Page 1

180
Electrical Safety Analyzer
Users Guide
PN 2185829
May 2008
© 2008 Fluke Corporation, All rights reserved. Printed in USA. Specifications subject to change without notice.
All product names are trademarks of their respective companies.
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and
workmanship for one year from the date of original purchase. During the warranty period, we will repair or at our option replace, at no charge, a product
that proves to be defective, provided you return the product, shipping prepaid,
to Fluke Biomedical. This warranty covers the original purchaser only and is
not transferable. The warranty does not apply if the product has been damaged
by accident or misuse or has been serviced or modified by anyone other than
an authorized Fluke Biomedical service facility. NO OTHER WARRANTIES,
SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE EXPRESSED
OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL,
INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES OR
LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE
OR THEORY.
This warranty covers only serialized products and their accessory items that
bear a distinct serial number tag. Recalibration of instruments is not covered
under the warranty
This warranty gives you specific legal rights and you may also have other
rights that vary in different jurisdictions. Since some jurisdictions do not allow
the exclusion or limitation of an implied warranty or of incidental or consequential damages, this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or other decision-maker of competent jurisdiction, such holding will not affect the validity
or enforceability of any other provision.
07/07
Page 3
Notices
All Rights Reserved
© Copyright 2007, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language without the written
permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and
other printed materials for use in service training programs and other technical publications. If
you would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for
damage. If damage is found, stop unpacking the instrument. Notify the carrier and ask for an
agent to be present while the instrument is unpacked. There are no special unpacking instructions,
but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email
techservices@flukebiomedical.com
1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical
damage is found, retain all packing materials in their original condition and contact the carrier
immediately to file a claim. If the instrument is delivered in good physical condition but does not
operate within specifications, or if there are any other problems not caused by shipping damage,
please contact Fluke Biomedical or your local sales representative.
or call 1-800-648-7952 or
Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and
items bearing a distinct serial number tag) are eligible for partial refund and/or credit.
Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules,
etc.) are not eligible for return or refund. Only products returned within 90 days from the date
of original purchase are eligible for refund/credit. In order to receive a partial refund/credit of a
product purchase price on a serialized product, the product must not have been damaged by the
customer or by the carrier chosen by the customer to return the goods, and the product must be
returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not in
“as new” and resalable condition, are not eligible for credit return and will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of
15 %. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a
minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will be applied to all returns.
Page 4
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to
our factory location. When you return an instrument to Fluke Biomedical, we recommend using
United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure
your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost
shipments or instruments that are received in damaged condition due to improper packaging or
handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double-walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive
material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order Entry Group at 1-800-648-7952 or 1-425-446-
6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-993-5853
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
material around the instrument.
, or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s
manufacturing specifications when it was shipped from the factory. Calibration measurements
are traceable to the National Institute of Standards and Technology (NIST). Devices for which
there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may
result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Page 5

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment
by Fluke Biomedical. Changes made to the information in this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The 180 Electrical Safety Analyzer is manufactured in Everett, Washington by Fluke
Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Page 6

Page 7

Table of Contents
Chapter Title Page
1 Introduction and Specifications.............................................. 1-1
Introduction .......................................................................................... 1-3
Simplicity.......................................................................................... 1-3
Versatility ......................................................................................... 1-3
General Safety Considerations.............................................................. 1-4
Symbols ............................................................................................ 1-4
Warnings and Cautions..................................................................... 1-4
Key Features......................................................................................... 1-6
Instrument Familiarity .......................................................................... 1-7
Specifications........................................................................................ 1-10
Accessories ........................................................................................... 1-12
2 Operation, Maintenance, and Service..................................... 2-1
Preliminary Steps.................................................................................. 2-3
Preparing the Analyzer for Use......................................................... 2-3
Verifying the Power Outlet Connections .......................................... 2-4
Measuring Line Voltage ....................................................................... 2-5
Measuring Device Current.................................................................... 2-6
Measuring Chassis Grounding Resistance ............................................ 2-7
Measuring Leakage Current.................................................................. 2-8
Ground Leakage Current................................................................... 2-8
Chassis (Enclosure) Leakage Current ............................................... 2-9
Lead-to-Ground (Patient Source) Current......................................... 2-10
Lead-to-Lead (Auxiliary) Current..................................................... 2-12
Lead Isolation (Patient Sink) Current ............................................... 2-13
Point-to-Point Measurements................................................................ 2-14
Leakage Current................................................................................ 2-14
Isolation Current ............................................................................... 2-15
Resistance ......................................................................................... 2-16
Maintenance.......................................................................................... 2-17
Avoiding Damage............................................................................. 2-17
Cleaning............................................................................................ 2-17
Service and Calibration......................................................................... 2-18
Packing Instructions.......................................................................... 2-18
Shipping............................................................................................ 2-19
Appendices
A Abbreviations................................................................................ A-1
i
Page 8

180
Users Guide
ii
Page 9

List of Tables
Table Title Page
1-1. Symbols ................................................................................................ 1-4
1-2. Components and Controls of the Analyzer ........................................... 1-8
1-3. Standard Accessories ............................................................................ 1-12
1-4. Optional Accessories ............................................................................ 1-12
List of Figures
Figure Title Page
1-1. Fluke Biomedical 180 Electrical Safety Analyzer ................................ 1-7
2-1. Block Diagram of a Typical DUT......................................................... 2-3
iii
Page 10

