphcare.com/sleep-apnea/
cpap-devices/warranty-cpap/
Page 30

28
9. TROUBLESHOOTING
If you feel that your device is not operating correctly, please refer to the following suggestions. If the problem
persists, please consult your healthcare provider. Do not attempt to repair the device yourself.
Warnings
To avoid electric shock:
• Do not modify the device or accessories.
• Do not take apart the device. Taking the device apart, for example by unscrewing the underside of the
device, will damage pressure seals and electrical components.
9.1 DEVICE TROUBLESHOOTING
Problem Possible cause Solution
My therapy won’t start, and
there is no display on the
display screen.
The power cord may not be
plugged in correctly.
Push the power cord connector firmly
to confirm it is inserted correctly into
the power supply and into the rear of
the device.
The power cord may be damaged. If damaged, stop using your device
immediately and contact your
healthcare provider for a replacement
power cord.
Has there been a storm?
Has there been a power outage/
power surge?
Check your circuit breaker or fuse, and
reset as required.
If the display screen does not turn on,
return the device to your healthcare
provider.
My therapy won’t start, but
there is a display on the
display screen.
There may be water in the blower,
preventing it from starting.
Turn o at the power supply and
unplug the device.
Remove the water chamber. Keep
the device lid open and tip the device
upside down to clear the water from
the device.
Place the water chamber back in the
device. Restart the device.
Is there an error message on the
display? If so, there may be a fault
with your device or accessories.
Refer to section 9.3 - Error Messages
on SleepStyle Screen, identify the
error code and take the appropriate
corrective action.
No error message. Turn the device o at the power
supply, wait a few seconds then
reconnect to power. If the problem
continues, contact your healthcare
provider.
Page 31

29
Problem Possible cause Solution
The pressure is fluctuating
or insucient air is being
delivered from the device.
Your mask may not be fitted
correctly, causing leaks.
Ensure your mask is correctly fitted.
Refer to your mask’s Use and Care
Guide for fitting instructions, or
contact your healthcare provider.
The air inlet filter is dirty or the air
inlet has become blocked.
Replace the air filter. Remove any
blockage from the air inlet.
There may be water in the
breathing tube.
Disconnect the breathing tube and
hang with both ends pointing to the
floor until all water in the breathing
tube has been cleared.
The device lid may not be closed
correctly or the chamber seal may
not be fitted to the water chamber
correctly.
Ensure the water chamber is in the
device.
Refer to section 6 - Caring for Your
Device for detailed instructions on
assembly.
The device is noisy. Air is leaking out of the device or
breathing tube.
Make sure:
The device lid has been closed
properly;
The breathing tube and mask are
connected correctly;
There are no air leaks and
condensation in the breathing tube.
Noise changes while you breathe The device adjusts the motor speed
to maintain the correct pressure as
you breathe in and out, this is normal
behavior.
The heater-plate and/
or water chamber base is
warm to the touch, even
though the device isn’t
being used.
This is normal and should not cause concern.
The power supply is located directly underneath the heater-plate. In stand-
by mode, it generates approximately 5 W of power. This may cause the
feeling of warmth.
The water chamber is also fully insulated by the device, which can cause
heat to be retained.
There is a build-up of water
on the heater-plate.
When therapy has stopped, the
device will cool, which may cause
condensation to form on the
heater-plate.
To reduce condensation, please keep
the device plugged in and switched
on at the power supply after stopping
therapy.
Before each use, remove the water
chamber and dry the chamber
housing of the device with a cloth. If
the water build-up becomes excessive,
please contact your healthcare
provider.
Page 32

30
Problem Possible cause Solution
I don’t think my humidifier
is working.
The humidity level is incorrect. Check if the humidity level is above
0. See section 3.6 – Comfort Settings
for more information on changing the
humidity setting.
The water chamber may be empty. Check if there is water in the water
chamber. See section 2.2 - Setting Up
Your Device for instructions on filling
your water chamber.
The ThermoSmart
breathing tube is not
warming up.
The ThermoSmart breathing tube
is not connected to the device
correctly.
Remove the ThermoSmart breathing
tube from the device and reconnect. Make sure that the electrical
connectors click together with the
ThermoSmart connection.
When connected correctly, the
ThermoSmart icon
will appear on
your home screen:
The humidity level is incorrect. Check if the humidity level is above
0. See section 3.6 – Comfort Settings
for more information on changing the
humidity setting.
9.2 SCREEN ICONS TROUBLESHOOTING
Problem Possible cause Solution
The ThermoSmart icon has
a line through it
There may be an error with the
ThermoSmart breathing tube.
You will still be treated and get
humidity, but it may not be optimal.
Remove the ThermoSmart breathing
tube from the device and reconnect. Make sure that the electrical
connectors click together with the
ThermoSmart connection.
When connected correctly, the
ThermoSmart icon
will appear on
your home screen:
If the problem persists, please consult
your healthcare provider.
There is a gap where the
ThermoSmart icon usually
appears.
The ThermoSmart breathing tube
may not be connected correctly.
You may be using a standard
breathing tube.
Consult your healthcare provider for
more information.
Page 33

