Page 1

ESA612
Electrical Safety Analyzer
Users Manual
FBC-0031
March 2009, Rev. 2, 3/13
© 2009, 2013 Fluke Corporation. All rights reserved. Specifications are subject to change without notice.
All product names are trademarks of their respective companies.
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one year from the date of
original purchase OR two years if at the end of your first year you send the instrument to a Fluke Biomedical service center
for calibration. You will be charged our customary fee for such calibration. During the warranty period, we will repair or at our
option replace, at no charge, a product that proves to be defective, provided you return the product, shipping prepaid, to
Fluke Biomedical. This warranty covers the original purchaser only and is not transferable. The warranty does not apply if the
product has been damaged by accident or misuse or has been serviced or modified by anyone other than an authorized
Fluke Biomedical service facility. NO OTHER WARRANTIES, SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE
EXPRESSED OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR
CONSEQUENTIAL DAMAGES OR LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE OR THEORY.
This warranty covers only serialized products and their accessory items that bear a distinct serial number tag. Recalibration
of instruments is not covered under the warranty.
This warranty gives you specific legal rights and you may also have other rights that vary in different jurisdictions. Since
some jurisdictions do not allow the exclusion or limitation of an implied warranty or of incidental or consequential damages,
this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or
other decision-maker of competent jurisdiction, such holding will not affect the validity or enforceability of any other provision.
7/07
Page 3
Notices
All Rights Reserved
Copyright 2008 2013, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system,
or translated into any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and other printed materials for use in service
training programs and other technical publications. If you would like other reproductions or distributions, submit a written request to Fluke
Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop unpacking
the instrument. Notify the carrier and ask for an agent to be present while the instrument is unpacked. There are no special unpacking
instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or
broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 648-7952 or 1-425446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all packing materials in
their original condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not
operate within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local
sales representative.
Page 4

Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items bearing a distinct serial number tag) are
eligible for partial refund and/or credit. Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules, etc.)
are not eligible for return or refund . Only products returned within 90 days from the date of original purchase are eligible for refund/credit. In
order to receive a partial refund/credit of a product purchase price on a serialized product, the product must not have been damaged by the
customer or by the carrier chosen by the customer to return the goods, and the product must be returned complete (meaning with all manuals,
cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not
in “as new” and resalable condition, are not eligible for credit return and will be returned to the customer. The Return Procedure (see below)
must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15 %. Products returned in excess
of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20 %. Additional charges for damage and/or
missing parts and accessories will be applied to all returns.
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to our factory location. When you return an
instrument to Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that
you insure your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost shipments or instruments that are
received in damaged condition due to improper packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double–walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Page 5
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order
Entry Group at 1-800-648-7952 or 1-425-446-6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service or
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-99 FLUKE (1-888-993-5853)
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications when it was
shipped from the factory. Calibration measurements are traceable to the National Institute of Standards and Technology (NIST). Devices for
which there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or improper
operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Page 6

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made to the
information in this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical
for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The ESA612 Electrical Safety Analyzer is manufactured at Fluke Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Page 7

Table of Contents
Title Page
Introduction .................................................................................................................... 1
Safety Information .......................................................................................................... 3
Intended Use .................................................................................................................. 4
Unpacking the Analyzer ................................................................................................. 5
Instrument Familiarization .............................................................................................. 6
How to Hold the Product ................................................................................................ 10
Connecting to Line Power .............................................................................................. 10
Connecting a DUT to the Analyzer ................................................................................. 11
Turning the Analyzer On ................................................................................................ 11
Accessing the Analyzer’s Functions ............................................................................... 13
Setting Up the Analyzer .................................................................................................. 14
Setting Polarity Switching Delay ................................................................................ 14
Setting the Display Contrast ...................................................................................... 15
Setting up the Beeper ................................................................................................ 15
Viewing Instrument Information ................................................................................. 15
i
Page 8

ESA612
Users Manual
Viewing Memory ....................................................................................................... 16
Setting the GFCI Limit ............................................................................................... 16
Performing Electrical Safety Tests ................................................................................. 16
Setting the Test Standard ......................................................................................... 16
Performing Mains Voltage Testing ............................................................................ 17
Performing a Ground Wire (Protective Earth) Resistance Test ................................. 17
Performing an Insulation Resistance Test ................................................................ 23
Performing a Current Consumption Test .................................................................. 29
Performing Leakage Current Tests ........................................................................... 29
Measuring Earth Leakage Current ....................................................................... 30
Performing a Chassis (Enclosure) Leakage Test ................................................. 33
Performing a Lead-to-Ground (Patient) Leakage Test ......................................... 35
Performing Lead-to-Lead (Patient Auxiliary) Leakage Tests ................................ 37
Performing a Lead Isolation (Mains on Applied Part) Leakage Test ......................... 40
Performing an Alternative Equipment Leakage Test ................................................. 43
Performing an Alternative Applied Part Leakage Test .............................................. 44
Performing a Direct Equipment Leakage Test .......................................................... 46
Performing a Direct Applied Part Leakage Test ........................................................ 49
Performing a Differential Leakage Current Test ........................................................ 52
Using the 1-to-10 Adapter .............................................................................................. 54
Making Point-To-Point Measurements ........................................................................... 58
Measuring Voltage .................................................................................................... 58
Measuring Resistance .............................................................................................. 58
Measuring Current .................................................................................................... 59
Simulating ECG Waveforms .......................................................................................... 59
Using Memory ............................................................................................................... 62
Storing Data into Memory ......................................................................................... 62
Viewing Memory Data ............................................................................................... 63
Deleting Data from Memory ...................................................................................... 64
ii
Page 9

Contents (continued)
Controlling the Analyzer Remotely ................................................................................. 64
Maintenance ................................................................................................................... 65
Testing and Replacing the Fuses ................................................................................... 65
Cleaning the Analyzer .................................................................................................... 67
Replaceable Parts .......................................................................................................... 68
Accessories .................................................................................................................... 70
Specifications ................................................................................................................. 71
Detailed Specifications ................................................................................................... 72
iii
Page 10

ESA612
Users Manual
iv
Page 11

List of Tables
Table Title Page
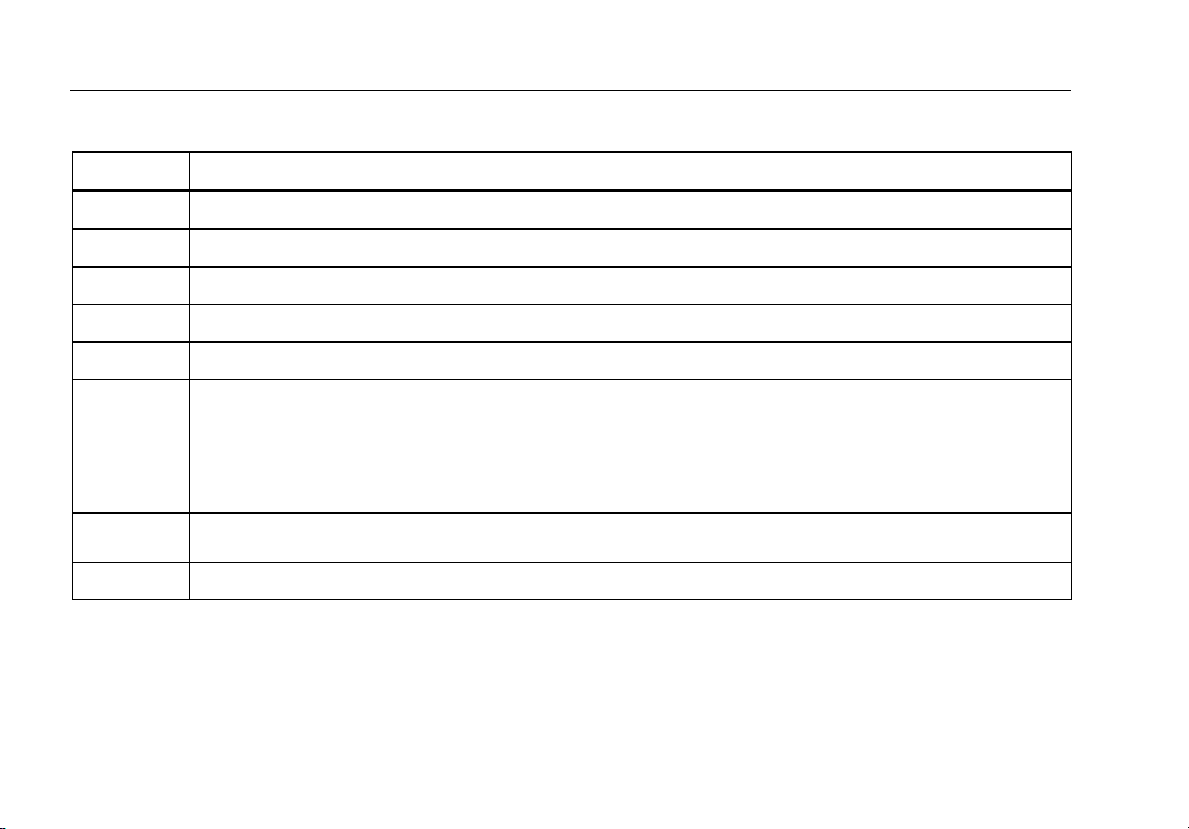
1. Symbols ................................................................................................................................. 2
2. Top-Panel Controls and Connections .................................................................................... 6
3. Side and Top-Panel Connections .......................................................................................... 9
4. Schematic Abbreviations ....................................................................................................... 21
5. Test Names Based on Selected Standard ............................................................................. 29
6. Replaceable Parts ................................................................................................................. 68
7. Accessories ........................................................................................................................... 70
v
Page 12

ESA612
Users Manual
vi
Page 13

List of Figures
Figure Title Page
1. Front-Panel Controls and Connections ................................................................................. 6
2. Side and Top-Panel Connections .......................................................................................... 8
3. Product Handle ...................................................................................................................... 10
4. Analyzer Ready for Operation ............................................................................................... 11
5. DUT Connections to the Analyzer ......................................................................................... 12
6. Leakage Current Menu .......................................................................................................... 13
7. Setup Menu ........................................................................................................................... 14
8. Mains Voltage Test Menu ...................................................................................................... 17
9. DUT Ground Resistance Measurement................................................................................. 18
10. Ground Wire (Protective Earth) Resistance Measurement Connections ............................... 20
11. Ground Wire (Protective Earth) Resistance Measurement Schematic .................................. 22
12. Insulation Resistance Measurement ..................................................................................... 23
13. Mains to Protective-Earth Insulation Resistance Test Schematic .......................................... 24
14. Applied Parts to Protective-Earth Insulation Test Schematic ................................................. 25
15. Mains to Applied Parts Insulation Test Schematic ................................................................. 26
vii
Page 14