180
Users Guide
iv
Page 11

Chapter 1
Introduction and Specifications
Contents Page
Introduction.................................................................................. 1-3
Simplicity ................................................................................. 1-3
Versatility ................................................................................. 1-3
General Safety Considerations..................................................... 1-4
Symbols .................................................................................... 1-4
Warnings and Cautions............................................................. 1-4
Key Features ................................................................................ 1-6
Instrument Familiarity.................................................................. 1-7
Specifications ............................................................................... 1-10
Accessories................................................................................... 1-12
1-1
Page 12

180
Users Guide
1-2
Page 13

Introduction and Specifications
Introduction
1
Introduction
The Fluke Biomedical 180 Electrical Safety Analyzer, hereafter referred to as
the “Analyzer”, is a highly versatile and portable instrument. It is used for the
basic electrical safety evaluation of electrical systems, medical devices and
physiological instrumentation. Its compact handheld size makes it an ideal
addition to a service technician or engineer's toolbox for that "after service
test" as well as serving as a bench top instrument for the laboratory. It does not
sacrifice functions or accuracy, and its low cost permits putting one on each
bench.
Simplicity
The Analyzer is simple to use. A single master function switch, directly
labeled with the test to be performed, leads the user through a complete
measurement procedure. A single range meter for each measurement avoids
potential erroneous readings.
The Analyzer utilizes simple, yet sophisticated, electronics for true-rms
measurement of current and voltage. Input impedance uses the AAMI ES11993 test load to compensate for high frequency components in the
measurement. Resistance measurements are made with a four-wire Kelvin
bridge to eliminate errors due to cable length and connector resistance.
Versatility
Unique to the Analyzer is its capability to make a broad range of point-to-point
measurements. These include leakage current and/or voltage gradients and
resistance between two points. Also, the Analyzer provides the voltage and
measuring provision for the independent measurement of the isolation current
of a device. Thus the Analyzer provides the additional versatility for
evaluation of electrical systems, system installation, separate components,
isolation of probes, transducers, and conventional leakage current
measurements.
1-3
Page 14
180
Users Guide
General Safety Considerations
This instrument and related documentation must be reviewed for
familiarization with safety markings and instructions before you operate the
instrument.
Symbols
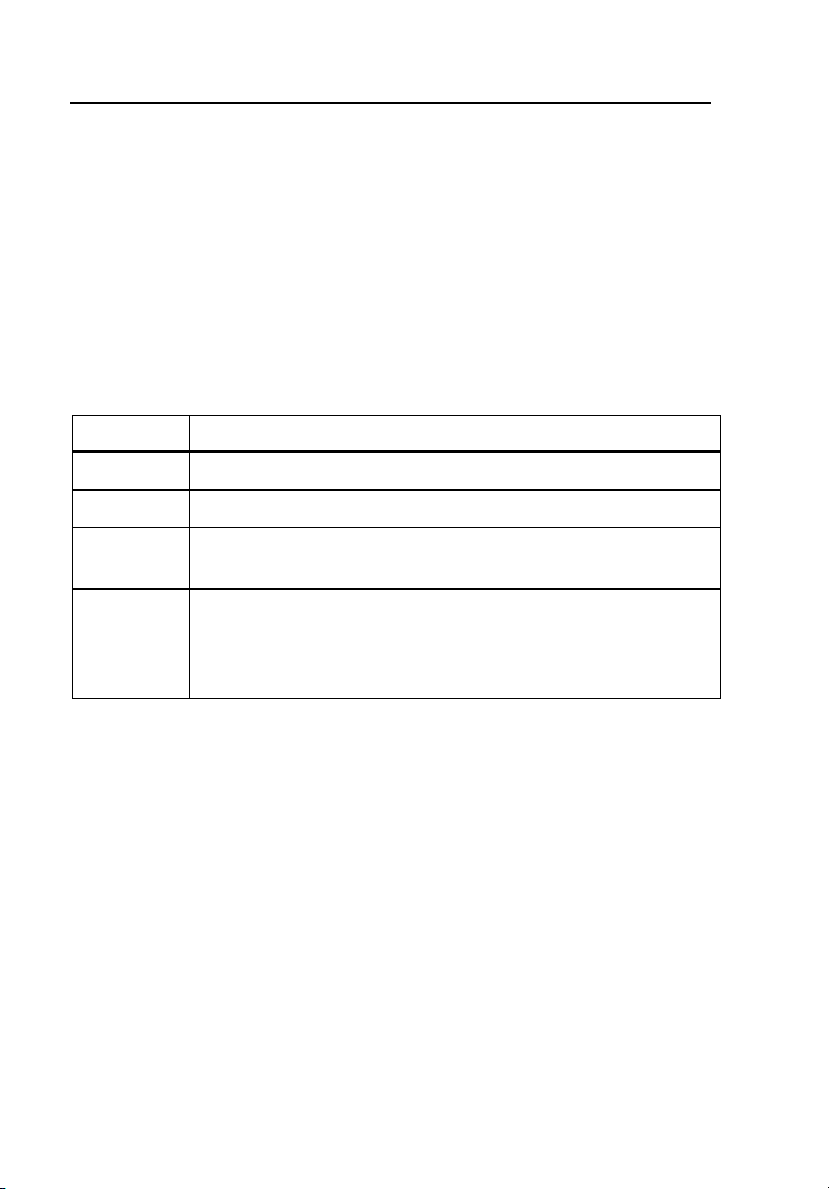
Table 1-1 describes the symbols used in association with this instrument.
Table 1-1. Symbols
Symbol Description
X Hazardous voltage
W Important information; refer to manual.
~
CAT I
Do not dispose of this product as unsorted municipal waste. Go
to Fluke’s website for recycling information.
IEC Measurement Category I – CAT I equipment designed to
protect against transients in equipment on circuits not directly
connected to MAINS. Under no circumstances should the
terminals of the Analyzer be connected to any MAINS voltage.
Warnings and Cautions
A Warning identifies hazardous conditions and actions that could cause
bodily harm or death.
A Caution identifies conditions and actions that could damage the Analyzer,
the equipment under test, or cause permanent loss of data.
1-4
Page 15

