Page 1

ESA620
Electrical Safety Analyzer
Users Manual
FBC-0028
January 2008, Rev. 2, 3/13
© 2008, 2013 Fluke Corporation. All rights reserved. Specifications are subject to change without notice.
All product names are trademarks of their respective companies.
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one year from the date of
original purchase OR two years if at the end of your first year you send the instrument to a Fluke Biomedical service center
for calibration. You will be charged our customary fee for such calibration. During the warranty period, we will repair or at our
option replace, at no charge, a product that proves to be defective, provided you return the product, shipping prepa id, to
Fluke Biomedical. This warranty covers the original purchaser only and is not transferable. T he warranty does not apply if
the product has been damaged by accident or misuse or has been serviced or modified by anyone other than an authorized
Fluke Biomedical service facility. NO OTHER WARRANTIES, SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE
EXPRESSED OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR
CONSEQUENTIAL DAMAGES OR LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE OR THEORY.
This warranty covers only serialized products and their accessory items that bear a distinct serial number tag. Recalibration
of instruments is not covered under the warranty.
This warranty gives you specific legal rights and you may also have other rights that vary in different jurisdictions. Since
some jurisdictions do not allow the exclusion or limitation of an implied warranty or of incidental or consequ ential damages,
this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or
other decision-maker of competent jurisdiction, such holding will not affect the validity or enforceability of any other provision.
7/07
Page 3

Notices
All Rights Reserved
Copyright 2008, 2013, Fluke Biomedical. No part of this publicat ion may be reproduced, transmitted, transcribed, stored in a retrieval
system, or translated into any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and other printed materials for use in service
training programs and other technical publications. If you would like other reproductions or distribution s, submit a w ritten request to Fluke
Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop unpacking
the instrument. Notify the carrier and ask for an agent to be present while the instrument is unpacked. There are no special unp acking
instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or
broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 850-4608 or 1-440248-9300. In Europe, email techsupport.emea@flukebiomedical.com or call +31-40-2675314.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all pa cking materials in
their original condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not
operate within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local
sales representative.
Page 4
Returns and Repairs
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freigh t-prepaid to our factory location. When you return an
instrument to Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that
you insure your shipment for its actual replacement cost. Fluke B iomedical will not be responsible for lo st shipments or in struments that are
received in damaged condition due to improper packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the follow ing guide for repackaging:
Use a double–walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order
Entry Group at 1-440-498-2560.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service or
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4608 x2564
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-99 FLUKE (1-888-993-5853)
Email: service.status@fluke.com
To ensure the accuracy of the Product is maintained at a high level, Fluke Biomedica l recommends the product be calibrated a t least once
every 12 months. Calibration must be done by qualified personnel. Contact your local Fluke Biomedica l representative for calibration.
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-40-2675300
Email: servicedesk@fluke.nl
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
Page 5

Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomed ical’s manufacturing specifications when it w as
shipped from the factory. Calibration measurements are traceable to the National Institute of Standards and T echnology (NIST). Devices for
which there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in ele ctrical shock hazards or improper
operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made to the
information in this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical
for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The ESA620 Electrical Safety Analyzer is manufactured at Fluke Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Page 6

Page 7

Table of Contents
Title Page
Introduction .................................................................................................................... 1
Safety Information .......................................................................................................... 3
Intended Use .................................................................................................................. 4
Unpacking the Analyzer ................................................................................................. 5
Instrument Familiarization .............................................................................................. 6
Connecting to Line Power .............................................................................................. 10
Connecting a DUT to the Analyzer ................................................................................. 10
Turning the Analyzer On ................................................................................................ 10
Accessing the Analyzer’s Functions ............................................................................... 12
Setting Up the Analyzer .................................................................................................. 12
Selecting 2-Wire or 4-Wire Measurements ................................................................ 13
Setting the Default Measurement Current ................................................................. 13
Setting Polarity Switching Delay ................................................................................ 16
Setting the Display Contrast ...................................................................................... 16
Setting up the Beeper ................................................................................................ 16
i
Page 8

ESA620
Users Manual
Performing Electrical Safety Tests ................................................................................. 17
Setting the Test Standard ......................................................................................... 17
Performing an Accessible Voltage Test (IEC 61010 only) ........................................ 17
Performing Mains Voltage Testing ............................................................................ 18
Performing a Protective Earth Resistance Test ........................................................ 18
Performing an Insulation Resistance Test ................................................................ 23
Performing a Current Consumption Test .................................................................. 29
Performing Leakage Current Tests ........................................................................... 29
Measuring Earth Leakage Current ....................................................................... 30
Performing an Enclosure Leakage Test ............................................................... 32
Performing a Patient Leakage Test ...................................................................... 34
Performing Patient Auxiliary Leakage Tests ......................................................... 36
Performing a Mains on Applied Part Leakage Test ................................................... 38
Performing an Alternative Equipment Leakage Test ................................................. 40
Performing an Alternative Applied Part Leakage Test .............................................. 40
Performing a Direct Equipment Leakage Test .......................................................... 43
Performing a Direct Applied Part Leakage Test ........................................................ 45
Performing a Differential Leakage Current Test ........................................................ 47
Performing an Accessible Leakage Current Test (IEC 61010 only) .......................... 47
Making Point-To-Point Measurements ........................................................................... 49
Measuring Voltage .................................................................................................... 49
Measuring Resistance .............................................................................................. 49
Measuring Current .................................................................................................... 50
Simulating ECG Waveforms .......................................................................................... 50
Controlling the Analyzer Remotely................................................................................. 52
Maintenance .................................................................................................................. 52
Cleaning the Analyzer .................................................................................................... 53
Replaceable Parts ......................................................................................................... 54
Accessories ................................................................................................................... 56
ii
Page 9

Contents (continued)
Specifications ................................................................................................................. 57
Detailed Specifications ................................................................................................... 58
iii
Page 10

ESA620
Users Manual
iv
Page 11

List of Tables
Table Title Page
1. Symbols ................................................................................................................................. 2
2. Top-Panel Controls and Connections .................................................................................... 7
3. Rear-Panel Connections ....................................................................................................... 9
4. Schematic Abbreviations ....................................................................................................... 21
5. Test Names Based on Selected Standard ............................................................................. 29
6. Replaceable Parts ................................................................................................................. 54
7. Accessories ........................................................................................................................... 56
v
Page 12

ESA620
Users Manual
vi
Page 13

List of Figures
Figure Title Page
1. Top-Panel Controls and Connections .................................................................................... 6
2. Rear-Panel Connections ....................................................................................................... 8
3. Analyzer Ready for Operation ............................................................................................... 10
4. DUT Connections to the Analyzer ......................................................................................... 11
5. Leakage Current Menu .......................................................................................................... 12
6. Setup Menu ........................................................................................................................... 13
7. 2-Wire Earth Resistance Measurement Connections ............................................................ 14
8. 4-Wire Earth Resistance Measurement Connection .............................................................. 15
9. Mains Voltage Test Menu ...................................................................................................... 18
10. DUT Ground Resistance Measurement................................................................................. 20
11. Protective-Earth Resistance Measurement Schematic ......................................................... 22
12. Insulation Resistance Measurement ..................................................................................... 23
13. Mains to Protective-Earth Insulation Resistance Test Schematic .......................................... 24
14. Applied Parts to Protective-Earth Insulation Test Schematic ................................................. 25
15. Mains to Applied Parts Insulation Test Schematic ................................................................. 26
vii
Page 14

ESA620
Users Manual
16. Mains to Non-Earth Accessible Conductive Points Schematic ............................................. 27
17. Applied Parts to Non-Earth Conductive Points Schematic .................................................... 28
18. Leakage Current Main Menu ................................................................................................ 30
19. Earth Leakage Current Test Schematic ................................................................................ 31
20. Enclosure Leakage Current Test Schematic ......................................................................... 33
21. Patient Leakage Current Test Schematic ............................................................................. 35
22. Applied Parts Connection Posts Display ............................................................................... 36
23. Patient Auxiliary Leakage Current Test Schematic ............................................................... 37
24. Mains-On-Applied-Parts-Leakage-Current Test Schematic .................................................. 39
25. Alternative Equipment Leakage Current Test Schematic ...................................................... 41
26. Alternative Applied Part Leakage Test Schematic ................................................................ 42
27. Direct Equipment Leakage Test Schematic .......................................................................... 44
28. Direct Applied Parts Leakage Current Test Schematic ......................................................... 46
29. Differential Leakage Current Test Schematic ....................................................................... 48
30. Point-To-Point Function Menu .............................................................................................. 49
31. ECG Waveform Simulation Menu ......................................................................................... 50
32. ECG Monitor Connections .................................................................................................... 51
viii
Page 15