31
Problem Possible cause Solution
The InfoUSB icon has a line
through it
There may be an error with the
InfoUSB.
You will still be treated, but your
therapy data may not be recorded to
the InfoUSB.
Remove the InfoUSB from the InfoUSB
port and reinsert.
When connected correctly, the
InfoUSB icon
will appear on your
home screen:
If the problem persists, please consult
your healthcare provider.
There is a gap where
the InfoUSB icon usually
appears.
The InfoUSB may not be
connected correctly.
The Bluetooth icon has a
line through it
Bluetooth is turned “O” on your
device.
You will still be treated, but your
therapy data may not be available on
your SleepStyle app.
Refer to section 4.2 - View your
therapy data on the SleepStyle App or
website for instructions on changing
your Bluetooth setting.
Turning Bluetooth o and on again
on your mobile device may resolve
connectivity issues.
If the problem persists, please consult
your healthcare provider.
There may be an error with the
Bluetooth setting.
The modem icon has a line
through it
Modem is turned “O” on your
device.
You will still be treated, but your
therapy data may not be uploaded to
your healthcare provider.
Refer to section 5.1 – Modem for
instructions on changing your modem
setting.
Turning modem o and on again on
your SleepStyle device may resolve
connectivity issues.
If the problem persists, please consult
your healthcare provider.
The modem has failed to connect.
There is a gap where
the modem icon usually
appears.
Modem is not available on your
device model.
Consult your healthcare provider for
more information.
Page 34

32
9.3 ERROR MESSAGES ON SLEEPSTYLE SCREEN
If a fault is detected with your device or its accessories, an error message will appear on the display screen.
Identify the error code in the ranges specified below and follow the appropriate corrective action. If the error
persists or reoccurs, please consult your healthcare provider. Do not attempt to repair the device yourself.
Error codes
between
Description Corrective action
100–199 Your device may not be able to
provide eective therapy.
Your device may have shut down
or may not be able to provide
your prescribed pressure.
Turn the device o at the power supply. Wait 15
seconds, and then turn the device back on at the
power supply to restart the device.
400–499 Humidity may have been disabled. Your device is still safe to use without humidity. You
will still be treated at your prescribed pressure.
Turn the device o at the power supply. Wait 15
seconds, and then turn the device back on at the
power supply to restart the device.
510 or 512 There may be a problem with your
ThermoSmart breathing tube.
You will still be treated and get humidity, but it may
not be optimal.
Try reconnecting your ThermoSmart breathing tube.
When connected correctly, the ThermoSmart icon
will appear on the home screen:
Turn the device o at the power supply. Wait 15
seconds, and then turn the device back on at the
power supply to restart the device.
500–599
(excluding 510
or 512)
The ThermoSmart breathing tube
may have been disabled.
You will still be treated and get humidity, but it may
not be optimal.
Turn the device o at the power supply. Wait 15
seconds, and then turn the device back on at the
power supply to restart the device.
9.4 SLEEPSTYLE APP TROUBLESHOOTING
Try the following steps if you have trouble connecting to, or receiving data from, your SleepStyle device:
1. Ensure that your mobile device has Bluetooth turned on and is close to your SleepStyle device.
2. Check that Bluetooth is “On” on your SleepStyle device. If Bluetooth is “O”, there will be a line through
the Bluetooth icon on your home screen:
Page 35

33
If Bluetooth is “O”, see section 4.2 – View your therapy data on the SleepStyle App or website for
instructions on changing your Bluetooth setting.
3. Open the Bluetooth settings page on your mobile device. Try turning Bluetooth o and then back on
again, then check that your SleepStyle device appears in the paired devices list. If it is not currently
connected, try tapping on it to connect.
4. Turn the power to your SleepStyle device o for 10 seconds and then turn it back on. Re-launch the app,
ensuring that your mobile device is near your SleepStyle device*.
If you have a case or cover on your mobile device, try removing this and repeating the above steps. Ensure
that other potential sources of radio interference are minimized or removed.
If you have tried the above steps and still cannot connect, try deleting the pairing on your SleepStyle device
and starting again:
5. Open the Bluetooth settings page on your mobile device. Find your SleepStyle device in the paired
devices list. Delete the pairing.
6. On your SleepStyle device, hold Menu
for 5 seconds.
7. Press Menu
to scroll through the screens until you reach the “Clear all paired devices?” setting.
8. Press Down or Up to select Yes .
9. Press OK to confirm the selection. Wait for the following screen to appear:
10. Open the Bluetooth settings page on your mobile device and search for new devices. Your SleepStyle
should be shown in the list of discovered devices. Select “SleepStyle” and follow the instructions on your
mobile device.
If the problem continues, please contact your healthcare provider.
*Approximately 30 cm (1 ft).
Page 36