Introduction and Specifications
General Safety Considerations
XW Warning
To avoid possible electrical shock or personal injury,
follow these guidelines:
• Disconnect all patient connections before connecting
the device to be tested to the analyzer. Continued
connection may jeopardize patient safety by possible
application of measurement currents.
• Maintain care when making connections. Isolation test
utilizes 120 or 240 V ac applied to patient leads or to
external connections that are accessible to the tester.
Although the voltage is current limited by 120 kΩ
resistor per AAMI test procedure and is safe for
healthy intact skin contact, it can be felt and can result
in a startle reaction.
• Ensure the mains installation current rating is
adequate for the device under test. If the device under
test requires 20 A, the analyzer must be powered by a
20 A service to avoid overloading the mains
installation.
1
W Caution
To avoid possible damage to the Analyzer, follow these
guidelines:
• Perform the dual lead leakage test only with an
optional black test lead with the clamp with the red
insulation (Fluke Biomedical part numbers 2393448 or
2231563) in the jack labeled DUAL. Using a black test
lead with the clamp with the black insulation in the
DUAL jack may result in a blown internal fuse and false
readings of 000 to 002 μA, requiring the unit to be
returned for servicing.
Note
For point to point measurements, optional accessory cables must be
purchased.
1-5
Page 16

180
Users Guide
• Ensure that the device under test power requirements
are within the capabilities of the Analyzer, labeled as
20 A at 120 V ac and 10 A at 240 V ac. The Analyzer
requires that adapter cables match the appropriate
connector for line voltages other than 120V.
• Do not leave the device under test connected and
drawing high load current for extended periods. The
Analyzer is not designed for continuous
measurements and may overheat.
• Always pause in the off (middle) position when
switching polarity from normal to reverse. Inductive
loads of the device under test may create high voltage
transients when trying to reverse the direction of
current flow instantaneously.
Key Features
• Handheld instrument
• Test power line integrity
• Line voltage
• Instrument current
• Grounding resistance via 4-wire method
• Ground leakage current
• Enclosure (chassis) leakage current
• Patient (lead to ground) leakage current
• Patient (lead to lead) auxiliary current
• Patient isolation (sink) current
• Voltage gradients
• Device-to-device resistance
• Device-to-device leakage current
• Probe and transducer isolation current
• True-rms measurement
• AAMI test load
1-6
Page 17

Introduction and Specifications
Instrument Familiarity
1
Instrument Familiarity
The Analyzer is shown in Figure 1-1 , and Table 1-2 describes its labeled
components.
1
2
10
7
3
L
E
- GROUND
A
K
- CHASSIS
A
- LEAD -GND
G
E
120 VOLTS 20 AMP
5 MINUTES MAX
OPEN
- LEAD -LEAD
µA
- DUAL
NEUTRAL
CLOSED
REVERSED
OUTLET
OK
180
ELECTRICAL SAFETY ANALYZER
V - LINE VOLTS
A - CURRENT
- RESISTANCE
OUTLET
OPEN GND
NORMAL
OFF
rl
ra
REV
POL
la
ll
C
HAZARDOUS LIVE
VOLTAGE DURING
ISO TEST
DUAL
LEAD
la
all
ll
C
CHASSIS
ISO TEST
6
5
4
Figure 1-1. Fluke Biomedical 180 Electrical Safety Analyzer
9
12
11
8
fat01.eps
1-7
Page 18

180
Users Guide
Table 1-2. Components and Controls of the Analyzer
Label Name Function
Supplies power to the Electrical Safety
Analyzer and to the device under test (DUT).
A Power Cord
B Outlet Indicators
C Test Receptacle
D Outlet Switch
The measurement circuits are energized when
the power cord is plugged into an outlet. There
is no on/off switch.
Verify the polarity and wiring of the outlet to
which the Analyzer is connected. Only correctly
wired outlets should be used. Not applicable to
isolated power systems.
Supplies power to the DUT. This outlet is
powered if the OUTLET switch is set to
NORMAL or REVERSED and the NEUTRAL
switch is CLOSED.
If in the center off position, there is no power to
the DUT receptacle. This switch permits testing
with both normal (forward) and reversed
polarity of the line. It is recommended that the
switch be paused in the center OFF position
before changing polarity. Note: Failure to pause
the three-position switch in the OFF position
may cause switch damage or blow the internal
pico fuse.
E Neutral Switch
1-8
Permits making leakage current measurements
under the OPEN condition as required by UL
and IEC.
Page 19