Electrical Safety Analyzer
Introduction
The Fluke Biomedical ESA620 Electrical Safety Analyzer
(hereafter the Analyzer) is a full-featured, compact,
portable analyzer, designed to verify the electrical safety
of medical devices. The Analyzer tests to international
(IEC 60601-1, EN62353, AN/NZS 3551, IEC61010, VDE
751) and domestic (ANSI/AAMI ES1, NFPA 99) electricalsafety standards. The integrated ANSI/AAMI ES1,
IEC60601-1, and IEC61010 patient loads are easily
selectable.
The Analyzer performs the following tests:
• Mains (Line) voltage
• Protective Earth (or Ground Wire) Resistance
• Equipment current
• Insulation resistance
• Earth (Ground) leakage
• Enclosure (Chassis) leakage
• Patient (Lead to Ground) and patient auxiliary
(Lead to Lead) leakage
• Mains on applied parts leakage (Lead isolation)
• Differential leakage
• Direct equipment leakage
• Direct applied part leakage
• Alternative equipment leakage
• Alternative applied part patient leakage
• Accessible part leakage
• Accessible part voltage
• Point to point leakage, voltage, and resistance
• ECG simulation and performance waveforms
1
Page 16
ESA620
Users Manual
Table 1. Symbols
Symbol Description
Risk of Danger. Important information. See Manual.
Hazardous voltage. Risk of electric shock.
Conforms to relevant North American Safety Standards.
Conforms to relevant Australian EMC standards
Conforms to European Union directives
This product complies with the WEEE Directive (2002/96/EC) marking requirements. The affixed label
indicates that you must not discard this electrical/electronic product in domestic household waste. Product
Category: With reference to the equipment types in the WEEE Directive Annex I, this product is classed as
category 9 "Monitoring and Control Instrumentation" product. Do not dispose of this prod uct as unsorted
municipal waste. Go to Fluke’s website for recycling information.
2
CAT II
Measurement Category II is applicable to test and measuring circuits connected directly to utilization points
(socket outlets and similar points) of the low-voltage MAINS installation.
Accessible Functional Earth Terminal
Page 17

Electrical Safety Analyzer
Safety Information
Safety Information
In this manual, a Warning identifies conditions and
procedures that are dangerous to the user. A Caution
identifies conditions and procedures that can cause
damage to the Product or the equipment under test.
Warning
To avoid possible electrical shock or
personal injury, follow these guidelines:
• Use this Analyzer only in the manner
specified by the manufacturer or the
protection provided may be impaired.
• Read the Users Manual before operating
the Analyzer.
• Do not connect the Analyzer to a patient
or equipment connected to a patient. The
Analyzer is intended for equipment
evaluation only and should never be
used in diagnostics, treatment or in any
other capacity where the Analyzer would
come in contact with a patient.
• Do not use the product in wet locations,
around explosive gases or dust.
• Inspect the Analyzer before using it. Do
not use the Analyzer if abnormal
conditions of any sort are noted (such as
a faulty display, broken case, etc.)
• Inspect the test leads for damaged
insulation or exposed metal. Check test
lead continuity. Replace damaged leads
before using the Analyzer.
• When testing, always be sure to keep
your fingers behind the safety barriers
on the test leads.
• Never open the Analyzer's case.
Dangerous voltages are present. There
are no user replaceable parts in the
Analyzer.
• Have the Analyzer serviced only by
qualified personnel.
• Do not use the 15-20A adapter to power
devices rated in excess of 15A. Doing so
may overload the installation.
3
Page 18

ESA620
Users Manual
• The Analyzer must be properly earthed.
Only use a supply socket that has a
protective earth contact. If there is any
doubt as to the effectiveness of the
supply socket earth, do not connect the
Analyzer. Do not use a two-conductor
adapter or extension cord; this will break
the protective ground connection.
• Use extreme caution when working with
voltages above 30 volts.
• Use the proper terminals, functions and
ranges for the test being performed.
• Do not touch metal parts of the device
under test (DUT) during analysis. The
DUT should be considered an electrical
shock hazard when connected to the
Analyzer as some tests involve high
voltages, high currents, and/or the
removal of DUT earth bond.
Intended Use
The Analyzer is intended for use by trained service
technicians to perform periodic inspections on a wide
range of medical equipment. The testing procedures are
menu-driven, and simple to operate.
The Product is an electronic signal source and
measurement device for verifying the electrical safety of
medical devices. The Product also provides ECG
simulation and performance waveforms to verify patient
monitors are performing within their operating
specifications.
The Product provides the following function categories:
• ECG Functions
• ECG-Performance Testing
The intended user is a trained biomedical equipment
technician who performs periodic preventative
maintenance checks on patient monitors in service. Users
can be associated with hospitals, clinics, original
equipment manufacturers and independent service
companies that repair and service medical equipment.
The end user is an individual, trained in medical
instrumentation technology. This Product is intended to
be used in the laboratory environment, outside of the
patient care area, and is not intended for use on patients,
or to test devices while connected to patients. This
Product is not intended to be used to calibrate medical
equipment. It is intended for over the counter use.
4
Page 19