ESA612
Users Manual
16. Mains to Non-Earth Accessible Conductive Points Schematic ............................................. 27
17. Applied Parts to Non-Earth Conductive Points Schematic .................................................... 28
18. Leakage Current Main Menu ................................................................................................ 30
19. Earth Leakage Current Test Schematic ................................................................................ 32
20. Enclosure Leakage Current Test Schematic ......................................................................... 34
21. Lead-to-Ground (Patient) Leakage Current Test Schematic ................................................. 36
22. Applied Parts Connection Posts Display ............................................................................... 37
23. Lead-to-Lead (Patient Auxiliary) Leakage Current Test Schematic ...................................... 39
24. Lead Isolation (Mains On Applied Parts) Leakage Test Schematic ...................................... 42
25. Alternative Equipment Leakage Current Test Schematic ...................................................... 45
26. Alternative Applied Part Leakage Test Schematic ................................................................ 48
27. Direct Equipment Leakage Test Schematic .......................................................................... 50
28. Direct Applied Parts Leakage Current Test Schematic ......................................................... 51
29. Differential Leakage Current Test Schematic ....................................................................... 53
30. 1-to-10 Adapter Connections ................................................................................................ 55
31. ECG Lead Connection with 1-to-10 Adapter ......................................................................... 57
32. Point-To-Point Function Menu .............................................................................................. 58
33. ECG Waveform Simulation Menu ......................................................................................... 59
34. ECG Monitor Connections .................................................................................................... 61
35. Test Record ID Entry Screen ................................................................................................ 63
36. Fuse Access ......................................................................................................................... 66
viii
Page 15

Electrical Safety Analyzer
Introduction
The Fluke Biomedical ESA612 Electrical Safety Analyzer
(hereafter the Analyzer) is a full-featured, compact,
portable analyzer, designed to verify the electrical safety
of medical devices. The Analyzer tests to domestic
(ANSI/AAMI ES1, NFPA 99) and international (IEC62353,
AN/NZS 3551, and parts of IEC 60601-1) electrical-safety
standards. The integrated ANSI/AAMI ES1 and
IEC60601-1 patient loads are easily selectable.
The Analyzer performs the following tests:
• Line (Mains) voltage
• Ground Wire (or Protective Earth) Resistance
• Equipment current
• Insulation resistance
• Ground (Earth) leakage
• Chassis (Enclosure) leakage
• Lead to Ground (Patient) and Lead to Lead (Patient
Auxiliary) leakage
• Lead isolation (Mains on applied parts leakage)
• Differential leakage
• Direct equipment leakage
• Direct applied part leakage
• Alternative equipment leakage
• Alternative applied part patient leakage
• Point to point leakage, voltage, and resistance
• ECG simulation and performance waveforms
1
Page 16
ESA612
Users Manual
Table 1. Symbols
Symbol Description
Important information; refer to manual.
Hazardous voltage
Conforms to relevant Canadian and US standards
Conforms to relevant Australian EMC requirements
Conforms to European Union directives
This product complies with the WEEE Directive (2002/96/EC) marking requirements. The affixed label
indicates that you must not discard this electrical/electronic product in domestic household waste. Product
CAT II
2
Category: With reference to the equipment types in the WEEE Directive Annex I, this product is classed as
category 9 "Monitoring and Control Instrumentation" product. Do not dispose of this product as unsorted
municipal waste. Go to Fluke’s website for recycling information.
IEC Measurement Category II – CAT II equipment designed to protect against transients from energyconsuming equipment supplied from fixed installations.
Accessible Functional Earth Terminal
Page 17

Electrical Safety Analyzer
Safety Information
Safety Information
In this manual, a Warning identifies hazardous conditions
and actions that could cause bodily harm or death. A
Caution identifies conditions and actions that could
damage the Analyzer, the equipment under test, or cause
permanent loss of data.
Warning
To avoid possible electrical shock or
personal injury, follow these guidelines:
• Use this Analyzer only in the manner
specified by the manufacturer or the
protection provided may be impaired.
• Read the Users Manual before operating
the Analyzer.
• Do not connect the Analyzer to a patient or
equipment connected to a patient. The
Analyzer is intended for equipment
evaluation only and should never be used
in diagnostics, treatment or in any other
capacity where the Analyzer would come
in contact with a patient.
• Do not use the product in wet or damp
locations, around explosive gases or dust.
• Inspect the Analyzer before using it. Do
not use the Analyzer if abnormal
conditions of any sort are noted (such as a
faulty display, broken case, etc.)
• Inspect the test leads for damaged
insulation or exposed metal. Check test
lead continuity. Replace damaged leads
before using the Analyzer.
• When testing, always be sure to keep your
fingers behind the safety barriers on the
test leads.
• Never open the Analyzer's c ase.
Dangerous voltages are present. There are
no user replaceable parts in the Analyzer.
• Have the Analyzer serviced on l y by
qualified personnel.
3
Page 18

ESA612
Users Manual
• The Analyzer must be properly earthed.
Only use a supply socket that has a
protective earth contact. If there is any
doubt as to the effectiveness of the supply
socket earth, do not connect the Analyzer.
Do not use a two-conductor adapter or
extension cord; this will break the
protective ground connection.
• Do not use the 15-20 A adapter to power
devices rated in excess of 15 A. Doing so
may overload the installation.
• Use extreme caution when working with
voltages above 30 V.
• Use the proper terminals, functions and
ranges for the test being performed.
• Do not touch metal parts of the device
under test (DUT) during analysis. The DUT
should be considered an electrical shock
hazard when connected to the Analyzer as
some tests involve high voltages, high
currents, and/or the removal of DUT earth
bond.
Intended Use
The Product is an electronic signal source and
measurement device for verifying the electrical safety of
medical devices. The Product also provides ECG
simulation and performance waveforms to verify patient
monitors are performing within their operating
specifications.
The Product provides the following function categories:
• ECG Functions
• ECG-Performance Testing
4
Page 19