Introduction and Specifications
Instrument Familiarity
Table 1.2. Components and controls of the Analyzer (cont.)
This is a dual-function momentary switch that
must be held in position while performing the
test. The OPEN GND position will open ground
to the device for leakage current
measurements. The ISO TEST position will
Lift Ground/ISO
F
Test Switch
G Function Switch
energize the selected patient lead at 110
percent line voltage, current limited, to measure
the isolation current when the main FUNCTION
switch is in the LEAD ISO position. With the
FUNCTION switch in the DUAL position, the
isolation test voltage is supplied to the DUAL
connector for measuring the isolation current of
a probe or transducer.
Provides direct, one-step selection of the
measurement to be made. These are line V ac,
instrument current, grounding resistance,
ground (internal) and chassis (external)
leakage currents, and the patient leakage
currents. These include lead to ground
(source), lead to lead (auxiliary) and isolation
(sink) current. A dual position is provided to
measure leakage current between two points or
isolation current of probes and transducers
independent of their instruments.
1
Directs the selected patient lead measurement
H Lead Switch
I Meter
Universal
J
Patient Lead
Terminals
to the desired lead. When testing a 10-lead
device, a second pass will be required for the C
leads.
This is a large, ½ inch, high-contrast LCD, 3½digit display of the measured parameter. This
will read up to 1999 with decimal points added
where required.
Provide means for connection of the patient
leads for leakage current measurement.
1-9
Page 20

180
Users Guide
Table 1.2. Components and controls of the Analyzer (cont.)
Chassis
K
Connector
L Dual Connector
Specifications
Provides a means for inputting the chassis
cable with its clip for connection to the DUT
chassis or enclosure. The chassis ground
resistance is measured with the FUNCTION
switch in the RESISTANCE position, and the
chassis leakage current is measured in the
CHASSIS position.
Used to make point-to-point measurements
with optional leads available for purchase. For
leakage and voltage measurements, the black
cable with the clamp with black insulation is
attached to the CHASSIS connector, and the
black cable with the clamp with red insulation to
the DUAL connector. For point-to-point
resistance measurements, the black cables
with clamps with black insulation are attached
to the DUAL and CHASSIS connectors.
The following are specifications for the Analyzer. Please contact your Fluke
Biomedical service representative for more information regarding the device
specifications.
Line Voltage
Range .....................................................................90 – 240 V ac 50/60 Hz
Accuracy................................................................. ±3 % of reading, ±1 LSD
Load Current
Range .....................................................................0 – 19.99 A
Accuracy.................................................................±5 % of reading, ±1 LSD
1-10
Page 21

Introduction and Specifications
Specifications
1
Ground Resistance
Range .................................................................... 0 – 2.00 Ω
Accuracy ................................................................ ±1 % of reading, ±1 LSD
Range .................................................................... 2.01 – 19.99 Ω
Accuracy ................................................................ ±3 % of reading, ±1 LSD
Current source ....................................................... 10 mA dc
Type....................................................................... 4-wire bridge
Leakage Current
Range .................................................................... 0 – 1999 μA
Accuracy:
DC and 25 Hz to 1 kHz ...................................... ±1 % of reading, ±3 LSD
1.0 kHz to 100 kHz ............................................ ±2.5 % of reading, ±3 LSD
100 kHz to 1 MHz .............................................. ±5 % of reading, ±3 LSD
Type measurement............................................ True rms
Impedance ......................................................... 1000 Ω, AAMI ESI 1993
Isolation Test
Voltage................................................................... 110 % Line voltage ±5%
Current................................................................... limited by 120 kΩ resistor
Current Capacity
Line 90 to 140 V ac................................................ 20 A max. on time 5 min.
Line over 140 V ac................................................. 10 A
15 A max. on time 30 min.
Current Consumption
ESA 180................................................................. 0.1 A @ 120 V – 60 Hz
Environmental
Operating Temperature ......................................... 15 – 40 °C
Storage Temperature............................................. -20 – 65 °C
Relative Humidity................................................... 90 % max.
Mains voltage range .............................................. 90 to 240 V ac
1-11
Page 22

180
Users Guide
Accessories
The following are accessories for the Analyzer. To order, contact your Fluke
Biomedical equipment dealer and use the Fluke Biomedical part numbers
provided. Table 1-3 lists standard accessories shipped with the Analyzer; Table
1-4 lists optional accessories that must be ordered.
Table 1-3. Standard Accessories
Description Quantity Shipped Part Number
Users Guide 1 2185829
8-foot black cable –
with large clamp with
black insulation
Table 1-4. Optional Accessories
Description Part Number
8-foot black cable – with large clamp
with black insulation (used for dual
lead resistance)
16-foot black cable – with large
clamp with black insulation
8-foot black cable – with large clamp
with red insulation
16-foot black cable – with large
clamp with red insulation
Soft carrying case 2248864
220 V Adapter kit 2185787
1 2392409
2392409
2392411
2392448
2231563
1-12
Page 23