Electrical Safety Analyzer
Unpacking the Analyzer
Unpacking the Analyzer
Carefully unpack all items from the box and check that
you have the following items:
• ESA620
• Getting Started Manual
• Users Manual CD
• Carrying case
• Power cord
• 15 – 20 A Adapter (USA only)
• Test lead set
• TP1 Test probe set (USA, Australia, and Israel only)
• TP74 Test probe set (Europe only)
• Ansur demo CD
• Alligator clip set
• Null post adapter
• Data transfer cable
5
Page 20
ESA620
Users Manual
Instrument Familiarization
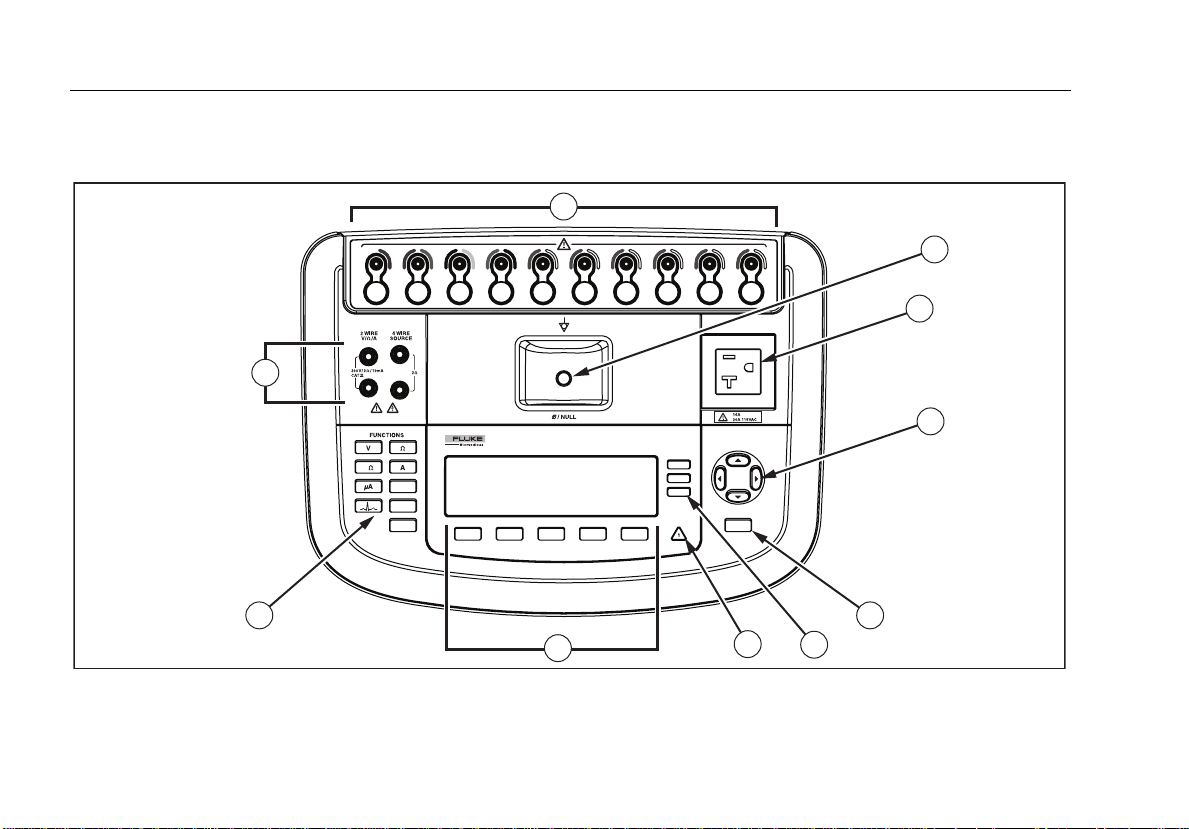
Figure 1 and Table 2 describes the top-panel controls and connections of the Analyzer.
1
RA LL LA RL V1 V2 V3 V4 V5 V6R F L N C1 C2 C3 C4 C5 C6
10
2
3
6
230 VAC
ELECTRICAL SAFETY ANALYZER
ESA620
M
POINT TO
POINT
STANDARDS
SETUP
F1 F2 F3 F4 F5
POLARITY
NEUTRAL
EARTH
TEST
9
8
7
6
4
5
faw02.eps
Figure 1. Top-Panel Controls and Connections
Page 21
Electrical Safety Analyzer
Instrument Familiarization
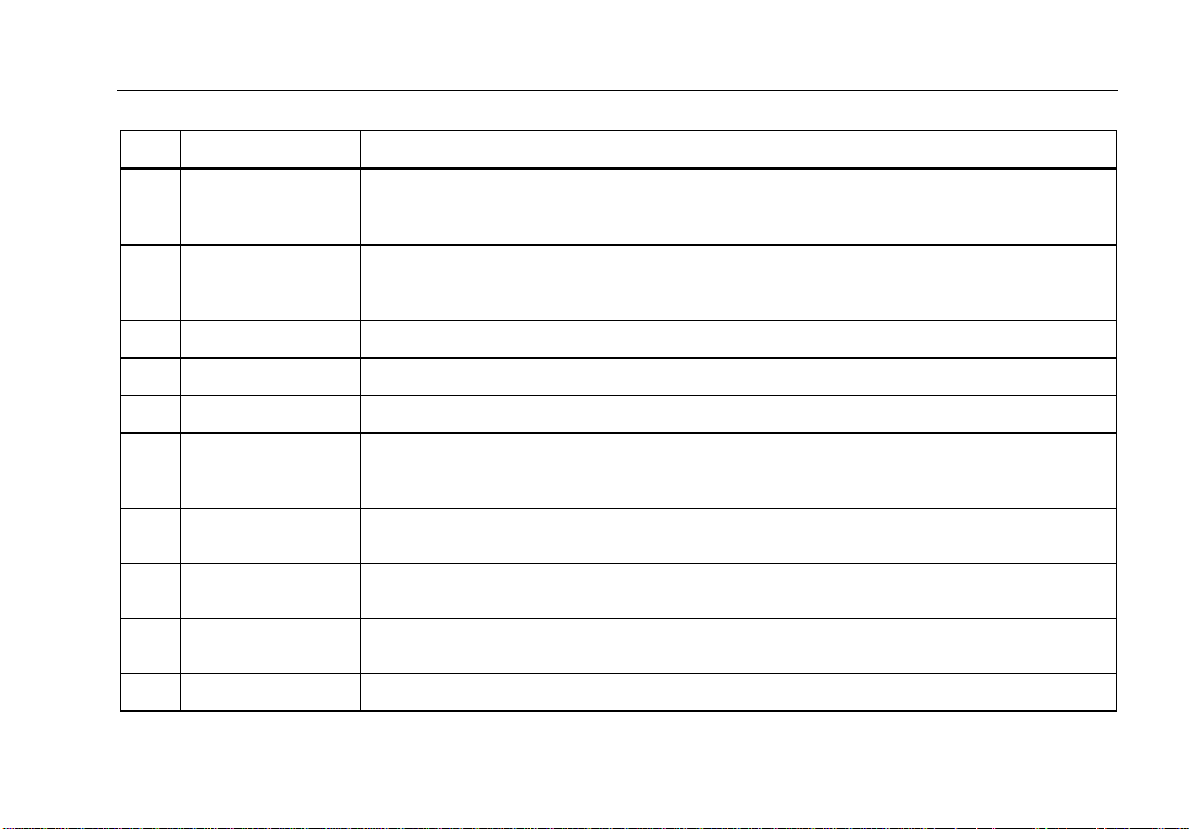
Table 2. Top-Panel Controls and Connections
Item Name Description
ECG/Applied Parts
1
Posts
Nulling Jack
2
Equipment Outlet
3
Navigation Buttons
4
Test Button
5
Equipment Outlet
Configuration
6
Buttons
High Voltage
7
Indicator
Function Softkeys
8
Test Function
9
Buttons
Input Jacks
10
Connection posts for Device Under Test (DUT) leads, like ECG leads. Used to test for
leakage current through leads and to supply ECG signals and performance waveforms to a
DUT.
Connection for zeroing test lead resistance. Use the probe attached to the test lead to put
into the null jack. Use the null post adapter when you use the alligator clip attached to the
test lead.
Equipment outlet, specific to the version of the Analyzer, which provides a DUT connection.
Cursor control buttons for navigating menus and lists.
Initiates selected tests.
Controls the wiring of the equipment outlet. Opens and closes the neutral and ground
connection and reverses the polarity of the neutral and hot connection.
Indicates when high voltage is applied to the ECG/Applied Parts posts or L1 and L2 of the
Test Receptacle.
Keys F1 through F5 are used to select from a number of selections that appear in the LCD
display above each function softkey.
Selects the various Analyzer test functions.
Test lead connectors.
7
Page 22
ESA620
Users Manual
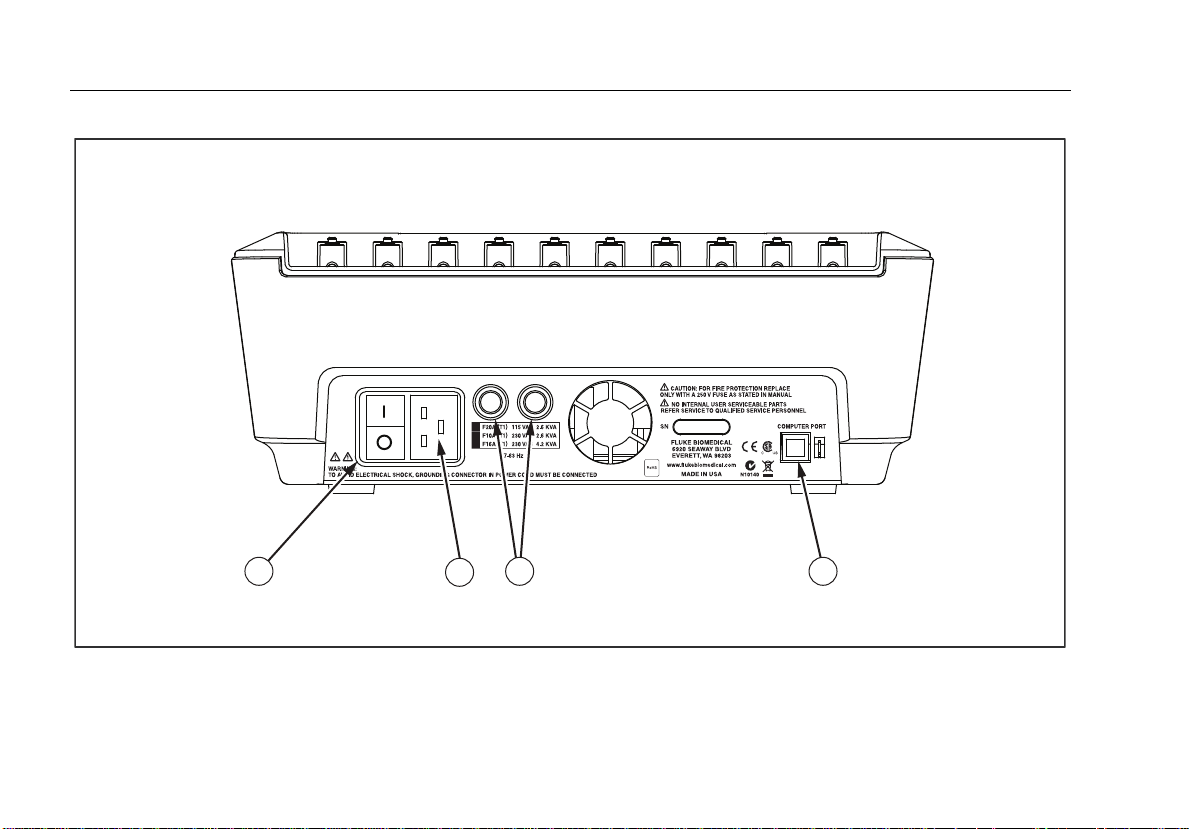
Figure 2 and Table 3 describe the rear-panel connections of the Analyzer.
8
1
2
43
faw01.eps
Figure 2. Rear-Panel Connections
Page 23
Electrical Safety Analyzer
Instrument Familiarization
Table 3. Rear-Panel Connections
Item Name Description
1 AC Power Switch Turns ac power on and off
2 AC Power Input Connector
3 Line Power Fuse Holders The line power fuses.
USB Device Port (B-style
4
connector)
A grounded male three-prong (IEC 320 C20) connector that accepts the
line-power cord.
Digital connection for controlling the Analyzer from a PC or instrument
controller.
9
Page 24