Electrical Safety Analyzer
Unpacking the Analyzer
The intended user is a trained biomedical equipment
technician who performs periodic preventative
maintenance checks on patient monitors in service. Users
can be associated with hospitals, clinics, original
equipment manufacturers and independent service
companies that repair and service medical equipment.
The end user is an individual, trained in medical
instrumentation technology.
This Product is intended to be used in the laboratory
environment, outside of the patient care area, and is not
intended for use on patients, or to test devices while
connected to patients. This Product is not intended to be
used to calibrate medical equipment. It is intended for
over-the-counter use.
Unpacking the Analyzer
Carefully unpack all items from the box and check that
you have the following items:
• ESA612
• Getting Started Manual
• Users Manual CD
• Carrying case
• Power cord
• 15 – 20 A Adapter (USA only)
• ESA USA Accessory Kit (USA, Australia, and Israel
only)
• ESA EUR Accessory Kit
• Ansur demo CD
• Null Post Adapter
• 5-to-5 Banana to ECG Adapter (BJ2ECG)
• Transfer cable
5
Page 20
ESA612
Users Manual
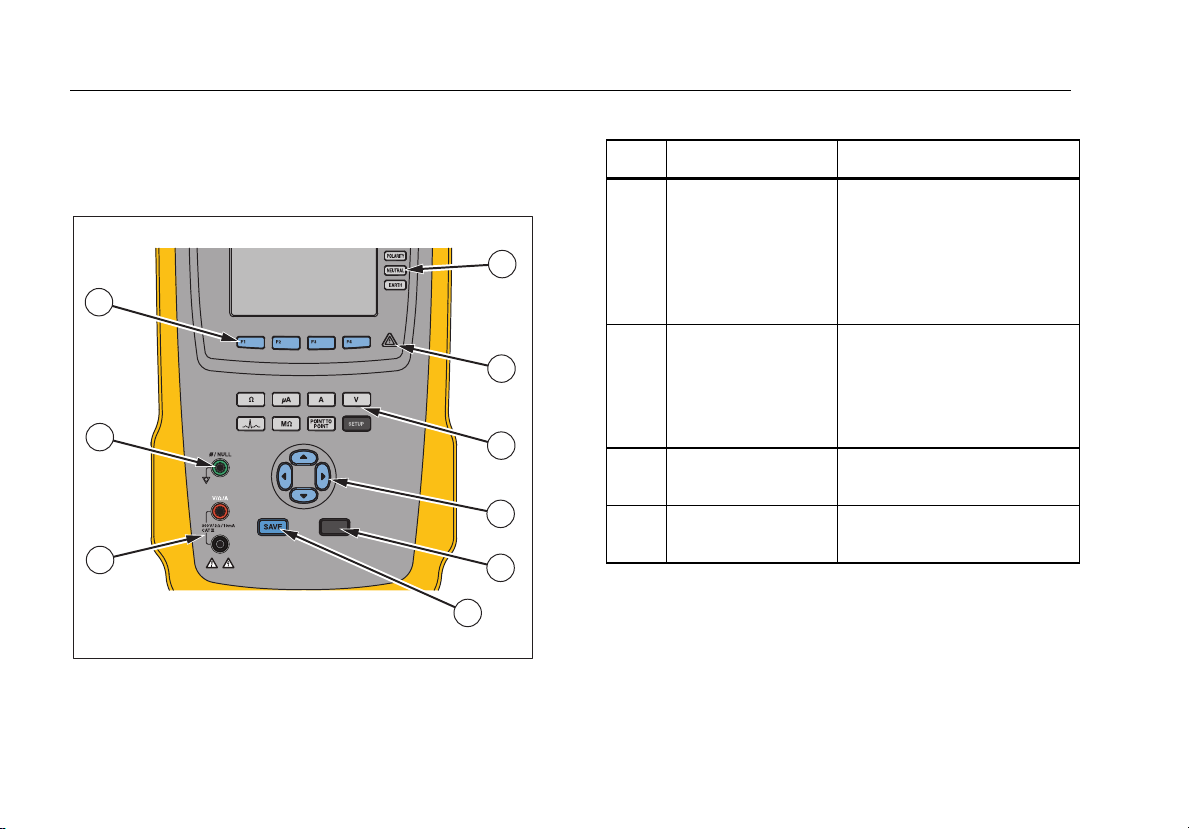
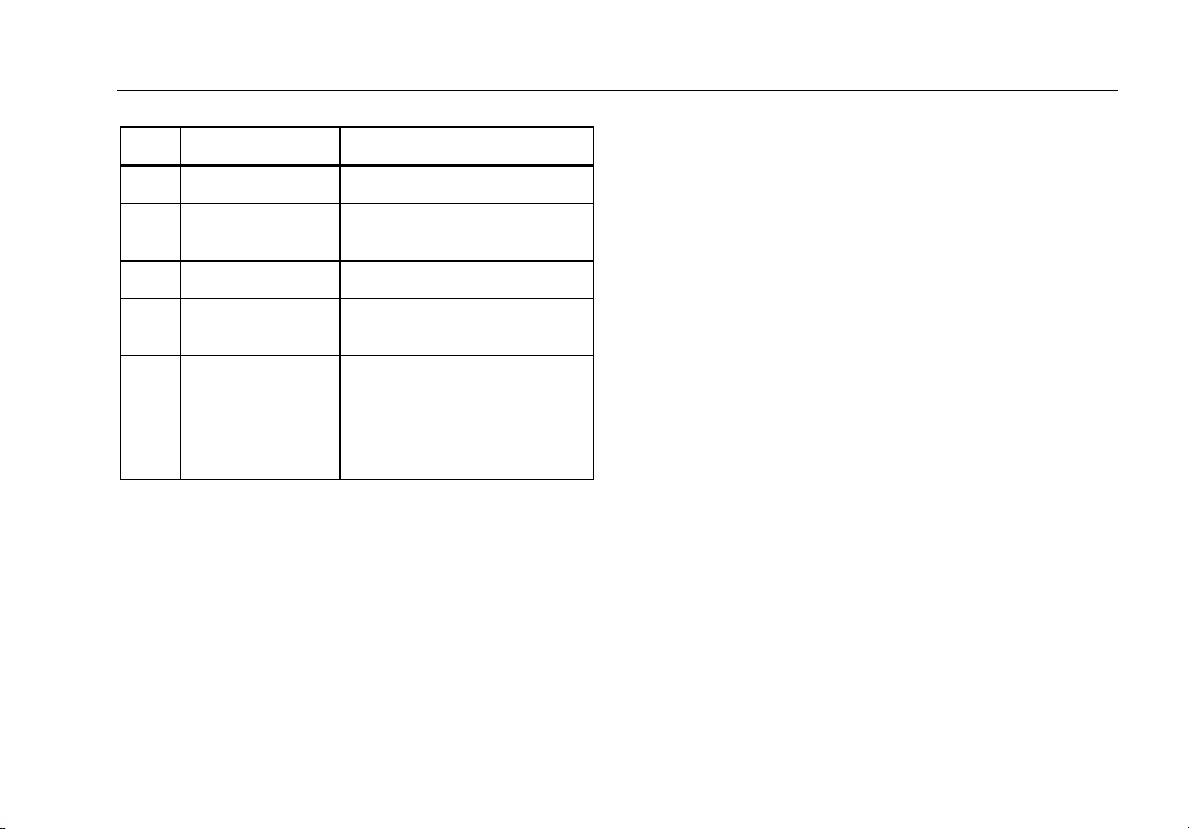
Instrument Familiarization
Figure 1 and Table 2 describes the front-panel controls
and connections of the Analyzer.
1
9
2
8
TEST
7
Figure 1. Front-Panel Controls and Connections
3
4
5
6
fis116.eps
Table 2. Top-Panel Controls and Connections
Item Name Description
Controls the configuration of
Equipment Outlet
Configuration
1
Buttons
the equipment outlet. Opens
and closes the neutral and
ground connection and
reverses the polarity of the
neutral and hot connection.
Indicates when high voltage
High Voltage
2
Indicator
is applied to the
ECG/Applied Parts posts or
L1 and L2 of the Test
Receptacle.
Test Function
3
Buttons
Navigation Buttons
4
Selects the various Analyzer
test functions.
Cursor control buttons for
navigating menus and lists.
6
Page 21
Electrical Safety Analyzer
Instrument Familiarization
Item Name Description
Test Button
5
Save Button
6
Input Jacks
7
Nulling Jack
8
Function Softkeys
9
Initiates selected tests.
Saves the measurement or
ECG waveform to memory.
Test lead connectors.
Connection for zeroing test
lead resistance.
Keys F1 through F4 are used
to select from a number of
selections that appear in the
LCD display above each
function softkey.
7
Page 22
ESA612
Users Manual
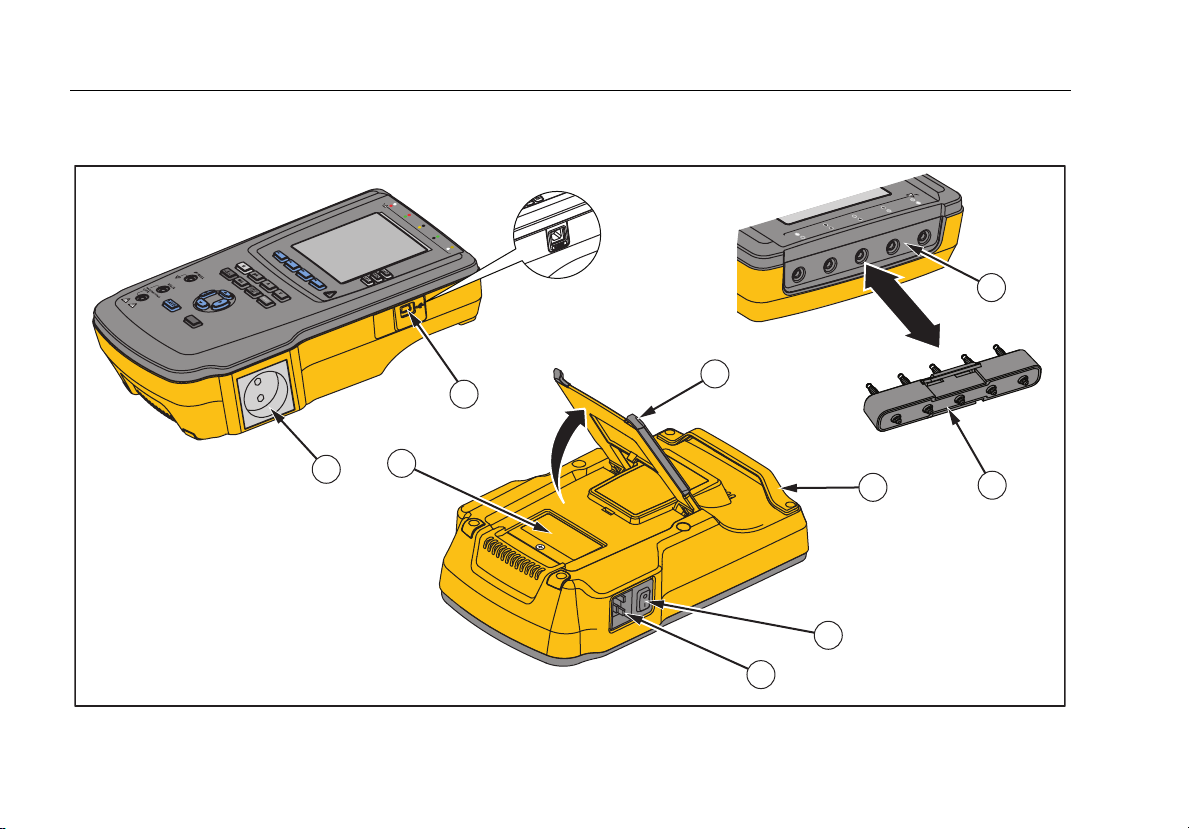
Figure 2 and Table 3 describe the side and top-panel connections of the Analyzer.
RA
RFLNC1
LL LA RL
V1
R
RA
FLN
LL LA RL
C1
V1
TEST
1
3
*Old versions have a B style connector for USB port.
Figure 2. Side and Top-Panel Connections
8
Old Version*
7
4
2
9
8
5
6
fis110.eps
Page 23
Electrical Safety Analyzer
Instrument Familiarization
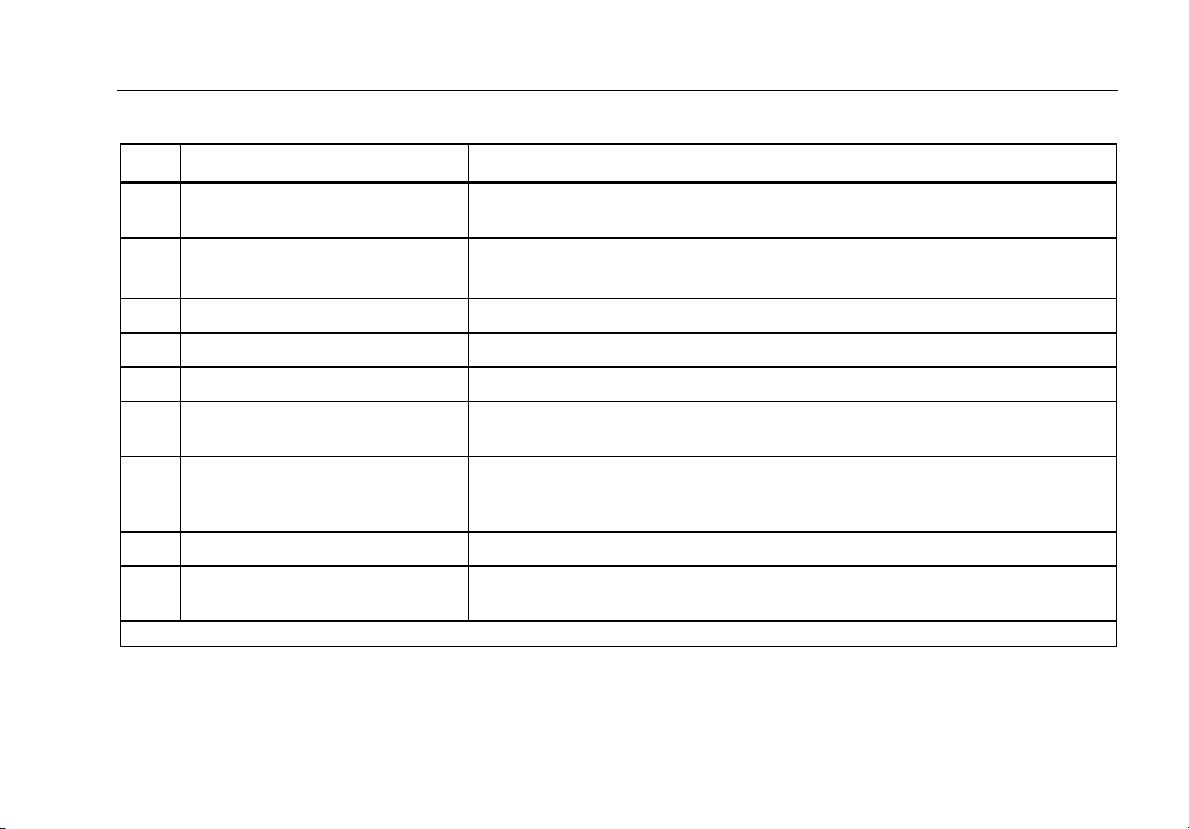
Table 3. Side and Top-Panel Connections
Item Name Description
1 Equipment Outlet
USB Device Port
2
(Mini B-style connector)1
3 Fuse Access Door Covers the equipment outlet fuses.
4 Tilt Stand Support for holding the Analyzer in a tilted position.
5 AC Power Switch Turns ac power on and off.
6 Power Input Connector
7 ECG/Applied Parts Jacks
8 Banana Jack to ECG Adapter Adapter for connecting ECG snap leads to the Analyzer.
9 Carry Handle
1 Older versions of the Analyzer have a B-style USB port connector.
Equipment outlet, specific to the version of the Analyzer, which provides a DUT
connection.
Digital connection for controlling the Analyzer from a PC or instrument
controller.
A grounded male three-prong (IEC 60320 C19) connector that accepts the
line-power cord.
Connection posts for Device Under Test (DUT) applied parts, such as ECG
leads. Used to test for leakage current through leads and to supply ECG
signals and performance waveforms to a DUT.
Handle to transport Analyzer. Note: There is no handle on old versions of the
analyzer.
9
Page 24
ESA612
Users Manual
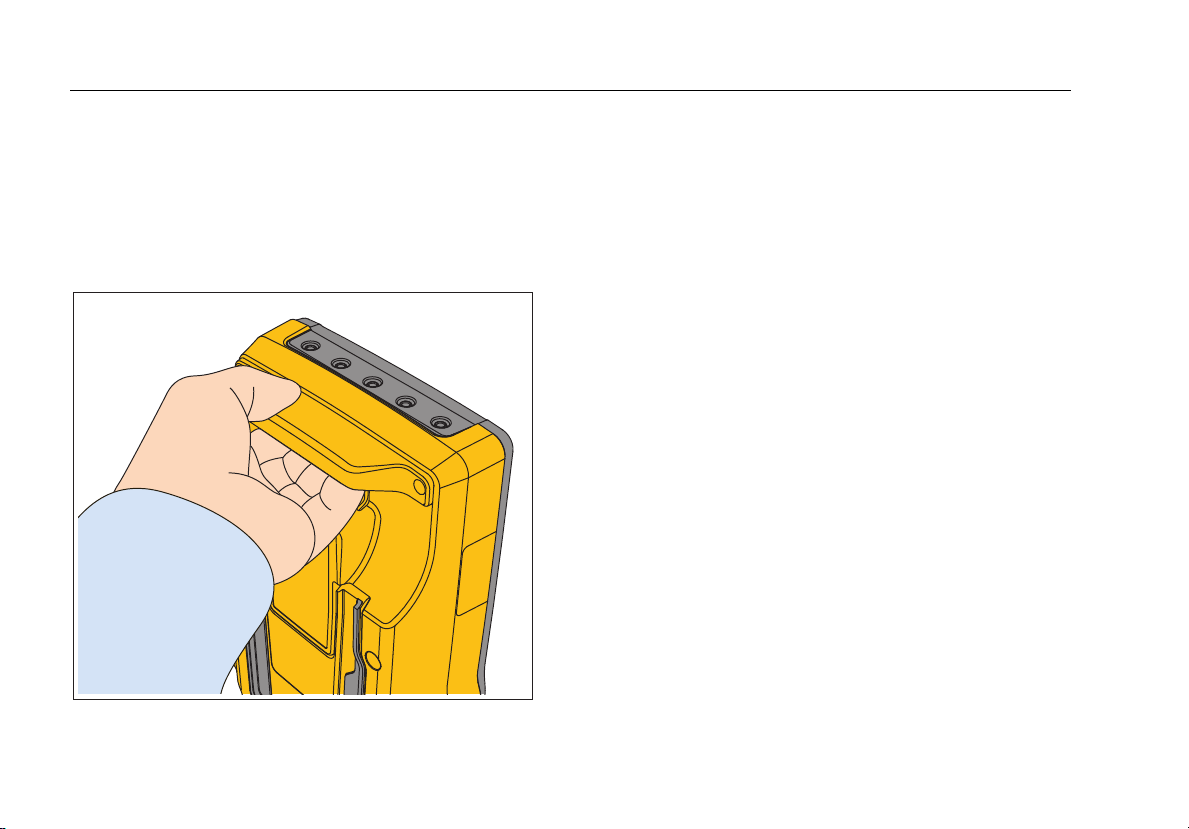
How to Hold the Product
When you move the Analyzer, use the handle in the
bottom case to hold it. See Figure 3.
Note
There is no handle on old versions of the
Analyzer.
Figure 3. Product Handle
fis122.eps
Connecting to Line Power
Warning
To avoid shock hazard and for proper
Analyzer operation, connect the factory
supplied three-conductor line power cord to
a properly grounded power outlet. Do not
use a two-conductor adapter or extension
cord; this will break the protective ground
connection.
Connect the Analyzer to a properly grounded three-prong
outlet. The Analyzer will not properly test a DUT when the
ground lead is open.
The Analyzer is intended for use with single-phase,
grounded power. It is not intended for dual, split-phase or
three-phase power configurations. But it can be used with
any power system that supplies the correct voltages for
single-phase and is grounded, or is an isolated power
system.
10
Page 25
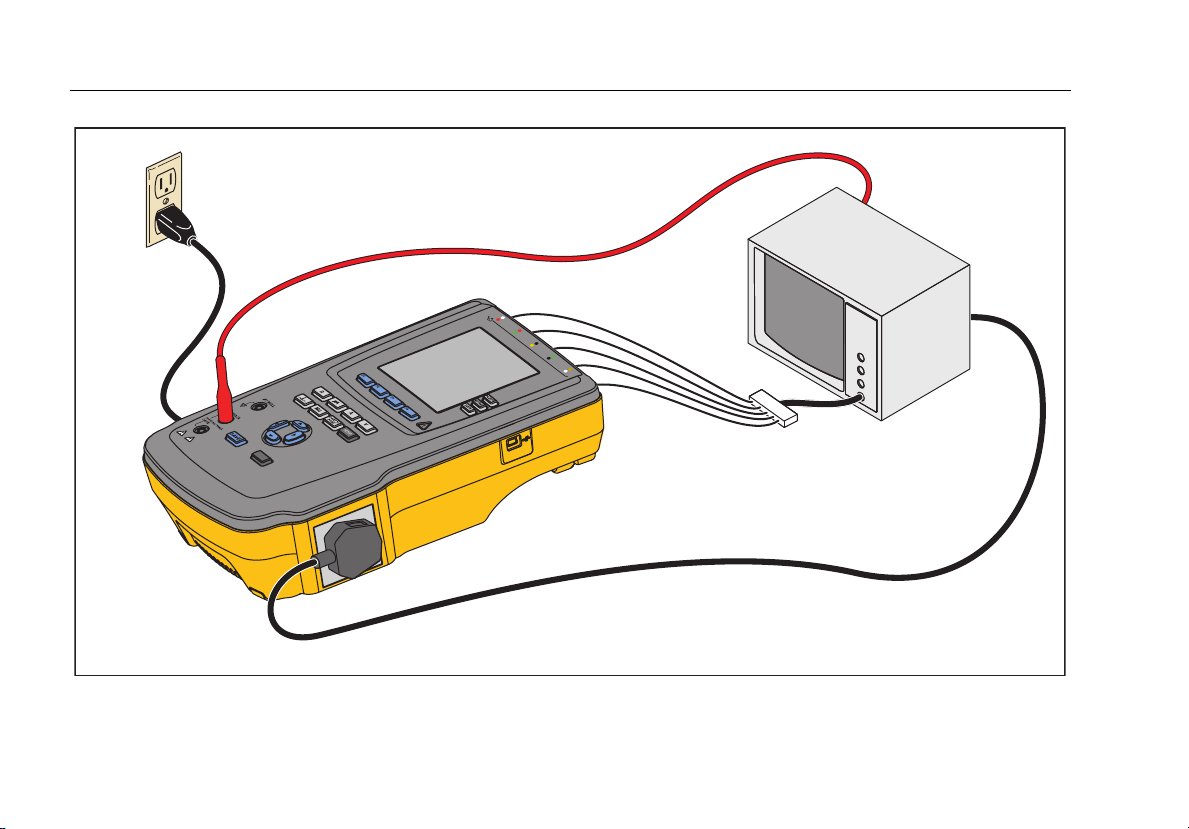
Electrical Safety Analyzer
Connecting a DUT to the Analyzer
Connecting a DUT to the Analyzer
A Device Under Test (DUT) can be connected in a
number of different ways depending on the device and
the number of connections needed for a full electrical
safety test. Figure 5 shows a DUT connected to the test
receptacle, applied parts posts, and a separate
connection to the DUT’s enclosure or protective earth
ground.
Turning the Analyzer On
Note
To ensure the high voltage indicator is working,
look for it to illuminate during the power-up self
test.
Press the power switch on the left-side panel so the “I”
side of the ac power switch is depressed. The Analyzer
will perform a series of self tests and then display the
message shown in Figure 4 when the self test has
completed successfully.
Figure 4. Analyzer Ready for Operation
fis101.jpg
11
Page 26
ESA612
Users Manual
Connect ESA612
to grounded
mains socket.
To protective earth or
any exposed conductive
surface on the enclosure
RA
R
LL
F
LA
L
RL
N
V1
C1
TEST
Connect the DUT ac power cord to the
equipment outlet on the Analyzer
12
Figure 5. DUT Connections to the Analyzer
fis113.eps
Page 27
Electrical Safety Analyzer
Accessing the Analyzer’s Functions
During the self-test, the Analyzer checks its ac mains
input for proper polarity, ground integrity and voltage
level. The high voltage indicator illuminates briefly during
the self test. If the polarity is reversed, the Analyzer
indicates this condition and allows the polarity to be
reversed internally. If the ground is open, the Analyzer
displays this fault. If the mains voltage is too high or too
low, the Analyzer displays this fault and does not continue
until the supply voltage is corrected and the Analyzer
power cycled off and then on again.
Accessing the Analyzer’s Functions
For each test and setup function, the Analyzer uses a
series of menus to access various Analyzer test and
setup variables. As shown in Figure 6, the Analyzer
indicates various leakage current tests along the bottom
of the display. An Exit selection is also indicated as a way
of backing out of the leakage current tests. Pressing a
softkey (F1 through F4) under a specific test will cause
the analyzer to setup for or perform the selected test.
In addition to the function softkeys, the Analyzer test
functions may require using the navigation buttons to
select parameters as well. In the example above, the
leakage selection has next to it. This icon indicates the
selection is controlled by pressing or . In this
example, the leakage current measurement is switched
between AC+DC, AC only, or DC only. The applied parts
indicator has on the left end and on the right end.
These icons indicate the use of and to select an
applied part.
fis102.jpg
Figure 6. Leakage Current Menu
The three buttons along the right side of the display
() control the wiring of the Analyzer’s
test receptacle for some electrical tests. The present state
of these three buttons is displayed along the right edge of
the display whenever these controls are active.
13
Page 28