Chapter 2
Operation, Maintenance, and Service
Contents Page
Preliminary Steps ......................................................................... 2-3
Preparing the Analyzer for Use ................................................ 2-3
Verifying the Power Outlet Connections.................................. 2-4
Measuring Line Voltage............................................................... 2-5
Measuring Device Current ........................................................... 2-6
Measuring Chassis Grounding Resistance ................................... 2-7
Measuring Leakage Current......................................................... 2-8
Ground Leakage Current .......................................................... 2-8
Chassis (Enclosure) Leakage Current....................................... 2-9
Lead-to-Ground (Patient Source) Current ................................ 2-10
Lead-to-Lead (Auxiliary) Current ............................................ 2-12
Lead Isolation (Patient Sink) Current....................................... 2-13
Point-to-Point Measurements....................................................... 2-14
Leakage Current ....................................................................... 2-14
Isolation Current....................................................................... 2-15
Resistance................................................................................. 2-16
Maintenance ................................................................................. 2-17
Avoiding Damage..................................................................... 2-17
Cleaning.................................................................................... 2-17
Service and Calibration ................................................................ 2-18
Packing Instructions ................................................................. 2-18
Shipping.................................................................................... 2-19
2-1
Page 24

180
Users Guide
2-2
Page 25

Operation, Maintenance, and Service
Preliminary Steps
2
Preliminary Steps
The Analyzer measures electrical parameters, as described below. These
voltages and currents are a natural phenomenon, and their presence within
reasonable limits does not constitute a hazard. However, it is necessary to
measure their values to determine if there is a significant change from previous
measurements or from the device specifications.
Figure 2-1 is a block diagram of a typical line-operated instrument with patient
connections. All measured parameters are labeled, and the following topics are
keyed to the labels in the diagram.
C
L
Z
I
I
L
Hot
Neutral
Ground
V
L
Z
L
R
L
C
L
I
E
R
G
Grounded
Data and
Control
Circuits
Chassis
Isolated
Patient
Circuits
I
A
I
I
120/220
VAC
I
P
I
C
Earth
Enclosure
fat02.eps
Figure 2-1. Block Diagram of a Typical DUT
Preparing the Analyzer for Use
WX Warning
To avoid possible electric shock, burning of the skin, or
personal injury to the patient, disconnect all patient
connections to the device before starting the following
procedure.
2-3
Page 26

180
Users Guide
Prepare the Analyzer for use as follows:
1. To start from the same position each time, place the Analyzer's switches in
the following starting positions:
FUNCTION switch V- LINE VOLTS
LEAD switch RL
NEUTRAL switch CLOSED
OUTLET switch OFF [center]
2. Plug the Analyzer into a properly rated outlet.
The Analyzer is equipped with a hospital-grade power plug. Grounding
reliability can be achieved only when the Analyzer is connected to an
equivalent power receptacle marked "Hospital Grade." Grounding is
important for personnel safety and to make some of the tests offered by
the Analyzer. Do not circumvent this step for any reason.
Verifying the Power Outlet Connections
Note
Not applicable to isolated power systems.
Three neon lamps indicate the polarity and condition of the outlet being used,
as shown in the following chart:
REV
● ● ● ○ ○ ○
OK ● ○ ○ ○ ● ●
○ ● ○ ○ ● ●
Correct
wiring
Reverse
polarity
Open
ground
Open hot
Open
neutral
Hot /
ground
reversed
Note
Review all power outlets that do not indicate "correct wiring" and
have them corrected by a qualified electrician.
If the line is found faulty, correct the problem before proceeding. If the line
checks OK, plug the device to be tested into the Analyzer's line receptacle.
2-4
Page 27

Operation, Maintenance, and Service
Measuring Line Voltage
2
WX Warning
To avoid electrical shock due to faulty DUT, DO NOT touch
the metal chassis, any accessible conductive part, or
terminal of the DUT with mains power applied until all
applicable tests have been completed and the product is
verified as compliant. Keep fingers behind guards on
supplied test accessories and/or remove power from the
DUT before making necessary connections.
Measuring Line Voltage
Line Voltage [VL] is the mains power supplied by the electrical distribution
system of the hospital. It is a three-wire system of HOT, NEUTRAL, and
GROUND, with NEUTRAL, like GROUND, returned to true earth at their
entry into the building.
The Analyzer measurement is made between the HOT and NEUTRAL wires
via transformer coupling to isolate the measuring circuits from the line. As
recommended, measurement of the line voltage with the device under test
(DUT) OFF and then ON indicates whether the line is adequate for the device.
Use the following steps to measure line voltage:
1. From the recommended start position, with the FUNCTION switch in the
V - LINE VOLTS position, observe that the meter displays the line
voltage with a resolution of one volt.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat03.eps
2. With the OUTLET switch in the NORMAL position and the DUT turned
on, the meter continues to display line voltage, but under the load of the
2-5
Page 28

180
Users Guide
device being tested. Depending on the device's operating current and the
electrical supply wiring, the voltage differential may be significant.
3. Check the value under load against the device's ratings to ensure that the
actual value remains within prescribed limits. An excessive drop also
suggests that a dedicated line of increased capacity should be run to the
instrument.
Measuring Device Current
Device, or Instrument, Current [IL] is the current used by the DUT. When
turned on, the device should be operated in its various modes to determine the
worst condition to track. Verify that the current of the DUT is within the
current rating of the Analyzer being used.
Measurement is made in the HOT wire via transformer coupling to ensure that
the total current is measured, as it is possible that the NEUTRAL and
GROUND wire could share the return path.
Use the following steps to measure device current:
1. Switch the FUNCTION switch to A - CURRENT. The meter displays the
device's current to 19.99 A.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat04.eps
2. Place the OUTLET switch in the NORMAL position; the NEUTRAL
switch remains in the CLOSED position.
3. Turn on the device and place it in its maximum load condition to obtain
the proper reading.
4. Log this data to note changes in values that indicate early problems.
2-6
Page 29