Finland
Tel: +358 (0)405 406618
Fax: +46 (0)8 36 6310
France
Tel: +33 1 6446 5201
Fax: +33 1 6446 5221
Germany
Tel: +49 7181 98599 0
Fax: +49 7181 98599 66
India
Tel: +91 80 4284 4000
Fax: +91 80 4123 6044
Irish Republic
Tel: 1800 409 011
Italy
Tel: +39 06 7839 2939
Fax: +39 06 7814 7709
Japan
Tel: +81 3 5117 7110
Fax: +81 3 5117 7115
Korea
Tel: +82 2 6205 6900
Fax: +82 2 6309 6901
Northern Ireland
Tel: 0800 132 189
Russia
Tel and Fax: +7 495 782 21 50
Spain
Tel: +34 902 013 346
Fax: +34 902 013 379
Sweden
Tel: +46 8 564 76 680
Fax: +46 8 36 63 10
Manufacturer
Fisher & Paykel Healthcare Ltd
15 Maurice Paykel Place
East Tamaki, Auckland 2013
PO Box 14 348, Panmure
Auckland 1741
New Zealand
Tel: +64 9 574 0100
Fax: +64 9 574 0158
Email: info@fphcare.co.nz
Web: www.fphcare.com
Australia (Sponsor)
Fisher & Paykel Healthcare Pty Limited
19-31 King Street, Nunawading,
Melbourne, Victoria 3131.
Tel: +61 3 9879 5022
Fax: +61 3 9879 1598
Austria
Tel: 0800 29 31 23
Fax: 0800 29 31 22
Benelux
Tel: +31 40 216 3555
Fax: +31 40 216 3554
Brazil
Fisher & Paykel do Brasil
Rua Sampaio Viana, 277 cj 21, Paraíso,
04004-000
São Paulo – SP, Brazil
Tel: +55 11 2548 8002
China
代理人/售后服务机构:
费雪派克医疗保健(广州)有限公
司,广州高新技术产业开发区科学城
科丰路31号G12栋301号
电话: +86 20 32053486
传真: +86 20 32052132
Switzerland
Tel: 0800 83 47 63
Fax: 0800 83 47 54
Taiwan
Tel: +886 2 8751 1739
Fax: +886 2 8751 5625
Turkey
Fisher Paykel Sağlık Ürünleri
Ticaret Limited Şirketi,
Alinteri Bulvari 1161/1 Sokak
No. 12-14, P.O. Box 06371 Ostim,
Ankara, Turkey
Tel: +90 312 354 34 12
Fax: +90 312 354 31 01
UK
Fisher & Paykel Healthcare Ltd
Unit 16, Cordwallis Park
Clivemont Road, Maidenhead
Berkshire SL6 7BU, UK
Tel: +44 1628 626 136
Fax: +44 1628 626 146
USA/Canada
Tel: +1 800 446 3908
or +1 949 453 4000
Fax: +1 949 453 4001
REF 185048408 Rev A 2017–02 © 2017 Fisher & Paykel Healthcare Limited
SleepStyle, SensAwake, ThermoSmart, and F&P InfoUSB are trademarks of Fisher & Paykel Healthcare Ltd.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG Inc. and any use of such marks by
Fisher & Paykel Healthcare is under license. Other trademarks and trade names are those of their respective owners.
Android and Google Play are trademarks of Google Inc.
”Made for iPhone” means that an electronic accessory has been designed to connect specifically to iPhone, and has been
certified by the developer to meet Apple performance standards. Apple is not responsible for the operation of this device
or its compliance with safety and regulatory standards. Please note that the use of this accessory with iPhone may aect
wireless performance. Apple, OS X, Mac and iPhone are trademarks of Apple Inc., registered in the U.S. and other countries.
App Store is a service mark of Apple Inc., registered in the U.S. and other countries.
Windows is either a registered trademark or trademark of Microsoft Corporation in the United States and/or other countries.
For patent information, see www.fphcare.com/ip.
 Loading...
Loading...