ESA612
Users Manual
Figure 6 shows polarity is settable between normal,
reversed, and off. Neutral is also settable to closed or
open. Earth condition is not displayed, which indicates it
can not be changed. However, earth is internally opened
during this test.
Setting Up the Analyzer
There are a number of Analyzer parameters that are
adjusted through a setup function as well as the ability to
save a record by ID and date. To access the first Setup
menu shown in Figure 7, press .
fis114.bmp
Figure 7. Setup Menu
Note
See the Using Memory section later in this
manual for a description of how to enter a test
record ID.
The setup parameters have been grouped into six
categories: Instrument, Display, Sound, Instrument Info,
Calibration, and Diagnostics.
Setting Polarity Switching Delay
When switching the polarity of the Analyzer’s test
receptacle, a delay can be set to control the actual switch
time. To set the polarity delay:
1. From the setup menu, press the softkey labeled
More to reveal additional menu selections.
2. Press the softkey labeled Instrument to reveal the
instrument setup selections.
3. Press the softkey labeled Polarity Delay to open the
scroll box above the softkey label.
4. Press or to adjust the delay from 1 to 5
seconds in 1 second steps.
14
Page 29

Electrical Safety Analyzer
Setting Up the Analyzer
5. Press the softkey labeled Done to exit the switching
polarity delay setup function.
Setting the Display Contrast
There are two methods for setting the display contrast.
From the Select a Test…. menu or through the setup
menu.
Whenever the Analyzer displays its start-up menu (Select
a test…), pressing or will increase or decrease the
display’s contrast respectively. Press the softkey labeled
Done to exit contrast setup.
Another way to adjust the contrast is through the
Analyzer’s setup menu.
1. From the setup menu, press the softkey labeled
More twice to set F1 to the display contrast function.
2. Press the softkey labeled Display Contrast.
3. Press or to increase or decrease the display’s
contrast respectively.
4. Press the softkey labeled Done to exit contrast
setup.
Setting up the Beeper
To enable or disable the beeper:
1. From the setup menu, press the softkey labeled
More twice to set F2 to the beeper on/off function.
2. Press the softkey labeled Beeper to switch the
beeper on and off.
3. Press the softkey labeled Done to go back to the
setup menu.
Viewing Instrument Information
To view information about the Analyzer:
1. From the setup menu, press the softkey labeled
More twice to set F3 to the instrument information
function.
2. Press the softkey labeled Instrument Information.
3. After viewing the displayed information, press the
softkey labeled Done to exit the information screen.
15
Page 30