Operation, Maintenance, and Service
Measuring Chassis Grounding Resistance
Measuring Chassis Grounding Resistance
Note
This test is only applicable to devices utilizing three-wire (grounded)
power cords.
2
Ground Wire Resistance [R
] (grounding resistance) is the resistance from the
G
device's conductive "grounded" chassis to the grounding terminal on the
receptacle into which it is plugged. The resistance is largely composed of the
GROUND wire in the power cable and is directly proportional to its length.
Use the following steps to measure chassis grounding resistance:
1. Connect the standard cable supplied with unit that has a black cable with
the clamp with black insulation to the CHASSIS connector on the front
panel of the Analyzer.
2. Clamp the clip of the cable to the DUT's exposed chassis or the enclosure,
if conductive. Resistance is measured between the clip on the black
chassis cable and the grounding pin receptacle of the Analyzer.
Take care to ensure that bare metal is reached and that both jaws of the
clip are in contact with the chassis. Metal labels or incidental conductive
hardware should not be used for this test.
If a non-conducting enclosure is used and no chassis is readily accessible,
a safety ground terminal can be used.
3. Once connection is made, rotate the FUNCTION switch to
Ω - RESISTANCE and read its value directly in ohms to 19.99 Ω.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat05.eps
2-7
Page 30

180
Users Guide
Note
The OUTLET switch should be in the OFF (center) position for this
measurement.
Measuring Leakage Current
Leakage current is the flow of current through or over the surface of an
insulating material or insulator. For example, if a person comes in contact with
an operational device, leakage current is the amount of current that flows from
the point where the person came in contact with the device product, through
the person’s body, and back to ground. Measurement of leakage current is
required on all mains power products.
Ground Leakage Current
Ground Leakage Current [IE] (internal chassis current) is the current that flows
in the ground wire of the power cable to return the chassis leakage current to
true earth ground. It is only applicable to devices utilizing three-wire
(grounded) power cords.
This current does not constitute a hazard as long as the ground wire remains
intact and the current does not become excessive. Leakage current is due to the
proximity of the hot wire or line potential components to the chassis
represented by Z
components.
, a combination of capacitance, CL, and resistance, RL,
L
Note
The connection is made internally in the Analyzer, so no external
connectors are required for this test.
Use the following steps to measure ground leakage current:
1. Set the FUNCTION switch to GROUND. The leakage current is
displayed to 1999 μA (microamperes).
2-8
Page 31

Operation, Maintenance, and Service
Measuring Leakage Current
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
2
fat06.eps
2. Make measurements under all combinations of the OUTLET switch,
NORMAL and REVERSE; the NEUTRAL switch CLOSED and
OPEN; and with the device power turned ON and OFF. Power to the
outlet is OFF when the NEUTRAL switch is in the OPEN position.
Note
Be sure to pause in the OFF (middle) position when switching the
OUTLET switch from the NORMAL to the REVERSE position.
Chassis (Enclosure) Leakage Current
Chassis [Enclosure] Leakage Current [IC] flows between the accessible
conductive chassis or enclosure and earth (ground) measured through a
1,000 Ω impedance.
The Analyzer measures chassis leakage from the exposed metal part on the
DUT, through the black cable and the AAMI load, back to the ground.
Use the following steps to measure chassis leakage current:
1. Connect the standard cable (supplied with unit) that has a black cable with
the clamp that has black insulation to the CHASSIS connector on the
front of the Analyzer.
2. Clamp the clip on the cable in turn to accessible conductive sections of the
chassis and the enclosure. Metal labels or incidental conductive hardware
are not applicable for this test.
If a non-conducting enclosure is used, present standards require
connection to the enclosure via a 200 cm² conductive foil in intimate
contact with the enclosure. This can be accomplished with a 14 X 14 cm
2-9
Page 32

180
Users Guide
(5.5 X 5.5 in) piece of aluminum foil taped to the surface and the cable
clipped to the foil.
3. To make the measurement, place the FUNCTION switch in the
CHASSIS position and read the display in microamperes.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
4. Make measurements under all combinations of the OUTLET switch,
NORMAL and REVERSE; the GROUND switch CLOSED and OPEN;
the NEUTRAL switch CLOSED and OPEN; and with the device power
turned ON and OFF. Power to the outlet is OFF when the NEUTRAL
switch is in the OPEN position.
fat07.eps
Note
Be sure to pause in the OFF (middle) position when switching the
OUTLET switch from the NORMAL to the REVERSE position.
Lead-to-Ground (Patient Source) Current
Lead-to-Ground [IP] (patient source) current would flow between an individual
patient lead and ground if the patient were to come into contact with earth
ground. An example is a patient with leads attached touching ground such as
an electric bed.
Note
Although originally required only for devices incorporating intracardiac electrodes or conductive pathways directly to the heart, leadto-ground current has found its way into standards for all devices
having patient-applied parts.
2-10
Page 33