ESA620
Users Manual
Connecting to Line Power
Warning
To avoid shock hazard and for proper
Analyzer operation, connect the factory
supplied three-conductor line power cord to
a properly grounded power outlet. Do not
use a two-conductor adapter or extension
cord; this will break the protective ground
connection.
Connect the Analyzer to a properly grounded three-prong
outlet. The Analyzer will not properly test a DUT when the
ground lead is open.
The Analyzer is intended for use with single-phase,
grounded power. It is not intended for dual, split-phase or
three-phase power configurations. But it can be used with
any power system that supplies the correct voltages for
single-phase and is grounded.
Connecting a DUT to the Analyzer
A Device Under Test (DUT) can be connected in a
number of different ways depending on the device and
the number of connections needed for a full electrical
safety test. Figure 4 shows a DUT connected to the test
receptacle, applied parts posts, and a separate
connection to the DUT’s enclosure or protected earth
ground.
Turning the Analyzer On
Note
To ensure the high voltage indicator is working,
look for it to illuminate during the power-up self
test.
Press the power switch on the back panel so the “I” side
of the ac power switch is depressed. The Analyzer will
perform a series of self tests and then display the
message shown in Figure 3 when the self test has
completed successfully.
faw05.eps
Figure 3. Analyzer Ready for Operation
10
Page 25

Electrical Safety Analyzer
Turning the Analyzer On
To protective earth or enclosure
Figure 4. DUT Connections to the Analyzer
faw03.eps
11
Page 26

ESA620
Users Manual
During the self-test, the Analyzer checks its ac mains
input for proper polarity, ground integrity and voltage
level. The high voltage indicator illuminates briefly during
the self test. If the polarity is reversed, the Analyzer
indicates this condition and allows the polarity to be
reversed internally. If the ground is open, the Analyzer
displays this fault. If the mains voltage is too high or too
low, the Analyzer displays this fault and does not continue
until the supply voltage is corrected and the ESA620
power cycled off and then on again.
Accessing the Analyzer’s Functions
For each test and setup function, the Analyzer uses a
series of menus to access various Analyzer test and
setup variables. As shown in Figure 5, the Analyzer
indicates various leakage current tests along the bottom
of the display. An Exit selection is also indicated as a way
of backing out of the leakage current tests. Pressing a
softkey (F1 through F5) under a specific test will cause
the analyzer to setup for or perform the selected test.
faw04.eps
Figure 5. Leakage Current Menu
In addition to the function softkeys, the Analyzer test
functions may require using the navigation buttons to
select parameters as well. In the example above, the
leakage selection has next to it. This icon indicates the
selection is controlled by pressing or . In this
example, the leakage current measurement is switched
between AC+DC, AC only, or DC only. The applied parts
indicator has on the left end and on the right end.
These icons indicate the use of and to select an
applied part.
The three buttons along the right side of the display
() control the wiring of the Analyzer’s
test receptacle for some electrical tests. The present state
of these three buttons is displayed along the right edge of
the display whenever these controls are active.
Setting Up the Analyzer
There are a number of Analyzer parameters that are
adjusted through a setup function. To access the Setup
menu shown in Figure 6, press .
12
Page 27

Electrical Safety Analyzer
Setting Up the Analyzer
Setting the Default Measurement Current
The default test current selection for the Protective Earth
(Ground Wire Resistance) Test can be set between
200 mA and 25 A ac. To change the default current:
faw13.eps
Figure 6. Setup Menu
The setup parameters have been grouped into six
categories: Instrument, Display, Sound, Instrument Info,
Calibration, and Diagnostics.
Selecting 2-Wire or 4-Wire Measurements
The 2-Wire and 4-Wire resistance measurement setting is
under the Instrument setup functions. To switch between
them:
1. Press the softkey labeled Instrument from the setup
menu to reveal the instrument setup selections.
2. Press the softkey labeled Resistance to switch the
type of resistance measurement between 2-Wire and
4-Wire method. See Figure 7 for 2-wire connections
and Figure 8 for 4-wire connections.
Note
Optional Kelvin Test Leads are available to
make a 4-wire measurement with this Analyzer.
See the Accessories section later in this manual.
3. Press the softkey Back then the softkey labeled Exit
to exit the setup function.
1. Press the softkey labeled Instrument from the setup
menu to reveal the instrument setup selections.
2. Press the softkey labeled Test Current to switch
between 200 mA and 25 A ac.
3. Press the softkey Back then the softkey labeled Exit
to exit the setup menu.
13
Page 28

ESA620
Users Manual
To protective earth or enclosure
14
Figure 7. 2-Wire Earth Resistance Measurement Connection s
faw12.eps
Page 29

Electrical Safety Analyzer
Setting Up the Analyzer
To protective earth or enclosure
Kelvin Test Lead
Figure 8. 4-Wire Earth Resistance Measurement Connection
faw11.eps
15
Page 30

ESA620
Users Manual
Setting Polarity Switching Delay
When switching the polarity of the Analyzer’s test
receptacle, a delay can be set to control the actual switch
time. To set the polarity delay:
1. Press the softkey labeled Instrument from the setup
menu to reveal the instrument setup selections.
2. Press the softkey labeled Polarity Delay to open the
scroll box above the softkey label.
3. Press or to adjust the delay from 0 to 5
seconds in 1 second steps.
4. Press the softkey Back then the softkey labeled Exit
to exit the setup function.
Setting the Display Contrast
There are two methods for setting the display contrast.
From the “Select a Test….” menu or through the setup
menu.
Whenever the Analyzer displays its start-up menu (Select
a test…), pressing or will increase or decrease the
display’s contrast respectively. Press the softkey labeled
Done to exit contrast setup.
Another way to adjust the contrast is through the
Analyzer’s setup menu.
1. Press the softkey labeled Display from the setup
menu.
2. Press the softkey labeled Contrast.
3. Press or to increase or decrease the display’s
contrast respectively.
4. Press the softkey labeled Done to exit contrast
setup.
Setting up the Beeper
In addition to being enabled or disabled, the Analyzer’s
beeper volume can be set as well. To setup the beeper:
1. Press the softkey labeled Sound from the setup
menu.
2. Press the softkey labeled Beeper to switch the
beeper on and off.
3. Press the softkey labeled Volume to open a scroll
box above the softkey label.
4. Press or to increase or decrease the volume
respectively.
5. Press the softkey labeled Done to go back to the
setup menu.
16
Page 31

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing Electrical Safety Tests
The Analyzer is designed to perform a number of different
electrical and performance tests on biomedical
equipment. The following sections describe the various
tests and how to perform them using the Analyzer.
Setting the Test Standard
The Analyzer is designed to perform electrical safety
testing based on a number of different safety standards.
The IEC 60601 is the Analyzer’s default standard. To
select another standard:
1. Press .
2. Press or to scroll through the standard
selections.
3. When the desired standard is displayed, press the
softkey labeled Select.
To exit the standard selection menu without changing the
standards selection, press the softkey labeled Exit.
Some electrical tests may not be applicable for a specific
standard. In these cases, the Analyzer’s menu will not
display the excluded test as a selection.
Performing an Accessible Voltage Test (IEC 61010 only)
Note
The Accessible Voltage test selection will only
appear in the Analyzer’s menu when the
standard is set to IEC61010.
The Accessible Voltage test measures the voltage that
may exist between the DUT’s enclosure and protective
earth. To access the Accessible Voltage test, press .
1. Connect the DUT’s power cord to the Analyzer’s test
receptacle.
2. Connect a test lead from the Analyzer’s 2-WIRE
V/Ω/A jack and an exposed metal part on the DUT’s
enclosure. Any measured voltage is shown in the
Analyzer’s display.
The following outlet conditions apply when performing this
test:
• Normal polarity
• Normal polarity, earth open
• Normal polarity, neutral open
• Reversed polarity
• Reversed polarity, earth open
• Reversed polarity, neutral open
17
Page 32

ESA620
Users Manual
Performing Mains Voltage Testing
The Mains Voltage test measures the voltage on the
mains input through three separate measurements. To
access the Mains Voltage test, press . If the
selected standard is IEC61010, then an additional step is
required. Press the softkey labeled Mains Voltage. The
Mains Voltage test menu is shown in Figure 9.
faw14.eps
Figure 9. Mains Voltage Test Menu
Press each function softkey to perform each of the three
measurements: Live to neutral, neutral to earth, and live
to earth.
Note
Power to the test receptacle is off during the
Mains Voltage test.
Performing a Protective Earth Resistance Test
The Protective-Earth-Resistance test measures the
impedance between the Analyzer’s test receptacle’s PE
terminal and the exposed conductive parts of the DUT
that are connected to the DUT’s Protective Earth.
Prior to conducting any leakage tests with the Analyzer, it
is best to test the integrity of the ground connection
between the Analyzer’s test receptacle ground and the
DUT’s Protective earth ground or enclosure with this test.
To access the Protective Earth (Ground Wire Resistance)
Test menu press .
Note
The DUT is powered off for this test.
The Protective-Earth (Ground Wire) resistance
measurement can be taken using either a 2-Wire or 4Wire resistance measurement. To select between the two
measurement methods, refer to the “Selecting 2-Wire or
4-Wire Measurements” section.
18
Page 33