ESA612
Users Manual
Viewing Memory
Refer to the Using Memory section later in the manual to
learn about viewing memory and how to store data in the
Analyzer.
Setting the GFCI Limit
To set the GFCI current limit:
1. From the setup menu, press the softkey labeled
More to reveal additional menu selections.
2. Press the softkey labeled Instrument to reveal the
instrument setup selections.
3. Press the softkey labeled GFCI Limit to open the
scroll box above the softkey label.
4. Press or to adjust the current limit from 5 to
25 mA.
5. Press the softkey labeled Done to exit the GFCI Limit
setup function.
Performing Electrical Safety Tests
The Analyzer is designed to perform a number of different
electrical and performance tests on biomedical
equipment. The following sections describe the various
tests and how to perform them using the Analyzer.
Setting the Test Standard
The Analyzer is designed to perform electrical safety
testing based on a number of different safety standards:
AAMI ES1/NFPA99, IEC62353, IEC60601-1, and
AN/NZS 3551. AAMI is the Analyzer’s default standard.
To select another standard:
1. Press .
2. From the setup menu, press the softkey labeled
More to reveal additional menu selections.
3. Press the softkey labeled Instrument to reveal the
instrument setup selections.
4. Press the softkey labeled Standard to open the
scroll box above the softkey label.
5. Press or to scroll through the standard
selections.
16
Page 31

Electrical Safety Analyzer
Performing Electrical Safety Tests
6. When the desired standard is displayed, press the
softkey labeled Done.
Some electrical tests may not be applicable for a specific
standard. In these cases, the Analyzer’s menu will not
display the excluded test as a selection.
Performing Mains Voltage Testing
The Mains Voltage test measures the voltage on the
mains input through three separate measurements. To
access the Mains Voltage test, press . The Mains
Voltage test menu is shown in Figure 8.
fis104.jpg
Figure 8. Mains Voltage Test Menu
Press each function softkey to perform each of the three
measurements: Live to neutral, neutral to earth, and live
to earth.
Note
Power to the test receptacle is off during the
Mains Voltage test.
Performing a Ground Wire (Protective Earth) Resistance Test
The Ground Wire (Protective-Earth) Resistance test
measures the impedance between the Analyzer’s test
receptacle’s PE terminal and the exposed conductive
parts of the DUT that are connected to the DUT’s
Protective Earth.
Prior to conducting any leakage tests with the Analyzer, it
is best to test the integrity of the ground connection
between the Analyzer’s test receptacle ground and the
DUT’s Protective earth ground or enclosure with this test.
To access the Ground Wire (Protective Earth) ∅/Null
Resistance Test menu press .
Note
The DUT is powered off for this test.
17
Page 32

ESA612
Users Manual
To perform a ground wire resistance test:
1. Ensure the power cord from the DUT is plugged into
the Analyzer’s test receptacle.
2. Press to reveal the resistance function menu.
3. Connect one end of a test lead to the V/Ω/A jack as
shown in Figure 10.
4. If using an accessories probe, connect it to the other
end of the test lead and place the probe tip into the
∅/Null jack. If using an alligator clip accessory,
connect it to the other end of the test lead, place the
null post adapter in the ∅/Null jack, and clamp the
alligator clip to the null post adapter.
5. Connect the other end of the test lead to ∅/Null jack.
6. Press the softkey labeled Zero Leads. The Analyzer
zeroes out the measurement to cancel the test lead
resistance.
7. Connect the test lead coming from the ∅/Null jack to
the DUT enclosure or protective earth connection.
8. The measured resistance is displayed as shown in
Figure 9 after the DUT connection(s) is/are made.
fis105.jpg
Figure 9. DUT Ground Resistance Measurement
Warning
To avoid electric shock, remove the null post
adapter from the ∅/Null jack after a test lead
zero is performed. The ∅/Null jack becomes
potentially hazardous during some of the test
conditions.
18
Page 33

Electrical Safety Analyzer
Performing Electrical Safety Tests
A low resistance reading is required to confirm a good
ground connection through the power cord. Refer to the
appropriate electrical safety standard for the specific limit
value to be followed.
Figure 10 shows the electrical connections between the
Analyzer and the DUT. Table 4 lists the abbreviations
used in the schematics and their descriptions.
19
Page 34

ESA612
Users Manual
To protective earth or any exposed
conductive surface on the enclosure
RA
RF
LL
LA
L
RL
NC1
V1
TEST
Connect the DUT ac power cord to the
equipment outlet on the Analyzer
Figure 10. Ground Wire (Protective Earth) Resistance Measurement Connections
fis112.eps
20
Page 35

Electrical Safety Analyzer
Performing Electrical Safety Tests
Table 4. Schematic Abbreviations
Abbreviation Meaning
MD Measuring Device (ESA612 Analyzer)
FE Functional Earth
PE Protective Earth
Mains Mains Voltage Supply
L1 Live Conductor
L2 Neutral Conductor
DUT Device Under Test
DUT_L1 Device Under Test Live conductor
DUT_L2 Device Under Test neutral conductor
DUT_PE Device Under Test protective earth
REV POL Reversed mains supply polarity
LEAD GND Lead to ground, used in Patient leakage test
MAP Mains on Applied Part
MAP REV Reverse mains on applied part source voltage
PE Open Open protective earth
Test Voltage
21
Page 36

ESA612
Users Manual
DUT_L1
DUT_L2
DUT_PE
MD
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST LEAD
Figure 11. Ground Wire (Protective Earth) Resistance Measurement Schematic
faw26.eps
22
Page 37

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing an Insulation Resistance Test
The five insulation resistance tests take measurements on
mains (L1 & L2) to Protective earth, applied parts to
Protective earth, mains to applied parts, mains to nonearthed accessible conductive points, and applied parts to
non-earthed accessible conductive points.
To access the Insulation Resistance Test menu,
press .
All Insulation Resistance Tests can be performed using
500 or 250 volts dc. To change the test voltage from the
Insulation Resistance Test menu, press the softkey
labeled More. Pressing the softkey labeled Change
Voltage will cause the test voltage to toggle between 250
and 500 volts dc.
Note
Exiting and re-entering the Insulation Resistance
Test menu causes the test voltage to return to its
default value of 500 volts dc.
As shown in Figure 12, three of the five tests are shown
over function soft keys F1 through F3. To access the other
two tests or test voltage selection, press the softkey
labeled More. The softkey labeled Back will move the
menu back up to the top-level insulation resistance test
menu.
Figure 12. Insulation Resistance Measurement
After selecting one of the tests by pressing the appropriate
softkey, press to apply the selected voltage to the
DUT and take the resistance measurement.
Figures 13 through 17 shows the electrical connections
between the Analyzer and DUT for the five insulation
resistance tests.
Note
The DUT is powered off for this test.
fis106.jpg
23
Page 38

ESA612
Users Manual
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
MD
DUT_L1
DUT_L2
DUT_PE
Figure 13. Mains to Protective-Earth Insulation Resistance Test Schematic
faw17.eps
24
Page 39

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
DUT_PE
MD
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
Figure 14. Applied Parts to Protective-Earth Insulation Test Schematic
faw18.eps
25
Page 40

ESA612
Users Manual
MD
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
Figure 15. Mains to Applied Parts Insulation Test Schematic
faw19.eps
26
Page 41

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
MD
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST
LEAD
Figure 16. Mains to Non-Earth Accessible Conductive Points Schematic
faw20.eps
27
Page 42

ESA612
Users Manual
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
MD
FE
faw21.eps
Figure 17. Applied Parts to Non-Earth Conductive Points Schematic
28
Page 43

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Current Consumption Test
To measure the current consumed by the DUT, press
. The Analyzer displays the current flowing through
the mains connections of the test receptacle.
Performing Leakage Current Tests
The Analyzer measures leakage current for a number of
different DUT configurations. In addition to the leakage
found on the enclosure and the earth connection, the
Analyzer can measure leakage on each connected applied
part and combinations of connected applied parts.
Table 5. Test Names Based on Selected Standard
IEC60601 AAMI/NFPA 99
Protective Earth Resistance Ground Wire Resistance
Earth Leakage Current Ground Wire Leakage Current
Touch or Enclosure Leakage Current Chassis Leakage Current
Patient Leakage Current Lead to Ground Leakage Current
Patient Auxiliary Leakage Current Lead to Lead Leakage Current
Mains on Applied Part (MAP) Leakage Current Isolation Leakage Current
Which leakage tests are available depends on which
standard is selected. See the Selecting the Test Standard
section earlier in this manual to change the standard the
Analyzer is using.
Table 5 lists six leakage current tests that have different
names based on which standard is selected.
Press to access the leakage current main menu
shown in Figure 18.
29
Page 44

ESA612
Users Manual
fis117.jpg
Figure 18. Leakage Current Main Menu
Note
The display shown in Figure 18 is the main
leakage current menu when AAMI is the selected
standard.
All leakage currents, with the exception of Lead Isolation
(Mains on Applied parts), are displayed in one of three
ways: AC+DC, AC Only, or DC only. The initial result is
displayed in the appropriate parameter based on the
standard selected. To change the displayed parameter,
press or . The present measurement method is
displayed to the right of the current measurement while
leakage current tests are conducted.
Measuring Earth Leakage Current
Note
The Ground Wire (Earth) Leakage test is
available for AAMI, 60601, and not IEC 62353.
To measure the current flowing in the DUT’s protective
earth circuit, press the softkey labeled Ground Wire
(pending the standard) from the leakage current main
menu. Figure 19 shows the electrical connections between
the Analyzer and the DUT during a Ground Wire Leakage
Current Test.
Within the Ground Wire Leakage Current test there are a
few combination measurements that can be performed.
Pressing switches the polarity of the mains voltage
applied to the Analyzer’s test receptacle between Normal,
Off, Reverse, and Off. Pressing opens and closes
the neutral connection to the Analyzer’s test receptacle.
There is no need to open up the test receptacle earth
(ground), since this is done internally during the
measurement.
30
Page 45

Electrical Safety Analyzer
Performing Electrical Safety Tests
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Reversed Polarity
• Reversed Polarity, Open Neutral
IEC60601-1 specifies that the applied parts should be
connected for this measurement. Enable this
measurement by pressing or which grounds and
ungrounds all applied parts connection posts.
31
Page 46

ESA612
Users Manual
L1
MAINS
L2
MAINS
ON
PE
L2
MD
APPLIED PART
DUT_L1
DUT_L2
REV
POL
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
Figure 19. Earth Leakage Current Test Schematic
Note
Ground wire leakage is the same schematic without the Applied Parts switch.
FE
faw27.eps
32
Page 47