Operation, Maintenance, and Service
Measuring Leakage Current
2
Use the following steps to measure lead-to-ground current:
1. Connect the patient leads to the corresponding snaps on the top of the
Analyzer. If the device has 10 leads, connect the limb leads and C1 (V1)
initially and repeat with C2 (V2) through C6 (V6). Lead nomenclature for
this test is not important.
2. Select LEAD-GND with the FUNCTION switch and read leakage current
in microamperes for any combination of the patient lead selected by the
LEAD switch.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat08.eps
3. Make measurements under all combinations of the OUTLET switch,
NORMAL and REVERSE; the LIFT GROUND switch CLOSED and
OPEN; and the device power turned ON and OFF.
4. Rotate the LEAD switch to each lead to test individually and then to ALL
for testing with all leads connected together.
LEADLEAD
lala
ra
rlrl
ll
c
allall
fat17.eps
Current measured should be the same for all leads, including the ALL
position, as the current represents the isolation impedance to the patient
circuit.
2-11
Page 34

180
Users Guide
Lead-to-Lead (Auxiliary) Current
Lead-to-Lead [IA] (auxiliary) current flows from any patient lead to any other
patient lead and to all other leads connected together. The current can be dc or
ac or a combination of both. Measurements are made with a true-rms converter
to provide the common base necessary for accurate readout with a variety of
common wave forms.
Under normal conditions, the current is primarily input bias current,
measurement current, or lead off sensing current. The worst-case condition is
measured from the individual lead to all others connected together. This is the
measurement made by the Analyzer.
Use the following steps to measure lead-to-lead current:
1. Connect the patient leads to the snaps on top of the Analyzer.
2. Set the FUNCTION switch in the LEAD-LEAD position.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat09.eps
3. Make readings for individual leads, selecting by the LEAD switch. The
single lead carrying the most current is the reference lead, R
, acting as
L
the return for the other leads.
LEADLEAD
lala
ra
rlrl
ll
c
allall
fat12.eps
2-12
Page 35

Operation, Maintenance, and Service
Measuring Leakage Current
Note
The ALL position has no meaning in this test.
4. Make measurements under all combinations of the OUTLET switch,
NORMAL and REVERSE; GROUND CLOSED and the LIFT
GROUND switch OPEN; and the device power turned ON and OFF.
5. If the DUT utilizes a 10-lead patient input, test each C (V) lead in turn,
individually.
2
Lead Isolation (Patient Sink) Current
Lead Isolation [II] (patient sink) current would flow into the DUT if the patient
were to come into contact with full line voltage. An example is an electric bed
that has become ungrounded and has a short to the frame.
WX Warning
To avoid possible electric shock, burning of the skin, or
personal injury to the patient, take care when handling the
patient leads. High voltage, 110 percent of line volts, with
respect to earth ground is accessible at the patient
connections (snaps) during part of this test. The patient
lead isolation current can flow in individual leads or all
leads connected together if line volts come into contact
with the patient.
Use the following steps to measure lead isolation current:
1. Attach the patient leads to the snaps on top of the Analyzer, making sure
that ground is intact.
2. Set the FUNCTION switch in the LEAD ISO position.
2-13
Page 36

180
Users Guide
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
3. Test individual leads, selecting by the LEAD switch.
4. To apply the high voltage to the lead safely, press the LIFT
GROUND/ISO TEST switch to ISO TEST. The current is limited with a
120 kΩ resistor for user protection.
5. While ISO TEST is energized, read the isolation current in
microamperes.
6. Perform the test using the OUTLET switch in both NORMAL and
REVERSE, the NEUTRAL switch CLOSED, and with the DUT ON
and OFF.
fat10.eps
Point-to-Point Measurements
The Analyzer provides the capability of making point-to-point leakage current,
isolation current, and resistance measurements between selected points,
otherwise known as a dual lead measurement.
Note
Additional optional accessory cables must be purchased to perform
these tests.
Leakage Current
Leakage Current between two points is also measured for permanently
installed equipment as additional verification of the integrity of the installation.
These measurements are equivalent, as the relationship between volts and
current across 1,000 Ω is one mV per μA.
2-14
Page 37

Operation, Maintenance, and Service
Point-to-Point Measurements
2
Use the following steps to measure leakage current between two points:
1. To isolate the measurement circuit from ground, do not plug any device
into the Analyzer's receptacle.
2. Connect the black cable with the clamp with black insulation to the
CHASSIS connector and the black cable with the clamp with red
insulation to the DUAL connector.
3. Set the FUNCTION switch in the DUAL position.
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
fat11.eps
4. Clip the two cables to the two points for which the leakage current is to be
measured. The meter displays the leakage current between the two points
to 1999 µA. The number displayed is also the voltage gradient in
millivolts between the two points based on a volt meter with an input
impedance of 1000 Ω.
Isolation Current
Isolation testing of probes and transducers making internal contact to a patient
is provided to ensure the reliability of the isolation barrier. These devices
incorporate electrical circuits that can introduce or sink hazardous currents to a
patient who comes into contact with line potential or who becomes grounded,
as previously described.
Use the following steps to measure isolation current between two points:
1. To isolate the measurement circuit from ground, do not plug any device
into the Analyzer's receptacle.
2-15
Page 38