Electrical Safety Analyzer
Performing Electrical Safety Tests
To perform a protective-earth resistance test:
1. Ensure the power cord from the DUT is plugged into
the Analyzer’s test receptacle.
2. Press to reveal the resistance function menu.
3. Connect one end of a test lead to the 2-WIRE V/Ω/A
jack as shown in Figure 7. A low resistance reading
is required to confirm a good ground connection
through the power cord. Refer to the appropriate
electrical safety standard for the specific limit value to
be followed.
For the 4-Wire resistance measurement, skip steps 4
and 5.
4. Connect the other end of the test lead to the nulling
jack in the middle of the top panel of the Analyzer.
Note
Use the supplied null post adapter when you null
the test lead with the alligator clip.
5. Press the softkey labeled Zero Leads. The Analyzer
zeroes out the measurement to cancel the test lead
resistance.
6. Connect the test lead coming from the 2-WIRE V/Ω/A
jack to the DUT enclosure or protective earth
connection. For a 4 Wire measurement, make
another test lead connection from the RED 4-Wire
Source jack to the same DUT or protective earth
connection the other lead is on as shown in Figure 8.
The optional Kelvin Lead Set is designed specifically
for 4-Wire resistance measurements. See the
Accessories section for ordering information.
The procedure changes at this point depending on which
of the two test currents are selected for this test.
For testing with a 200 mA test current:
1. If not already selected, press the softkey labeled
Low.
2. The measured resistance is displayed as shown in
Figure 10 after the DUT connection(s) is/are made.
19
Page 34

ESA620
Users Manual
For testing with a >10 A test current:
3. If not already selected, press the softkey labeled
High.
4. Press to apply the current to the DUT. Test
current is applied until a stable reading is taken
(approximately three seconds).
5. The measured resistance is displayed.
faw06.eps
Figure 10. DUT Ground Resistance Measurement
A low resistance reading is required to confirm a good
ground connection through the power cord. Refer to the
appropriate electrical safety standard for the specific limit
value to be followed.
Figure 11 shows the electrical connections between the
Analyzer and the DUT. Table 4 lists the abbreviations
used in the schematics and their descriptions.
20
Page 35

Electrical Safety Analyzer
Performing Electrical Safety Tests
Table 4. Schematic Abbreviations
Abbreviation Meaning
MD Measuring Device
FE Functional Earth
PE Protective Earth
Mains Mains Voltage Supply
L1 Hot Conductor
L2 Neutral Conductor
DUT Device Under Test
DUT_L1 Device Under Test hot conductor
DUT_L2 Device Under Test neutral conductor
DUT_PE Device Under Test protective earth line
REV POL Reversed mains supply polarity
LEAD GND Lead to ground, used in Patient leakage test
MAP Mains on Applied Part
MAP REV Reverse mains on applied part source voltage
PE Open Open protective earth
Test Voltage
21
Page 36

ESA620
Users Manual
DUT_L1
DUT_L2
DUT_PE
MD
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST LEAD
Figure 11. Protective-Earth Resistance Measurement S ch ematic
faw26.eps
22
Page 37

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing an Insulation Resistance Test
The five insulation resistance tests take measurements
on mains (L1 & L2) to Protective earth, applied parts to
Protective earth, mains to applied parts, mains to nonearthed accessible conductive points, and applied parts
to non-earthed accessible conductive points.
To access the Insulation Resistance Test menu,
press .
All Insulation Resistance Tests can be performed using
500 or 250 volts dc. To change the test voltage from the
Insulation Resistance Test menu, press the softkey
labeled More. Pressing the softkey labeled Change
Voltage will cause the test voltage to toggle between 250
and 500 volts dc.
Note
Exiting and re-entering the Insulation Resistance
Test menu causes the test voltage to return to its
default value of 500 volts dc.
faw15.eps
Figure 12. Insulation Resistance Measurement
As shown in Figure 12, three of the five tests are shown
over function soft keys F1 through F3. To access the
other two tests or test voltage selection, press the softkey
labeled More. The softkey labeled Back will move the
menu back up to the top-level insulation resistance test
menu.
After selecting one of the tests by pressing the
appropriate softkey, press to apply the selected
voltage to the DUT and take the resistance measurement.
Figure 13 through 17 shows the electrical connections
between the Analyzer and DUT for the five insulation
resistance tests.
Note
The DUT is powered off for this test.
23
Page 38

ESA620
Users Manual
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
MD
DUT_L1
DUT_L2
DUT_PE
Figure 13. Mains to Protective-Earth Insulation Resistance Test Schematic
faw17.eps
24
Page 39

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
DUT_PE
MD
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
Figure 14. Applied Parts to Protective-Earth Insulation Test Schematic
faw18.eps
25
Page 40

ESA620
Users Manual
MD
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
Figure 15. Mains to Applied Parts Insulation Test Schematic
faw19.eps
26
Page 41

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
MD
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST
LEAD
Figure 16. Mains to Non-Earth Accessible Conductive Points Schematic
faw20.eps
27
Page 42

ESA620
Users Manual
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST
LEAD
MD
Figure 17. Applied Parts to Non-Earth Conductive Points Schematic
faw21.eps
28
Page 43

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Current Consumption Test
To measure the current consumed by the DUT, press
. The Analyzer displays the current flowing through
the mains connections of the test receptacle.
Performing Leakage Current Tests
The Analyzer measures leakage current for a number of
different DUT configurations. In addition to the leakage
found on the enclosure and the earth connection, the
Analyzer can measure leakage on each connected
applied part and combinations of connected applied parts.
Table 5. Test Names Based on Selected Standard
IEC60601 AAMI/NFPA 99
Protective Earth Resistance Ground Wire Resistance
Earth Leakage Current Ground Wire Leakage Current
Touch or Enclosure Leakage Current Chassis Leakage Current
Patient Leakage Current Lead to Ground Leakage Current
Patient Auxillary Leakage Current Lead to Lead Leakage Current
Mains on Applied Part (MAP) Leakage Current Isolation Leakage Current
Which leakage tests are available depends on which
standard is selected. See the “Selecting the Test
Standard” section earlier in this manual to change the
standard the Analyzer is using.
The leakage current examples in this manual are those
found under the IEC 60601 standard. Table 5 lists six
leakage current tests that have different names based on
which standard is selected.
Press to access the leakage current main menu
shown in Figure 18.
29
Page 44

ESA620
Users Manual
faw16.eps
Figure 18. Leakage Current Main Menu
Note
The display shown in Figure 18 is the main
leakage current menu when IEC60601 is the
selected standard.
All leakage currents, with the exception of Mains on
Applied parts (Lead Isolation), are displayed in one of
three ways: AC+DC, AC Only, or DC only. The initial
result is displayed in the appropriate parameter based on
the standard selected. To change the displayed
parameter, press or . The present measurement
method is displayed in the upper left corner of the display
while leakage current tests are conducted.
Measuring Earth Leakage Current
Note
The Earth (or Ground Wire) Leakage test is
available for all standards except IEC 62353 and
IEC 61010.
To measure the current flowing in the DUT’s protective
earth circuit, press the softkey labeled Earth (pending the
standard) from the leakage current main menu. Figure 19
shows the electrical connections between the Analyzer
and the DUT during an Earth Leakage Current Test.
Within the Earth Leakage Current test there are a few
combination measurements that can be performed.
Pressing switches the polarity of the mains voltage
applied to the Analyzer’s test receptacle between Normal,
Off, Reverse, and Off. Pressing opens and closes
the neutral connection to the Analyzer’s test receptacle.
There is no need to open up the test receptacle earth
(ground), since this is done internally during the
measurement.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Reversed Polarity
• Reversed Polarity, Open Neutral
IEC60601-1 specifies that the applied parts should be
connected for this measurement. Enable this
measurement by pressing or which grounds and
ungrounds all applied parts connection posts.
30
Page 45

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
CONDUCTIVE PART
L2
MD
APPLIED PART
DUT_L1
DUT_L2
REV
POL
DUT_PE
Figure 19. Earth Leakage Current Test Schematic
APPLIED
PART
FE
faw27.eps
31
Page 46