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Chassis (Enclosure) Lea kage Test
Note
The Chassis (Enclosure) Leakage test is only
available for the IEC60601 or ANSI/AAMI ES1
1993 standard selections.
The Chassis (Enclosure) Leakage Test measures the
current flowing between the DUT’s enclosure and
protective earth. Figure 20 shows the electrical
connections between the Analyzer and the DUT.
To perform a Chassis (Enclosure) Leakage Test:
1. Connect a lead between the Analyzer’s V/Ω/A jack
and the DUT’s enclosure.
2. Press the softkey labeled Chassis from the Leakage
Current Test menu.
3. The Analyzer displays the measured current.
The Chassis Leakage test can be performed with a
number of fault conditions on the test receptacle. Press
to switch the test receptacle between Normal, Off,
Reverse, and Off. Press to open and close the
neutral connection to the receptacle. Press to open
and close the receptacle’s earth connection.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Earth
• Normal Polarity, Open Neutral
• Reversed Polarity
• Reversed Polarity, Open Earth
• Reversed Polarity, Open Neutral
IEC60601-1 specifies that the applied parts should be
connected for this measurement. Enable this
measurement by pressing or which grounds and
ungrounds all applied parts connection posts.
33
Page 48

ESA612
Users Manual
L1
MAINS
L2
MAINS
ON
PE
L2
EARTH
MD
APPLIED PART
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST LEAD
Figure 20. Enclosure Leakage Current Test Schematic
Note
Chassis leakage is the same schematic without the Applied Parts switch.
FE
faw28.eps
34
Page 49

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Lead-to-Ground (Patient) Leakage Test
Note
The Lead-to-Ground (Patient) Leakage Current
Test is not available for IEC 62353 standard
selections.
The Lead-to-Ground (Patient) Leakage Current test
measures the current flowing between a selected applied
part, selected group of applied parts, or ALL applied parts,
and the Mains PE. Figure 21 shows the electrical
connections between the Analyzer and the DUT.
To perform a Lead-to Ground (Patient) leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select one of the applied part groupings by pressing
or .
Note
Refer to the testing standard when deciding the
type of the applied parts and how they should be
grouped for testing.
4. Press the soft key labeled Select.
5. Press or to advance through each applied part
grouping, or the individual applied parts, to ground.
These are selected and measured.
The Lead-to-Ground Leakage test can be performed with
a number of fault conditions on the test receptacle. Press
to switch the test receptacle between Normal, Off,
Reverse, and Off. Press to open and close the
neutral connection to the receptacle. Press to open
and close the receptacle’s earth connection.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Normal Polarity, Open Earth
• Reversed Polarity
• Reversed Polarity, Open Neutral
• Reversed Polarity, Open Earth
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
35
Page 50

ESA612
Users Manual
L1
MAINS
L2
MAINS
ON
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
SELECT
RELAY*
PE
LEAD GND
SELECT RELAY
(REMOTE ONLY)
LEAD
PE
L2
EARTH
DUT_L1
DUT_L2
REV
POL
DUT_PE
MD
Figure 21. Lead-to-Ground (Patient) Leakage Current Test Schematic
FE
*Leads not selected are open.
gtv29.eps
36
Page 51

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing Lead-to-Lead (Patient Auxiliary) Leakage Tests
Note
The Lead-to-Lead (Patient Auxiliary) leakage test
is available when the IEC60601 or ANSI/AAMI
ES1-1993 standard is selected.
To measure the leakage current through each applied part
or lead and selected combination of lead connections (all
other or between two), press the softkey labeled Lead to
Lead from the Leakage Test main menu shown in
Figure 18. Figure 23 shows the electrical connections
between the Analyzer and the DUT during a Lead-to-Lead
(Patient Auxiliary) Leakage Current Test.
The Lead-to-Lead (Patient Auxiliary) Leakage test adds a
diagram of the applied parts connection posts to the
display, as shown in Figure 22. In the figure, the applied
parts post RA/R is shown above the other posts. This
indicates that the leakage measurement is being made
from RA/R to all others. To move to the next applied part
post, press . The first post will appear inline with the
other posts while the LL/F post appears above all others.
This indicates the second leakage measurement is being
made from LL/F to all others. Continue pressing or to
move from one connection post to another and noting the
measured current in the display.
After each post is isolated individually, the Lead-to-Lead
(Patient Auxiliary) Leakage test measures current of three
different combinations of posts tied together: RA/R and
LL/F, RA/R and LA/L, or LL/F and LA/L.
fis107.eps
Figure 22. Applied Parts Connection Posts Display
Within the Lead-to-Lead (Patient Auxiliary) Leakage test, a
number of fault measurements can be made. Pressing
switches the polarity of the mains voltage applied
to the Analyzer’s test receptacle between Normal, Off,
Reverse, and Off. Pressing opens and closes the
neutral connection to the Analyzer’s test receptacle.
Pressing opens and closes the earth or ground
connection to the Analyzer’s test receptacle.
37
Page 52

ESA612
Users Manual
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
38
Page 53

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
L2
EARTH
DUT_L1
DUT_L2
REV
POL
DUT_PE
DEVICE UNDER TEST
CONDUCTIVE PART
MD
PE
APPLIED
PART
+
LEAD
SELECT
RELAY*
- LEAD
SELECT
RELAY*
LEAD GND
SELECT RELAY*
(REMOTE ONLY)
FE
Figure 23. Lead-to-Lead (Patient Auxiliary) Leakage Current Test Schematic
*Leads not selected are open.
gtv30.eps
39
Page 54

ESA612
Users Manual
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Normal Polarity, Open Neutral
• Normal Polarity, Open Earth
• Reversed Polarity, Open Neutral
• Reversed Polarity, Open Earth
Performing a Lead Isolation (Mains on Applied Part) Leakage Test
Note
The Lead Isolation (Mains on Applied Part)
leakage test is available when the IEC60601 &
ANSI/AAMI standard is selected.
The Lead Isolation (Mains On Applied Parts) Leakage
Current test measures the current that flows in response
to an isolated AC voltage applied between a selected
applied part, group of applied parts, or ALL applied parts,
and Earth (and any conductive part connected to the RED
terminal). Figure 24 shows the electrical connections
between the Analyzer and the DUT during a Mains on
Applied Part Leakage Current Test.
Note
With 60601 standard selected, the MAP test
voltage is available in both Normal and Reverse
(180 degrees out of phase with mains).
To perform a Lead Isolation (Mains on Applied Part) test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
Note
Refer to the testing standard when deciding the
type of the applied parts and how they should be
grouped for testing.
4. Press the soft key labeled Select.
5. Press the soft key labeled Lead Isolation.
6. Press or to select the desired applied part
connection.
7. Press to apply the voltage and read the leakage
current in the display.
Pressing and scrolls through the applied part
connections or groupings. Press for each
connection configuration to thoroughly test the DUT.
40
Page 55

Electrical Safety Analyzer
Performing Electrical Safety Tests
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Reverse Polarity
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
41
Page 56

ESA612
Users Manual
L1
MAINS
L2
MAINS
On
PE
MAP (ISOLATION)
TRANSFORMER
Figure 24. Lead Isolation (Mains On Applied Parts) Leakage Test Schematic
REV
POL
MAP
REV
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
CONDUCTIVE PART
TEST
LEAD
MD
APPLIED
PART
LEAD
SELECT
RELAY*
FE
*Leads not selected are open.
gtv31.eps
42
Page 57

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing an Alternative Equipment Leakage Test
Note
The alternative equipment leakage test is
available when the EN62353 standard is
selected.
During the Alternative Equipment Leakage test, the
voltage source is applied between short-circuited
equipment outlet mains live, neutral, and equipment outlet
earth, the exposed conductive surface on the housing, and
all applied parts short-circuited together. Equipment is
separated from mains during the test. The current which
flows over the insulation of the DUT is measured.
This test is not applicable for equipment with internal
electrical power source. The switches in mains part shall
be closed during measurement.
To perform an alternative equipment leakage test:
1. Press .
2. Press the softkey labeled Alternative Equipment.
3. Press to apply the voltage and read the current
in the display.
Figure 25 shows the electrical connections between the
Analyzer and the DUT during an Alternative Equipment
Leakage Test.
The following outlet conditions apply when performing this
test:
• Closed Earth
• Open Earth
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
43
Page 58

ESA612
Users Manual
Performing an Alternative Applied Part Leakage Test
Note
The Alternative applied part leakage test is
available when the EN62353 standard is
selected.
During the Alternative Applied Part Leakage test, the test
voltage is applied between short-circuited applied parts of
a single function and the short-circuited equipment outlet
mains live, neutral, equipment outlet earth, and exposed
conductive surface on the housing. This test should only
be done for equipment with F-Type applied parts. For
equipment with multiple applied parts, test each group of
applied parts of a single function in turn with all others
floating during the test
to the Analyzer’s applied parts jacks and the lead selection
will float those not selected.
. All applied parts can be connected
44
Page 59

Electrical Safety Analyzer
Performing Electrical Safety Tests
DUT_L1
DUT_L2
DUT_PE
MD
DEVICE UNDER TEST
APPLIED
PART
FE
CONDUCTIVE PART
TEST
LEAD
Figure 25. Alternative Equipment Leakage Current Test Schematic
faw22.eps
45
Page 60

ESA612
Users Manual
To perform an alternative applied part leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
4. Press the soft key labeled Select.
5. Press the soft key labeled Alternative A.P..
6. Press to apply the test voltage and read the
current in the display.
7. Press or to advance to the next applied part
group(s) of a single function if applicable. Pressing
to read leakage current for each group.
Figure 26 shows the electrical connections between the
Analyzer and the DUT during an Alternative Applied Part
Leakage current test.
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
Performing a Direct Equipment Leakage Test
Note
The Direct Equipment Leakage test is available
when the EN62353 standard is selected.
The Direct Equipment Leakage Current test measures the
leakage current between all applied parts and the exposed
conductive surface on the housing, to mains earth.
To perform a direct equipment test:
1. Press .
The direct equipment test is the default test and should
already be selected.
2. Press to apply the voltage and read the leakage
current in the display
Figure 27 shows the electrical connections between the
Analyzer and the DUT during a Direct Equipment Leakage
Current Test.
46
Page 61

Electrical Safety Analyzer
Performing Electrical Safety Tests
The following outlet conditions apply when performing this
test:
• Normal Polarity, Closed Earth
• Normal Polarity, Open Earth
• Reversed Polarity, Closed Earth
• Reversed Polarity, Open Earth
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
47
Page 62

ESA612
Users Manual
DUT_L1
DUT_L2
DUT_PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
LEAD
SELECT
RELAY*
*Leads not selected
are open.
FE
MD
gtv23.eps
Figure 26. Alternative Applied Part Leakage Test Schematic
48
Page 63

Electrical Safety Analyzer
Performing Electrical Safety Tests
Performing a Direct Applied Part Leakage Test
Note
The Direct Applied Part Leakage test is available
when the EN62353 standard is selected.
The Direct Applied Part Leakage Current test measures
the leakage current between all applied parts of a single
function and the exposed conductive surface on the
housing, to mains earth. For equipment with multiple
applied parts, each group of a single function should be
tested each in turn with all other floating during the test.
This test should only be done for equipment with F-Type
applied parts.
For Type B applied part, see direct equipment leakage
schematic in Figure 27.
To perform a direct applied part leakage test:
1. Press .
2. Press the soft key labeled More.
3. Select the desired applied part groupings using
and .
4. Press the soft key labeled Select. The Direct A.P. test
should already be selected.
5. Press or to select the applied part test
configuration.
6. Press to apply the test voltage and read the
current in the display.
7. Press or to advance to the next group of applied
parts, if applicable.
Figure 28 shows the electrical connections between the
Analyzer and the DUT during a Direct Applied Part
Leakage Current Test.
The following outlet conditions apply when performing this
test:
• Normal Polarity
• Reversed Polarity
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
49
Page 64