180
Users Guide
2. Connect the black cable with the clamp with black insulation to the
CHASSIS connector and the black cable with the clamp with red
insulation to the DUAL connector.
3. Toggle the LIFT GROUND/ISO TEST switch to ISO TEST to apply
isolated line voltage between the two sides. The meter displays the
isolation current that flows.
Note
The actual method for making connection to either side of the
isolation barrier varies with the device to be tested; therefore, full
details are not provided.
W X Warning
To avoid possible electric shock, burning of the skin, or
personal injury take care when handling the cables. High
voltage, 110 percent of line volts, will be accessible
between the two cables when the LIFT GROUND/ISO TEST
switch is in the ISO TEST position.
Resistance
Resistance measurements between two points are made to verify the integrity
of permanently installed equipment whose ground cannot be broken to
measure the chassis leakage current. These are usually high-power devices that
can have high leakage currents and depend on the bonding of all chassis to a
common point for safety.
To measure resistance between two points, follow the steps below:
1. Disconnect any device attached to the Analyzer instrument receptacle.
2. Turn off any electrical devices to be tested.
3. Connect two black cables with clamps with black insulation to the
CHASSIS and DUAL connectors, and clip onto the two points to be
measured.
4. Set the FUNCTION switch to Ω - RESISTANCE, and the display will
show the resistance between the two points.
2-16
Page 39

Operation, Maintenance, and Service
Maintenance
- LINE VOLTS- LINE VOLTS
VV
AA
- CURRENT- CURRENT
- CURRENT- CURRENT
- RESISTANCE- RESISTANCE
- RESISTANCE- RESISTANCE
- GROUND- GROUND
- GROUND- GROUND
L
E
- CHASSIS- CHASSIS
- CHASSIS- CHASSIS
A
K
- LEAD -GND- LEAD -GND
- LEAD -GND- LEAD -GND
A
- LEAD -LEAD- LEAD -LEAD
- LEAD -LEAD- LEAD -LEAD
G
E
- LEAD ISO- LEAD ISO
- LEAD ISO- LEAD ISO
µAµA
- DUAL- DUAL
- DUAL- DUAL
2
fat05.eps
5. To compensate for possible dc components in the leakage current flowing
between the two points, reverse the two clips and average the readings.
Maintenance
The Analyzer requires little maintenance or special care; however, it is a
calibrated measuring instrument and should be treated as such. The following
describes how to maintain the Analyzer.
Avoiding Damage
Do not drop the instrument or subject it to any mechanical abuse that could
cause a shift in the calibrated settings.
W Caution
To avoid damage to the Analyzer or adverse affects on its
performance, follow these guidelines:
• Do not expose the system to temperature extremes.
Ambient temperatures should remain between 15° C
and 40° C. System performance may be adversely
affected if temperatures fluctuate above or below this
range.
Cleaning
Clean the exterior of the Analyzer occasionally with a cloth dampened with a
mild detergent solution. Take care to keep liquids out of the device.
2-17
Page 40

180
Users Guide
W Caution
To avoid damage to the Analyzer or adverse affects on its
performance, clean it only by gently wiping down with a
clean, lint-free cloth dampened with a mild detergent
solution. Do not spray liquids on or immerse the unit.
Carefully wipe down the cables and inspect them for damage and deterioration
of the insulation. Check the cable connections for integrity of the cable clamp
and strain relief.
Service and Calibration
If your new Analyzer fails to operate successfully, please contact Fluke
Biomedical Service Center immediately, as indicated under “Warranty and
Product Support.”
W Caution
To avoid damage to the Analyzer or adverse affects on its
performance, allow only qualified technical personnel to
service the Analyzer.
Annual calibration of the Analyzer by an authorized Fluke Biomedical Service
Center is recommended. Fluke Biomedical Service Centers have the
appropriate tools and procedures for performing calibrations as well as factoryauthorized updates.
International customers should contact their Fluke Biomedical dealers for
service/product support.
To obtain the name of your local dealer or service center, contact Fluke
Biomedical as indicated under “Return Procedures, Repair and calibration.”
Packing Instructions
If repairs are required, return the Analyzer to the factory or the nearest service
center, as follows:
1. Before returning the Analyzer for factory service, contact the Fluke
Biomedical Service Center for a required Return Authorization Number.
2. Provide the following information:
2-18
Page 41

Operation, Maintenance, and Service
Service and Calibration
• The Analyzer serial number
• The specific steps that reproduce your problem
• A daytime phone number
• Your name/company
• A fax number (if available)
3. Pack the instrument carefully, using the original packing materials. If the
original packing materials are not available, refer to “Return Procedures”
for a list of preferred materials or contact Fluke Biomedical for
replacement packing. Failure to pack the instrument properly could void
your warranty.
2
Shipping
1. Place the Return Authorization Number in a prominent place on the
outside of the packing box, and refer to the number in any correspondence
with Fluke Biomedical Service.
2. Enclose your return address and Return Authorization Number.
3. Insure the unit for full retail value and ship to the nearest Fluke
Biomedical service center.
2-19
Page 42

180
Users Guide
2-20
Page 43

Appendices
Appendix Title Page
A Abbreviations............................................................................... A-1
Page 44

180
Users Guide
Page 45

Appendix A
Abbreviations
Abbreviations
The following list includes abbreviations used in this document.
A ampere
ANSI American National Standards Institute
AAMI
dB decibel
°C degrees Celsius (centigrade)
DUT device under test
ECG electrocardiograph or electrocardiogram
EUT equipment under test
°F degrees Fahrenheit
Hz hertz
in inch
k kilo (10
kHz kilohertz
kΩ kilohm
LED light-emitting diode
M meg(a) (106)
MHz megahertz
MΩ megohm
Association for the Advancement of Medical
Instrumentation
3
)
A-1
Page 46

180
Users Guide
m meter
m milli (10-3)
mA milliampere
mm millimeter
mV millivolt
s second
-6
µ micro (10
µA microampere
µV microvolt
Ω ohm
)
A-2
 Loading...
Loading...