ESA620
Users Manual
Performing an Enclosure Leakage Test
Note
The Enclosure Leakage test is only available for
the IEC60601 & ANSI/AMMI ES60601-1,
ANSI/AAMI ES1 1993, and None standard
selections.
The Enclosure Leakage Test measures the current
flowing between the DUT’s enclosure and protective
earth. Figure 20 shows the electrical connections
between the Analyzer and the DUT
To perform an Enclosure (Chassis) Leakage Test:
1. Connect a lead between the Analyzer’s 2-WIRE
V/Ω/A jack and the DUT’s enclosure.
2. Press the softkey labeled Enclosure from the
Leakage Current Test menu.
3. The Analyzer displays the measured current.
The Enclosure Leakage test can be performed with a
number of fault conditions on the test receptacle. Press
to switch the test receptacle between Normal, Off,
Reverse, and Off. Press to open and close the
neutral connection to the receptacle. Press to
open and close the receptacle’s earth connection.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Earth
• Normal Polarity, Open Neutral
• Reversed Polarity
• Reversed Polarity, Open Earth
• Reversed Polarity, Open Neutral
IEC60601-1 specifies that the applied parts should be
connected for this measurement. Enable this
measurement by pressing or which grounds and
ungrounds all applied parts connection posts.
32
Page 47

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
CONDUCTIVE PART
L2
EARTH
MD
APPLIED PART
DUT_L1
DUT_L2
DUT_PE
Figure 20. Enclosure Leakage Current Test Schematic
APPLIED
PART
TEST LEAD
FE
faw28.eps
33
Page 48

ESA620
Users Manual
Performing a Patient Leakage Test
Note
The Patient Leakage Current Test is not
available for IEC 62353 or IEC 61010 standard
selections.
The Patient-Leakage Current test measures the current
flowing between a selected applied part, selected group
of applied parts, or ALL applied parts, and the Mains PE.
Figure 21 shows the electrical connections between the
Analyzer and the DUT.
To perform a patient leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select one of the applied part groupings by pressing
or .
Note
Refer to the testing standard when deciding the
type of the applied parts and how they should be
grouped for testing.
4. Press the soft key labeled Select.
5. Press or to advance through each applied part
grouping, or the individual applied parts, to ground.
These are selected and measured.
The Patient Leakage test can be performed with a
number of fault conditions on the test receptacle. Press
to switch the test receptacle between Normal, Off,
Reverse, and Off. Press to open and close the
neutral connection to the receptacle. Press to
open and close the receptacle’s earth connection.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Normal Polarity, Open Earth
• Reversed Polarity
• Reversed Polarity, Open Neutral
• Reversed Polarity, Open Earth
34
Page 49

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
CONDUCTIVE PART
PE
(REMOTE ONLY)
L2
EARTH
DUT_L1
DUT_L2
REV
POL
DUT_PE
MD
Figure 21. Patient Leakage Current Test Schematic
APPLIED
PART
LEAD
SELECT
RELAY*
LEAD GND
SELECT RELAY
FE
*Leads not selected are open.
gtv29.eps
35
Page 50

ESA620
Users Manual
Performing Patient Auxiliary Leakage Tests
Note
The Patient Auxiliary leakage test is available
when the AN/NZS3551, IEC60601, or
ANSI/AAMI ES1-1993 standard is selected.
To measure the leakage current through each applied
part or lead and selected combination of lead connections
(all other or between two), press the softkey labeled
Patient Auxiliary from the Leakage Test main menu
shown in Figure 18. Figure 23 shows the electrical
connections between the Analyzer and the DUT during a
Patient Auxiliary Leakage Current Test.
The Patient Auxiliary Leakage test adds a diagram of the
applied parts connection posts to the display, as shown in
Figure 22. In the Figure, the applied parts post RA/R is
shown above the other posts. This indicates that the
leakage measurement is being made from RA/R to all
others. To move to the next applied part post, press .
The first post will appear inline with the other posts while
the LL/F post appears above all others. This indicates the
second leakage measurement is being made from LL/F to
all others. Continue pressing or to move from one
connection post to another and noting the measured
current in the display.
After each post is isolated individually, the Patient
Auxiliary Leakage test measures current of three different
combinations of posts tied together: RA/R and LL/F, RA/R
and LA/L, or LL/F and LA/L.
faw10.eps
Figure 22. Applied Parts Connection Posts Display
Within the Patient Auxiliary Leakage test, a number of
fault measurements can be made. Pressing
switches the polarity of the mains voltage applied to the
Analyzer’s test receptacle between Normal, Off, Reverse,
and Off. Pressing opens and closes the neutral
connection to the Analyzer’s test receptacle. Pressing
opens and closes the earth or ground connection to
the Analyzer’s test receptacle.
36
Page 51

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
CONDUCTIVE PART
MD
PE
APPLIED
PART
+
LEAD
SELECT
RELAY*
- LEAD
SELECT
RELAY*
LEAD GND
SELECT RELAY*
(REMOTE ONLY)
FE
L2
EARTH
DUT_L1
DUT_L2
REV
POL
DUT_PE
Figure 23. Patient Auxiliary Leakage Current Test Schematic
*Leads not selected are open.
gtv30.eps
37
Page 52

ESA620
Users Manual
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Normal Polarity, Open Earth
• Reversed Polarity, Open Neutral
• Reversed Polarity, Open Earth
Performing a Mains on Applied Part Leakage Test
Note
The Mains on Applied Part leakage test is
available when the IEC60601 & ANSI/AAMI
ES60601-1 or AN/NZS 3551 standard is
selected.
The Mains-On-Applied-Parts-Leakage-Current test
measures the current that flows in response to an isolated
AC voltage applied between a selected applied part,
group of applied parts, or ALL applied parts, and Earth
(and any conductive part connected to the RED terminal).
Figure 24 shows the electrical connections between the
Analyzer and the DUT during a Mains on Applied Part
Leakage Current Test.
To perform a mains on applied part test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
Note
Refer to the testing standard when deciding the
type of the applied parts and how they should be
grouped for testing.
4. Press the soft key labeled Select.
5. Press the soft key labeled Mains on A. P.
6. Press or to select the desired applied part
connection.
7. Press to apply the voltage and read the
leakage current in the display.
Pressing and scrolls through the applied part
connections or groupings. Press for each
connection configuration to thoroughly test the DUT.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Reverse Polarity
38
Page 53

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
On
PE
MAP (ISOLATION)
TRANSFORMER
DEVICE UNDER TEST
CONDUCTIVE PART
TEST
LEAD
MD
APPLIED
PART
LEAD
SELECT
RELAY*
*Leads not selected are open.
REV
POL
MAP
REV
DUT_L1
DUT_L2
DUT_PE
Figure 24. Mains-On-Applied-Parts-Leakage-Current Test Schematic
FE
gtv31.eps
39
Page 54

ESA620
Users Manual
Performing an Alternative Equipment Leakage Test
Note
The alternative equipment leakage test is
available when the EN62353 & VDE 751
standard is selected.
During the Alternative Equipment Leakage test, the
voltage source is applied between short-circuited
equipment outlet mains live, neutral, and equipment outlet
earth, the exposed conductive surface on the housing,
and all applied parts short-circuited together. Equipment
is separated from mains during the test. The current
which flows over the insulation of the DUT is measured.
This test is not applicable for equipment with internal
electrical power source. The switches in mains part shall
be closed during measurement.
To perform an alternative equipment leakage test:
1. Press .
The alternative equipment test is the default test and
should already be selected.
2. Press to apply the voltage and read the current
in the display.
Figure 25 shows the electrical connections between the
Analyzer and the DUT during a Alternative Equipment
Leakage Test.
The following outlet conditions apply when performing this
test:
• Closed Earth
• Open Earth
Performing an Alternative Applied Part Leakage Test
Note
The Alternative applied part leakage test is
available when the EN62353 & VDE 751
standard is selected.
During the Alternative Applied Part Leakage test, the test
voltage is applied between short-circuited applied parts of
a single function and the short-circuited equipment outlet
mains live, neutral, equipment outlet earth, and exposed
conductive surface on the housing. This test should only
be done for equipment with F-Type applied parts. For
equipment with multiple applied parts, test each group of
applied parts of a single function in turn with all others
floating during the test
connected to the Analyzer’s applied parts jacks and the
lead selection will float those not selected.
. All applied parts can be
40
Page 55

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
DUT_PE
PE
OPEN
MD
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
Figure 25. Alternative Equipment Leakage Current Test Schematic
FE
faw22.eps
41
Page 56

ESA620
Users Manual
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
LEAD
SELECT
RELAY*
*Leads not selected
are open.
FE
Figure 26. Alternative Applied Part Leakage Test Schematic
MD
gtv23.eps
42
Page 57