ESA612
Users Manual
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
FE
faw24.eps
L1
MAINS
L2
MAINS
ON
PE
L2
MD
REV
POL
PE
DUT_L1
DUT_L2
DUT_PE
Figure 27. Direct Equipment Leakage Test Schematic
50
Page 65

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
L2
DUT_L1
DUT_L2
REV
POL
DUT_PE
Figure 28. Direct Applied Parts Leakage Current Test Schematic
LEAD
SELECT
RELAY*
*Leads not selected
are open.
FE
MD
gtv25.eps
51
Page 66

ESA612
Users Manual
Performing a Differential Leakage Current Test
Note
The Differential Leakage Current test is available
when the EN62353 standard is selected.
The differential leakage current test measures the
magnitudes of the differential current flowing in the
Equipment Outlet live and neutral, with power applied to
the equipment outlet. All applied parts should be
connected during this test, if equipment has applicable
applied parts.
To perform a differential leakage current test:
1. Press .
2. Press the soft key labeled Differential.
Figure 29 shows the electrical connections between the
Analyzer and the DUT during a Differential Leakage
Current test.
The following outlet conditions apply when performing this
test:
• Normal Polarity, Closed Earth
• Normal Polarity, Open Earth
• Reversed Polarity, Closed Earth
• Reversed Polarity, Open Earth
Note
If there are more than five applied parts to
connect to the Analyzer, see Using the 1-to-10
Adapter later in this manual.
52
Page 67

Electrical Safety Analyzer
Performing Electrical Safety Tests
L1
MAINS
L2
MAINS
ON
PE
DEVICE UNDER TEST
APPLIED
PART
CONDUCTIVE PART
TEST
LEAD
REV
POL
EARTH
DUT_L1
MD
DUT_L2
DUT_PE
Figure 29. Differential Leakage Current Test Schematic
FE
gtv32.eps
53
Page 68

ESA612
Users Manual
Using the 1-to-10 Adapter
The 1-to-10 Adapter, an optional accessory, is designed to
increase the number of lead or applied parts connections
to the Analyzer from five to 14. The adapter itself ties up to
ten leads together into a single lead that is plugged into
one of the input jacks of the Analyzer. The four remaining
Analyzer input jacks can also be used in conjunction with
the Adapter.
Figure 30 shows one application of the Adapter. The
Defibrillator/Monitor in the example has ten ECG leads,
two pacer leads, and two defibrillator paddles which need
to be tested together, and in groups if single function, for
current leakage per IEC62353. The example shows the
ECG leads to be snap type connectors and two BJ2ECG
adapters are shown plugged into the Adapter. If the ECG
leads did not have snap connectors, then the Universal
Snap to Banana Adapter can be used to make the
connections to the Adapter.
The common lead from the Adapter is plugged into the RA
st
jack (1
leads with alligator clips, connect the two defibrillator
paddles into the LL and LA Analyzer jacks and the two
pacer leads into the RL and V1 jacks. Using the selection
that ties all five Analyzer jacks together will test all
fourteen leads for leakage current. Using the selection of
applied part group of 1, 2, and 2 allows for testing of
groups of applied parts of single function.
jack) of the Analyzer. Using four sheathed test
54
Page 69

Electrical Safety Analyzer
Using the 1-to-10 Adapter
Defibrillator/Pacer
Use leads shorted
for electrical safety
testing only. Do not
short leads for ECG
simulation.
Caution: To prevent damage to the Product,
ESA612
do not apply more than 30 V dc to
DEFIB
OFF
PACER
the AP (ECG).
Check unkown connections using a
DMM before connecting to ESA612.
1-to-10 and
Alligator Clips
BJ2ECG
snap adapters
fis120.eps
Figure 30. 1-to-10 Adapter Connections
55
Page 70

ESA612
Users Manual
When performing an applied parts test using the
AAMI/NFPA-99 standard, the normal connections of RA,
LL, LA, and RL are made to their associated Analyzer
input jacks. Four adapters from the Universal Snap to
Banana Adapter set will be required for the first four
connections. The remaining chest leads are connected to
the Adapter and the common lead from the Adapter is
plugged into the V1 jack (5
Figure 31. This configuration allows for isolation of the RA,
LL, LA, and RL leads from each other and the remaining
chest leads, which are shorted together, while performing
leakage tests.
th
jack) of the Analyzer. See
56
Page 71

Electrical Safety Analyzer
Using the 1-to-10 Adapter
ECG Monitor
Use leads shorted
for electrical safety
testing only. Do not
short leads for ECG
simulation.
1-to-10 and
BJ2ECG
snap adapters
Figure 31. ECG Lead Connection with 1-to-10 Adapter
ESA612
fis121.eps
57
Page 72

ESA612
Users Manual
Making Point-To-Point Measurements
The Analyzer can make voltage, resistance, and low
current measurements through its Point-to-Point function.
To access the Point-to-Point function menu shown in
Figure 32, press . Softkeys F1 through F3 are used
to select the measurement function.
fis108.jpg
Figure 32. Point-To-Point Function Menu
Measuring Voltage
To make a voltage measurement:
1. Press the softkey labeled Voltage from the
Point-To-Point menu.
2. Insert test leads in the RED (V/Ω/A ) and BLACK
jacks.
3. Place the probe tips across the unknown voltage and
read the measurement in the Analyzer’s display.
The Analyzer will measure up to 300 volts ac.
Measuring Resistance
To make a resistance measurement:
1. Press the softkey labeled Resistance from the
Point-To-Point menu.
2. Insert test leads in the RED (V/Ω/A ) and BLACK
jacks.
3. Null lead resistance by shorting the leads together
and pressing the softkey labeled Zero Leads.
4. Place the probes across the unknown resistance and
read the measurement in the Analyzer’s display.
The Analyzer will measure resistances up to 2.0 Ω.
58
Page 73

Electrical Safety Analyzer
Simulating ECG Waveforms
Measuring Current
The Analyzer can make dc only, ac only, and ac+dc
current measurements up to 10 mA. To make a current
measurement:
1. Press the softkey labeled Leakage from the
Point-To-Point menu.
2. Using or select between ac only, dc only, or
ac+dc measurement mode.
3. Insert test leads in the RED (V/Ω/A ) and BLACK
jacks.
4. Place the leads on the two points the unknown
current may flow and read the measurement in the
Analyzer’s display.
Simulating ECG Waveforms
The Analyzer is capable of generating various waveforms
on the applied parts connection posts. These signals are
used to test the performance characteristics of ECG
monitors and ECG strip printers. See Figure 34 for proper
connections between the Analyzer and an ECG monitor.
For monitors using the snap style connectors, insert the
BJ2ECG adapter into the connectors at the top of the
Analyzer and connect the monitor leads to the snap
connectors on the adapter.
Note
If the ECG monitor/interpreter has banana posts
instead of snaps, use the optional universal snap
to banana adapter to connect to the Analyzer.
To access the ECG Simulation Waveform menu shown in
Figure 33, press . From this menu, a number of
different waveforms are selected through F1, and the rate
or frequency of the waveform is selected through F2.
fis109.jpg
Figure 33. ECG Waveform Simulation Menu
59
Page 74

ESA612
Users Manual
To select one of the predefined waveforms, press the
softkey labeled Wave Form. A scroll box with next to it
appears above the softkey label. Use or to scroll
through the different waveforms.
For all waveforms except VFIB and Triangle, the rate or
frequency of the waveform is adjusted through the softkey
labeled Frequency or Rate. For some waveforms, there
are more than two frequency or rate selections. For those
waveforms, pressing the softkey labeled Frequency or
Rate will open a scroll box above the softkey label with
next to it. Use or to select the frequency or rate. For
those waveforms that have only two frequency or rate
selections, the softkey labeled Frequency or Rate acts as
a toggle, where each press of the softkey switches to the
other value.
60
Page 75

Electrical Safety Analyzer
Simulating ECG Waveforms
ECG Monitor
RA LL
RFLNC1
LA
TEST
RL
V1
C1
V1
R
RA
FLN
LL LA RL
BJ2ECG
Adapter
Figure 34. ECG Monitor Connections
fis115.eps
61
Page 76

ESA612
Users Manual
Using Memory
The non-volatile memory of the Analyzer will store up to
500 measurements or ECG information for each of 100
different Test Records. Each test record can be recalled to
the display of the Analyzer or exported to a PC. Data
Viewer software, available at
www.flukebiomedical.com/biomedical/usen/Support/softwa
re, is required to upload memory data to a PC. Installation
and operator instructions are also located on the software
web page.
Note
It is recommended that memory data be
uploaded daily or as often as possible to reduce
upload time.
Storing Data into Memory
Each of the six measurement functions allow data storage
within the Analyzer. The ECG function allows storage of
the simulated waveforms only for reference of tests
performed. To store data, a Test Record ID should be
created first. To input a new Test Record ID:
1. Press to open the screen shown in Figure 35.
2. Press or to set the first character. Available
characters are 0-9, A-Z, and space.
3. Press to move to the next character position.
4. Fill in up to 15 character positions to identify a test
record.
5. Press (enter save button) to store the Test Record ID.
6. Use , , , and to enter the date of the Test
Record.
7. Press to store the date.
The Test Record ID is displayed in the upper-left
corner of the display.
Note
If no record ID data is entered, a default ID of
0000000000000001 and a date of --/--/-- is
assigned to the record.
62
Page 77

Electrical Safety Analyzer
Using Memory
Viewing Memory Data
Previously stored data for any Test Record is recalled to
the screen through the setup menus. To recall data:
1. Press .
2. Press the softkey labeled More to reveal additional
menu selections.
3. Press the softkey labeled View Memory.
4. Select the desired Test Record by pressing or
Figure 35. Test Record ID Entry Screen
8. After selecting a function, push to store the
measurement or ECG signal under the Test Record
ID.
After performing and saving all the tests for one Test
Record ID, enter a new Test Record ID to close the
previous record and start a new one.
Note
A previously closed record can not have
measurements or ECG signals added to it. Data
can only be stored under the most recently
opened record.
fis114.bmp
to scroll through the records listed in the display.
5. Press the softkey labeled View to view the data
stored for the selected record.
If the stored data is more than can fit on one screen, press
the softkey labeled Next Page to view the additional data.
63
Page 78