Electrical Safety Analyzer
Performing Electrical Safety Tests
To perform an alternative applied part leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
4. Press the soft key labeled Select.
5. Press the soft key labeled Alternative A.P..
6. Press to apply the test voltage and read the
current in the display.
7. Press or to advance to the next applied part
group(s) of a single function if applicable. Pressing
to read leakage current for each group.
Figure 26 shows the electrical connections between the
Analyzer and the DUT during an Alternative Applied Part
Leakage current test.
Performing a Direct Equipment Leakage Test
Note
The Direct Equipment Leakage test is available
when the EN62353 & VDE 751 or the None
standard is selected.
The Direct Equipment Leakage Current test measures the
leakage current between all applied parts and the
exposed conductive surface on the housing, to mains
earth.
To perform a direct equipment test:
1. Press .
2. Press the soft key labeled Direct Equipment.
3. Press to apply the voltage and read the
leakage current in the display.
Figure 27 shows the electrical connections between the
Analyzer and the DUT during a Direct Equipment
Leakage Current Test.
The following outlet conditions apply when performing this
test:
• Normal Polarity, Closed Earth
• Normal Polarity, Open Earth
• Reversed Polarity, Closed Earth
• Reversed Polarity, Open Earth
43
Page 58

ESA620
Users Manual
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
CONDUCTIVE PART
LEAD
L2
MD
REV
POL
PE
DUT_L1
DUT_L2
DUT_PE
Figure 27. Direct Equipment Leakage Test Schematic
TEST
APPLIED
PART
FE
faw24.eps
44
Page 59

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Direct Applied Part Leakage Test
Note
The Direct Applied Part Leakage test is available
when the EN62353 & VDE 751 or the None
standard is selected.
The Direct Applied Part Leakage Current test measures
the leakage current between all applied parts of a single
function and the exposed conductive surface on the
housing, to mains earth. For equipment with multiple
applied parts, each group of a single function should be
tested each in turn with all other floating during the test.
This test should only be done for equipment with F-Type
applied parts.
For Type B applied part, see direct equipment leakage
schematic in Figure 27.
To perform a direct applied part leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
4. Press the soft key labeled Select. The Direct A.P.
test should already be selected.
5. Press or to select the applied part test
configuration.
6. Press to apply the test voltage and read the
current in the display.
7. Press or to advance to the next group of applied
parts, if applicable.
Figure 28 shows the electrical connections between the
Analyzer and the DUT during a Direct Applied Part
Leakage Current Test.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Reversed Polarity
45
Page 60

ESA620
Users Manual
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
L2
DUT_L1
DUT_L2
REV
POL
DUT_PE
Figure 28. Direct Applied Parts Leakage Current Test Schematic
LEAD
SELECT
RELAY*
*Leads not selected
are open.
FE
MD
gtv25.eps
46
Page 61

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Differential Leakage Current Test
Note
The Differential Leakage Current test is available
when the EN62353 & VDE 751 or the None
standard is selected.
The differential leakage current test measures the
magnitudes of the differential current flowing in the
Equipment Outlet live and neutral, with power applied to
the equipment outlet. All applied parts should be
connected during this test, if equipment has applicable
applied parts.
To perform a differential leakage current test:
1. Press .
2. Press the soft key labeled Differential.
Figure 29 shows the electrical connections between the
Analyzer and the DUT during a Differential Leakage
Current test.
The following outlet conditions apply when performing this
test:
• Normal Polarity, Closed Earth
• Normal Polarity, Open Earth
• Reversed Polarity, Closed Earth
• Reversed Polarity, Open Earth
Performing an Accessible Leakage Current Test (IEC 61010 only)
Note
The Accessible Leakage Current test selection
will only appear in the Analyzer’s menu when the
standard is set to IEC61010.
To perform an accessible leakage current test:
1. Press .
2. Read the leakage current in the display.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Normal Polarity, Open Earth
• Reversed Polarity
• Reversed Polarity, Open Neutral
• Reversed Polarity, Open Earth
47
Page 62

ESA620
Users Manual
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
REV
POL
EARTH
DUT_L1
MD
DUT_L2
DUT_PE
Figure 29. Differential Leakage Current Test Schematic
FE
gtv32.eps
48
Page 63

Electrical Safety Analyzer
Making Point-To-Point Measurements
Making Point-To-Point Measurements
The Analyzer can make voltage, resistance, and low
current measurements through its Point-to-Point function.
To access the Point-to-Point function menu shown in
Figure 30, press . Softkeys F1 through F3 are used
to select the measurement function.
faw08.eps
Figure 30. Point-To-Point Function Menu
Measuring Voltage
To make a voltage measurement:
1. Press the softkey labeled Voltage from the
Point-To-Point menu.
2. Insert test leads in the RED and BLACK 2-Wire
V/Ω/A jacks.
3. Place the probe tips across the unknown voltage and
read the measurement in the Analyzer’s display.
The Analyzer will measure up to 300 volts ac.
Measuring Resistance
The Analyzer can make 2-Wire or 4-Wire resistance
measurements. To switch between these methods, see
the “Selecting 2-Wire or 4-Wire Measurements” section.
To make a resistance measurement:
1. Press the softkey labeled Resistance from the
Point-To-Point menu.
2. Insert test leads in the RED and BLACK 2-Wire
V/Ω/A jacks. For 4-Wire measurements, two
additional leads need to be inserted into the RED
and BLACK 4 Wire Source jacks.
3. Place the probes across the unknown resistance and
read the measurement in the Analyzer’s display.
The Analyzer will measure resistances up to 2.0 Ω.
49
Page 64

ESA620
Users Manual
Measuring Current
The Analyzer can make dc only, ac only, and ac+dc
current measurements up to 10 mA. To make a current
measurement:
1. Press the softkey labeled Leakage from the
Point-To-Point menu.
2. Using or select between ac only, dc only, or
ac+dc measurement mode.
3. Insert test leads in the RED and BLACK 2-Wire
V/Ω/A jacks.
Place the leads on the two points the unknown current
may flow and read the measurement in the Analyzer’s
display.
Simulating ECG Waveforms
The Analyzer is capable of generating various waveforms
on the applied parts connection posts. These signals are
used to test the performance characteristics of ECG
monitors and ECG strip printers. See Figure 32 for proper
connections between the Analyzer and an ECG monitor.
To access the ECG Simulation Waveform menu shown in
Figure 31, press . From this menu, a number of
different waveforms are selected through F1, and the rate
or frequency of the waveform is selected through F2.
faw09.eps
Figure 31. ECG Waveform Simulation Menu
To select one of the predefined waveforms, press the
softkey labeled Wave Form. A scroll box with next to it
appears above the softkey label. Use or to scroll
through the different waveforms.
For all waveforms except VFIB and Triangle, the rate or
frequency of the waveform is adjusted through the softkey
labeled Frequency or Rate. For some waveforms, there
are more than two frequency or rate selections. For those
waveforms, pressing the softkey labeled Frequency or
Rate will open a scroll box above the softkey label with
next to it. Use or to select the frequency or rate. For
those waveforms that have only two frequency or rate
selections, the softkey labeled Frequency or Rate acts
as a toggle, where each press of the softkey switches to
the other value.
50
Page 65

Electrical Safety Analyzer
Simulating ECG Waveforms
ECG Monitor
Figure 32. ECG Monitor Connections
faw07.eps
51
Page 66

ESA620
Users Manual
Controlling the Analyzer Remotely
Fluke Biomedical Ansur test automation software allows a
solutions-based approach to complete testing of the
medical device under test (DUT). Ansur helps create
standard work using the test template/sequence (which is
based on a user written test procedure), and integrates all
test results into a single test report which can be printed
or archived. Ansur allows for automatic comparisons to
the limits of the standard selected, indicating whether
results are passing or failing. Ansur manages test
procedures by allowing both manual and visual
automated test sequences.
The software works hand-in-hand with Fluke Biomedical
analyzers and simulators, creating a seamless integration
for:
• Visual inspections
• Preventive maintenance
• Work procedures
• Performance tests
• Safety tests
Ansur software utilizes plug-in modules to work with a
wide array of Fluke Biomedical instruments. The plug-in
module is a software interface to the Ansur test program.
The plug-in modules are available for purchase as an
optional accessory. Plug-ins provide test elements used
by Ansur. This has the benefit of using the same user
interface for all analyzers and simulators supported by an
Ansur plug-in.
When a new Fluke Biomedical analyzer or simulator is
purchased, simply update your existing Ansur software by
installing a new plug-in. Each plug-in module works only
with the options and capabilities needed for the
instrument being tested.
Maintenance
The Analyzer needs little maintenance or special care.
However, treat it as a calibrated measuring instrument.
Avoid dropping or other mechanical abuse that could
cause a shift in the calibrated settings.
52
Page 67

Electrical Safety Analyzer
Cleaning the Analyzer
Cleaning the Analyzer
Warning
To avoid electric shock, do not clean the
Analyzer plugged into mains or attached to a
DUT.
Caution
Do not pour fluid onto the Analyzer surface;
fluid seepage into the electrical circuitry may
cause the Analyzer to fail.
Caution
Do not use spray cleaners on the Analyzer;
such action may force cleaning fluid into the
Analyzer and damage electronic
components.
Clean the Analyzer occasionally utilizing a damp cloth
and mild detergent. Take care to prevent the entrance of
liquids.
Wipe down the adapter cables with the same care.
Inspect them for damage to and deterioration of the
insulation. Check the connections for integrity before
each use.
53
Page 68