ESA612
Users Manual
Deleting Data from Memory
To delete a Test Record and its associated data from
memory:
1. Press .
2. Press the softkey labeled More to reveal additional
menu selections.
3. Press the softkey labeled View Memory.
4. Select the desired Test Record by pressing or
to scroll through the records listed in the display.
5. Press the softkey labeled Delete.
Note
All Test Records can be deleted at this point by
pressing the softkey labeled Delete All.
6. When Delete? appears in the display, press the
softkey labeled Delete to remove the record from
memory. Otherwise press the softkey labeled Cancel
to return to the Test Record list and leave the
selected record in memory.
Note
Deleting the last or current record does not open
the previous record for additional data storage.
Only a new Test Record can be opened for data
recording after deleting the last record.
Controlling the Analyzer Remotely
Fluke Biomedical Ansur test automation software allows a
solutions-based approach to complete testing of the
medical device under test (DUT). Ansur helps create
standard work using the test template/sequence (which is
based on a user written test procedure), and integrates all
test results into a single test report which can be printed or
archived. Ansur allows for automatic comparisons to the
limits of the standard selected, indicating whether results
are passing or failing. Ansur manages test procedures by
allowing both manual and visual automated test
sequences.
64
Page 79

Electrical Safety Analyzer
Maintenance
The software works hand-in-hand with Fluke Biomedical
analyzers and simulators, creating a seamless integration
for:
• Visual inspections
• Preventive maintenance
• Work procedures
• Performance tests
• Safety tests
Ansur software utilizes plug-in modules to work with a
wide array of Fluke Biomedical instruments. The plug-in
module is a software interface to the Ansur test program.
The plug-in modules are available for purchase as an
optional accessory. Plug-ins provide test elements used
by Ansur. This has the benefit of using the same user
interface for all analyzers and simulators supported by an
Ansur plug-in.
When a new Fluke Biomedical analyzer or simulator is
purchased, simply update your existing Ansur software by
installing a new plug-in. Each plug-in module works only
with the options and capabilities needed for the instrument
being tested.
Fluke Biomedical recommends you download the latest
Ansur Software and plug-in module from
www.flukebiomedical.com to make sure the software and
Product are compatible.
Maintenance
The Analyzer needs little maintenance or special care.
However, treat it as a calibrated measuring instrument.
Avoid dropping or other mechanical abuse that could
cause a shift in the calibrated settings.
Testing and Replacing the Fuses
Warning
To prevent electric shock, remove all power
cords and test leads from the Analyzer before
opening the fuse door.
For electrical protection of the equipment outlet, the
Analyzer uses two fuses, one in the live (L1) line and one
in the neutral (L2) line.
65
Page 80

ESA612
Users Manual
To test the fuses, do the following while referring to
Figure 36:
1. Turn the Analyzer so the case bottom is facing up.
2. Flip up the tilt stand.
3. Remove the fuse door from the Analyzer by removing
the screw holding the fuse door with a #2 Phillips
head screwdriver and lifting the fuse door from the
Analyzer.
4. Remove the fuses from the Analyzer.
F1 - F2
fis111.eps
Figure 36. Fuse Access
5. Using a multimeter, measure the continuity of each
fuse.
If one or both fuses do not show continuity, replace
the fuse(s) with fuses that have the same current and
voltage rating. Appropriate fuse ratings are posted on
the case bottom label of the Analyzer. Table 6 lists
available fuses with Fluke Biomedical part numbers.
6. Reinstall the fuse door and secure it with the screw.
66
Page 81

Electrical Safety Analyzer
Cleaning the Analyzer
Cleaning the Analyzer
Warning
To avoid electric shock, do not clean the
Analyzer plugged into mains or attached to a
DUT.
Caution
Do not pour fluid onto the Analyzer surface;
fluid seepage into the electrical circuitry may
cause the Analyzer to fail.
Caution
Do not use spray cleaners on the Analyzer;
such action may force cleaning fluid into the
Analyzer and damage electronic components.
Clean the Analyzer occasionally utilizing a damp cloth and
mild detergent. Take care to prevent the entrance of
liquids.
Wipe down the adapter cables with the same care. Inspect
them for damage to and deterioration of the insulation.
Check the connections for integrity before each use.
67
Page 82

ESA612
Users Manual
Replaceable Parts
Table 6 lists the replaceable parts for the Analyzer.
Table 6. Replaceable Parts
Item Fluke Biomedical Part Number
ESA612 Getting Started Manual 3334511
ESA612 Users Manual CD 3334509
USA 2238680
UK 2238596
Australia 2238603
Power Cord
Null Post Adapter 3326842
Ansur, CD with demo version 2795488
5-to-5 Banana jack to ECG (BJ2ECG) adapter 3359538
Europe 2238615
France/Belgium 2238615
Thailand 2238644
Israel 2434122
Switzerland 3379149
68
Page 83

Electrical Safety Analyzer
Replaceable Parts
Table 6. Replaceable Parts (cont.)
Item Fluke Biomedical Part Number
Carrying Case
Data Transfer Cable
USA
Fuse
15 – 20 A Adapter
ESA USA/AUS/ISR Accessory Kit:
Test Lead Set
TP1 Test Probe Set
AC285 Alligator Clip Set
ESA EUR Accessory Kit:
Test Lead Set
TP74 Test Probe Set
AC285 Alligator Clip Set
To ensure safety, use exact replacement only.
Australia, Switzerland
Europe, UK, Thailand,
France/Belgium, Israel
T20A 250V Fuse (Time Lag), 1¼ in x ¼ in
T10A 250V Fuse (Time Lag), 1¼ in x ¼ in
T16A 250V Fuse (Time Lag), 6.3 mm x 32 mm
2248650
1626219
2183691
109298
3321245
2195732
3111008
3111024
69
Page 84

ESA612
Users Manual
Accessories
Table 7 lists the available accessories for the Analyzer.
Table 7. Accessories
Item Fluke Biomedical Part Number
Test Leads with Retractable Sheath 1903307
Ground Pin Adapters 2242165
1-to-10 ECG Adapter 3392119
Universal Snap to Banana Adapter 2462072
Ansur ESA612 Plug-In License 3454829
70
Page 85

Electrical Safety Analyzer
Specifications
Specifications
Temperature
Operating ............................................................ 10 °C to 40 °C (50 °F to 104 °F)
Storage ................................................................ -20 °C to 60 °C (-4 °F to 140 °F)
Humidity ................................................................. 10 % to 90 % non-condensing
Altitude
120 V ac mains supply voltage ........................... 5000 m
230 V ac mains supply voltage ........................... 2000 m
Display .................................................................... LCD display
Communications ................................................... USB device port for computer control
Modes of Operation ............................................... Manual and remote
Power
120 Volt power outlet .......................................... 90 to 132 V ac rms, 47 to 63 Hz, 20 A maximum
230 Volt power outlet .......................................... 180 to 264 V ac rms, 47 to 63 Hz, 16 A maximum
Weight .................................................................... 1.6 kg (3.5 lb)
Size ......................................................................... 28.5 cm x 17.6 cm x 8.4 cm (11.2 in x 6.9 in x 3.3 in)
Safety Standards
CE ....................................................................... IEC/EN61010-1 2
CSA ..................................................................... CAN/CSA-C22.2 No 61010-1; UL61010-1
Electromagnetic Compatibility Standards (EMC)
European EMC .................................................... EN61326-1
nd
Edition; Pollution degree 2
71
Page 86

ESA612
Users Manual
Detailed Specifications
Test Standard Selections ........................................ ANSI/AAMI ES-1, IEC62353, IEC60601-1, and AN/NZS 3551
Voltage
Ranges (Mains voltage) ...................................... 90.0 to 132.0 V ac rms
Range (Point-to-point voltage) ............................ 0.0 to 300.0 V ac rms
Accuracy ............................................................. ±(2 % of reading + 0.2 V)
Earth Resistance
Modes ................................................................. Two terminal
Test Current ........................................................ >200 mA ac
Range ................................................................. 0.000 to 2.000 Ω
Accuracy ............................................................. ±(2 % of reading + 0.015 Ω)
Equipment Current
Range ................................................................. 0.0 to 20.0 A ac rms
Accuracy ............................................................. ±(5 % of reading + (2 counts or 0.2 A, whichever is greater))
Duty cycle ........................................................... 15 A to 20 A, 5 min. on/5 min. off
Leakage Current
Modes* ................................................................ AC+DC (True-rms)
Patient Load Selection ........................................ AAMI ES1-1993 Fig. 1
180.0 to 264.0 V ac rms
10 A to 15 A, 7 min. on/3 min. off
0 A to 10 A, continuous
AC only
DC only
* Modes: AC+DC, AC only, and DC only available for all leakages with exception of MAP
that are available in True-rms (shown as AC+DC)
IEC 60601: Fig. 15
72
Page 87

Electrical Safety Analyzer
Detailed Specifications
Crest factor .......................................................... ≤3
Ranges ................................................................ 0.0 to 199.9 μA
Accuracy
DC to 1 kHz ..................................................... ±(1 % of reading + (1 μA or 1 LSD, whichever is greater))
1 to 100 kHz .................................................... ±(2 % of reading + (1 μA or 1 LSD, whichever is greater))
1 to 5 kHz (current > 1.6 mA) .......................... ±(4 % of reading + (1 μA or 1 LSD, whichever is greater))
100 kHz to 1 MHz ............................................ ±(5 % of reading + (1 μA or 1 LSD, whichever is greater))
Accuracy for Isolation, MAP, Direct AP, Alternative AP, and Alternative Equipment leakage tests all ranges are:
• At 120 VAC + (2.5 µA or 1 LSD, whichever is greater)
• At 230 VAC additional ± 3.0 % and + (2.5 µA or 1 LSD, whichever is greater)
For Alternative and Direct AP leakage tests, the leakage values are compensated for nominal mains as per 62353.
Therefore, the accuracy specified for other leakages is not applicable.
Mains on applied part test voltage ...................... 100 % ±7 % of Mains for AAMI, current limited to 1 mA ±25 % per AAMI
200 to 1999 μA
2.00 to 10.00 mA
Note
100 % ±7 % of Mains for IEC 62353 current limited to 3.5 mA ±25 % per IEC 62353
100 % ±7 % of Mains for IEC 60601-1 current limited to 7.5 mA ±25 % per IEC 60601-1
73
Page 88

ESA612
Users Manual
Differential leakage
Ranges ................................................................ 75 to 199 μA
Accuracy ............................................................. ±(10 % of reading + (2 counts or 20 μA, whichever is greater))
Insulation resistance
Ranges ................................................................ 0.5 to 20.0 MΩ
Accuracy
20 MΩ Range .................................................. ±(2 % of reading + 0.2 MΩ)
100 MΩ Range ................................................ ±(7.5 % of reading + 0.2 MΩ)
Source test voltage ............................................. 500 V dc (+20 %, -0 %) 2.0 ±0.25 mA short-circuit current or 250 V dc selectable
Maximum load capacitance ................................ 1 μF
ECG Performance Waveforms
Accuracy
Frequency ....................................................... ±2 %
Amplitude ........................................................ ±5 % of 2 Hz square wave only, fixed @ 1 mV Lead II configuration
Waveforms
ECG Complex ................................................. 30, 60, 120, 180, and 240 BPM
Ventricular Fibrillation
Square wave (50 % duty cycle) ...................... 0.125 Hz and 2 Hz
Sine wave ....................................................... 10, 40, 50, 60, and 100 Hz
Triangle wave .................................................. 2 Hz
Pulse (63 ms pulse width) ............................... 30 BPM and 60 BPM
200 to 1999 μA
2.00 to 20.00 mA
20.0 to 100.0 MΩ
74
 Loading...
Loading...