ESA620
Users Manual
Replaceable Parts
Table 6 lists the replaceable parts for the Analyzer.
Table 6. Replaceable Parts
Item Fluke Biomedical Part Number
ESA620 Getting Started Manual 2814971
ESA620 Users Manual CD 2814967
USA 2238680
UK 2238596
Australia 2238603
Power Cord
Ansur, CD with demo version 2795488
Test Probe Set
Europe 2238615
France/Belgium 2238615
Italy 2238615
Israel 2434122
USA, Australia, & Israel 650887
Europe 1541649
54
Page 69

Electrical Safety Analyzer
Replaceable Parts
Table 6. Replaceable Parts (cont.)
Item Fluke Biomedical Part Number
Null Post Adapter 3326842
Carrying Case 2814980
Data Transfer Cable 1626219
T20A 250V Fuse (Time Lag), 1¼ in x ¼ in 2183691
T10A 250V Fuse (Time Lag), 5 x 20 mm 3046641
T16A 250V Fuse (Time Lag), 5 x 20 mm 3056494
15 – 20 A Adapter 2195732
To ensure safety, use exact replacement only.
55
Page 70

ESA620
Users Manual
Accessories
Table 7 lists the available accessories for the Analyzer.
Table 7. Accessories
Item Fluke Biomedical Part Number
Test Leads with retractable sheath 1903307
Kelvin Test Lead Set for 4-wire ground 2067864
Ground Pin Adapters 2242165
ESA620 USA Accessory Kit:
Test Lead Set
TP1 Test Probe Set
AC285 Alligator Clip Set
ESA620 EUR/AUS/ISR Accessory Kit:
Test Lead Set
TP74 Test Probe Set
AC285 Alligator Clip Set
3111008
3111024
56
Page 71

Electrical Safety Analyzer
Specifications
Specifications
Temperature
Operating ............................................................ 10 °C to 40 °C (50 °F to 104 °F)
Storage ................................................................ -20 °C to 60 °C (-4 °F to 140 °F)
Humidity ................................................................. 10 % to 90 % non-condensing
Altitude ................................................................... To 5,000 meters @ 120 V ac (mains supply voltage)
To 2,000 meters @ 230 V ac (mains supply voltage)
Display .................................................................... LCD display
Communications ................................................... USB device port for computer control
Modes of Operation ............................................... Manual and remote
Power
120 Volt power outlet .......................................... 90 to 132 V ac rms, 47 to 63 Hz, 20 A maximum
230 Volt power outlet .......................................... 180 to 264 V ac rms, 47 to 63 Hz, 16 A maximum
Size (L x W x H) ...................................................... 32 cm x 23.6 cm x 12.7 cm (12.6 in x 9.3 in x 5 in)
Weight .................................................................... 4.7 kg (10.25 lb)
Safety Standards
CE ....................................................................... IEC/EN61010-1 2
CSA ..................................................................... CAN/CSA-C22.2 No 61010-1; UL61010-1
Electromagnetic Compatibility Standards (EMC)
European EMC .................................................... EN61326-1
nd
Edition; Pollution degree 2
57
Page 72

ESA620
Users Manual
Detailed Specifications
Voltage
Mains voltage
Ranges ............................................................ 0.0 to 300 V ac rms
Accuracy ......................................................... ±(2 % of reading + 1.0 V ac)
Accessible Voltage and Point to Point Voltage
Range ............................................................. 0.0 to 300 V ac rms
Accuracy ......................................................... ±(2 % of reading + 2 LSD)
Earth Resistance
Modes ................................................................. Two terminal and four terminal
Test Current ........................................................ >200 mA ac into 500 mΩ with open circuit voltage ≤ 24 V
Range ................................................................. 0.0 to 2.0 Ω
Accuracy
Two Terminal Mode
Test current >200 mA ac into 500 mΩ ........ ±(2 % of reading + 0.015 Ω) for 0.0 to 2.0 Ω
Test current 1-16 A ac ................................ ±(2 % of reading + 0.015 Ω) for 0.0 to 0.2 Ω
Four Terminal Mode
Test current >200 mA ac into 500 mΩ ........ ±(2 % of reading + 0.005 Ω) for 0.0 to 2.0 Ω
Test current 1-16 A ac ................................ ±(2 % of reading + 0.005 Ω) for 0.0 to 0.2 Ω
25 A short circuit ±10 % (with open circuit voltage 6 Vac at nominal mains)
±(5 % of reading + 0.015 Ω) for 0.2 to 2.0 Ω
±(5 % of reading + 0.005 Ω) for 0.2 to 2.0 Ω
58
Page 73

Electrical Safety Analyzer
Detailed Specifications
Additional error caused by series inductance
Resistance
0.000 Ω 0.000 Ω 0.030 Ω 0.040 Ω 0.050 Ω
0.020 Ω 0.000 Ω 0.025 Ω 0.030 Ω 0.040 Ω
0.040 Ω 0.000 Ω 0.020 Ω 0.025 Ω 0.030 Ω
0.060 Ω 0.000 Ω 0.015 Ω 0.020 Ω 0.025 Ω
0.080 Ω 0.000 Ω 0.010 Ω 0.015 Ω 0.020 Ω
0.100 Ω 0.000 Ω 0.010 Ω 0.010 Ω 0.015 Ω
>0.100 Ω 0.000 Ω 0.010 Ω 0.010 Ω 0.010 Ω
Equipment Current
Range .................................................................. 0 – 20 A ac rms
Accuracy.............................................................. 5 % of reading ± (2 counts or 0.2A, whichever is greater)
Duty cycle ............................................................ 15 A to 20 A, 5 min. on/5 min. off
Leakage Current
Modes* ................................................................ AC+DC (True-rms)
Patient Load Selection ........................................ AAMI ES1-1993 Fig. 1
0 µH 100 µH 200 µH 400 µH
Series Inductance
10 A to 15 A, 7 min. on/3 min. off
0 A to 10 A continuous
AC only
DC only
* Modes: AC+DC, AC only, and DC only available for all leakages with exception of MAP
that are available in True RMS (shown as AC+DC)
IEC 60601: Fig 15
IEC 61010: Fig A-1
59
Page 74

ESA620
Users Manual
Crest factor ......................................................... ≤3
Ranges ................................................................ 0.0 to 199.9 μA
Accuracy**
DC to 1 kHz ..................................................... ±(1 % of reading + (1 μA or 1 LSD, whichever is greater)
1 to 100 kHz .................................................... ±(2 % of reading + (1 μA or 1 LSD, whichever is greater)
100 kHz to 1 MHz ........................................... ±(5 % of reading + (1 μA or 1 LSD, whichever is greater)
** Map Voltage: Additional residual leakage up to 4 μA @120 V ac, 8 μA @240 V ac
Mains on applied part test voltage ...................... 110 % ±5 % of Mains, current limited to 7.5 mA ±25 % @ 230V for IEC 60601
For Alternative and Direct applied parts leakage tests, the leakage values are compensated for nominal mains as
per 62353. Therefore, the accuracy specified for other leakages is not applicable. The actual leakage readings
given during these tests will be higher.
Differential leakage
Ranges ................................................................ 50 to 199 μA
Accuracy ............................................................. ± 10 % of reading ± (2 counts or 20 μA, whichever is greater)
200 to 1999 μA
2.00 to 10.00 mA
100 % ±5 % of Mains for AAMI, current limited to 1 mA ±25 % @ 115V per AAMI
100 % ±5 % of Mains for 62353 current limited to 3.5 mA ±25 % @ 230V per 62353
Note
200 to 2000 μA
2.00 to 20.00 mA
60
Page 75

Electrical Safety Analyzer
Detailed Specifications
Insulation resistance
Ranges ................................................................ 0.5 to 20 MΩ
Accuracy
20 MΩ Range .................................................. ±(2 % of reading + 2 counts)
100 MΩ Range ................................................ ±(7.5 % of reading + 2 counts)
Source test voltage ............................................. 500 V dc (+20 %, -0 %) 1.5 mA short-circuit current or 250 V dc selectable
Maximum load capacitance ................................. 1 μF
ECG Performance Waveforms
Accuracy.............................................................. ±2 %
Waveforms
ECG Complex ................................................. 30, 60, 120, 180, and 240 BPM
Ventricular Fibrillation
Square wave (50 % duty cycle) ....................... 0.125 and 2
Sine wave ........................................................ 10, 40, 50, 60, and 100 Hz
Triangle wave .................................................. 2 Hz
Pulse (63 ms pulse width ................................ 30 and 60
20 to 100 MΩ
±5 % for amplitude of 2 Hz square wave only, fixed @ 1 mV Lead II configuration
61
Page 76

ESA620
Users Manual
62
 Loading...
Loading...